Abstract
OBJECTIVE
Because of reduced antioxidant defenses, β-cells are especially vulnerable to free radical and inflammatory damage. Commonly used antirejection drugs are excellent at inhibiting the adaptive immune response; however, most are harmful to islets and do not protect well from reactive oxygen species and inflammation resulting from islet isolation and ischemia-reperfusion injury. The aim of this study was to determine whether redox modulation, using the catalytic antioxidant (CA), FBC-007, can improve in vivo islet function post-transplant.
RESEARCH DESIGN AND METHODS
The abilities of redox modulation to preserve islet function were analyzed using three models of ischemia-reperfusion injury: 1) streptozotocin (STZ) treatment of human islets, 2) STZ-induced murine model of diabetes, and 3) models of syngeneic, allogeneic, and xenogeneic transplantation.
RESULTS
Incubating human islets with catalytic antioxidant during STZ treatment protects from STZ-induced islet damage, and systemic delivery of catalytic antioxidant ablates STZ-induced diabetes in mice. Islets treated with catalytic antioxidant before syngeneic, suboptimal syngeneic, or xenogeneic transplant exhibited superior function compared with untreated controls. Diabetic murine recipients of catalytic antioxidant–treated allogeneic islets exhibited improved glycemic control post-transplant and demonstrated a delay in allograft rejection. Treating recipients systemically with catalytic antioxidant further extended the delay in allograft rejection.
CONCLUSIONS
Pretreating donor islets with catalytic antioxidant protects from antigen-independent ischemia-reperfusion injury in multiple transplant settings. Treating systemically with catalytic antioxidant protects islets from antigen-independent ischemia-reperfusion injury and hinders the antigen-dependent alloimmune response. These results suggest that the addition of a redox modulation strategy would be a beneficial clinical approach for islet preservation in syngeneic, allogeneic, and xenogeneic transplantation.
Hypoxia is the leading cause of β-cell death during islet isolation and transplantation (1), with the highest percentage of islet graft loss and dysfunction occurring just days after transplantation (2,3). Because islets are a cellular transplant, devoid of intrinsic vasculature (1,4), they are exceptionally susceptible to ischemia-reperfusion injury. Islets are also increasingly vulnerable because they have inherently decreased antioxidant capacity (5–10), making them prone to oxidative/nitrosative/free radical damage. The antigen-independent complexities of islet transplantation increase the incidence of primary graft nonfunction and β-cell death, thus requiring protection for islets at early stages of the transplant procedure (11).
In addition to antigen-independent innate-mediated inflammatory injury, islet allografts are also plagued by the antigen-dependent T-cell mediated alloimmune response, which necessitates immunosuppressive drugs for allograft survival. Commonly used antirejection drugs are excellent at inhibiting the adaptive immune response, although most are harmful to islets and do not protect well from reactive oxygen species and inflammation during islet isolation and ischemia-reperfusion injury (12–14). In their review, Balamurugan et al. (13) concluded that successful islet transplantation in type 1 diabetes necessitates islet-sparing immunosuppressive agents that combat recurrent autoimmunity with low islet toxicity. Predominantly, the field of islet transplantation is devoid of cytoprotective agents that promote islet survival and function by inhibiting nonspecific innate-mediated inflammation during islet isolation and early inflammatory events in islet transplantation (11,13,15–19).
The first phase of immunity involves innate immune activation and subsequent proinflammatory signals required for optimal adaptive immune function (20–22), yet the majority of immunosuppressive drugs only target adaptive immune function (17,23), the second phase of immunity. A nontoxic, cell-permeable catalytic antioxidant (CA) redox modulator, FBC-007 [manganese(II) tetrakis (N-ethylpyridium-2-yl)porphyrin], is able to depress free radical and cytokine production by antigen-presenting cells (24) and T cells in transgenic and allospecific mouse models (20,25). Additionally, redox modulation inhibits cytotoxic lymphocyte target cell lysis by reducing the production of intracellular cytolytic molecules (perforin and granzyme B) in a mixed leukocyte reaction without toxicity (25), preserves and promotes human islet function in vitro (15,16), prevents the transfer of diabetes into young NOD.scid mice (26), and inhibits innate-immune nuclear factor (NF)-κB activation (24). Thus, islet-sparing agents, which decrease the production of free radicals and, therefore, inflammatory cytokines, may have a positive impact on islet function post-transplant.
Because islet transplantation can benefit from agents that inhibit early inflammatory cascades to preserve islet function (18), we hypothesize that redox modulation holds potential as a therapy in islet transplantation to decrease the incidence of β-cell primary nonfunction. To further test the effects of redox modulation using CA we treated human islets with streptozotocin (STZ) in vitro and treated mice in vivo with STZ, both in the presence or absence of CA, to mimic antigen-independent free radical damage and inflammation of post-transplant ischemia-reperfusion injury. To examine the effects of islet-directed CA treatment on innate-mediated (antigen-independent) primary islet nonfunction in vivo, we performed syngeneic (175 islets/recipient), suboptimal syngeneic (100 islets/recipient), allogeneic (300 islets/recipient), and xenogeneic (400–500 islets/recipient) islet transplants to assess islet function. Additionally, we performed (300 islets/recipient) islet transplants in diabetic recipients to assess islet function in the presence or absence of systemic redox modulation in an allogeneic transplant setting inclusive of both innate (antigen-independent) and adaptive (antigen-dependent) immune responses. Our results demonstrate that islet-directed and systemically delivered redox modulation, administered in the absence of an additional immunosuppressive regimen, preserve islet function post-transplant.
RESEARCH DESIGN AND METHODS
Human islets.
Human pancreata were obtained from CORE (Center for Organ Recovery and Education, Pittsburgh, PA) and were harvested using standard multiorgan recovery techniques, and islets were isolated as previously described (15).
In vitro human islet experiments.
Islet preparations were cultured in flasks at 37°C in an atmosphere of 5% CO2 in humidified air in human islet medium containing CMRL-1066 (Gibco-BRL, Carlsbad, CA), 5.5 mmol/l low glucose medium supplemented with 10% FCS, 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 2 mmol/l l-glutamine (Life Technologies, Grand Island, NY). Human islets were available to us after 3 days of culture. The islets were hand-picked on the fourth day using a dissecting microscope. Groups of 60 hand-picked islets were randomized to control and experimental groups. Each group was subcultured in 60 × 15-mm Falcon dishes at a concentration of 12 islets/ml for 8 h in the previously described islet media. The catalytic antioxidant group was treated with 68 μmol/l CA and the STZ group was treated with 11 mmol/l STZ, whereas the control group was cultured in islet media alone. The group treated with CA and STZ was treated with 68 μmol/l CA 20 min before the addition of 11 mmol/l STZ. We chose the dose of 68 μmol/l FBC-007 for use in these experiments based on the ability of FBC-007 to scavenge superoxide compared with the native manganese superoxide dismutase (MnSOD). On a per mass basis, 34 μmol/l is the concentration of FBC-007 that is needed to have the same activity as the endogenous MnSOD enzyme in most cells, save islet β-cells, which have reduced levels of MnSOD. We saw a benefit in doubling the dose of this nontoxic compound to 68 μmol/l for our studies.
Human islet viability.
Islet viability was determined by simultaneous staining of live and dead cells using a two-color fluorescence assay (acridine orange (green = live) and ethidium bromide (red = dead); Sigma, St. Louis, MO). After the 8-h incubation, all islets from each group were transferred into separate microcentrifuge tubes, washed with PBS, and spun at 2000 rpm for 2 min. Supernatants were carefully aspirated, leaving ∼25 μl to allow resuspension of the cell pellets. Next, 1.3 μl of dye mix (100 μg/ml acridine orange + 100 μg/ml ethidium bromide in PBS) was added to each tube to stain all islet cell nuclei. The tube was mixed gently, 25 μl cell suspension was transferred to a microscope slide, and a cover slip was placed on top of the suspension. Cells were visualized at ×10 magnification using a fluorescence microscope with an excitation of 450–490 nm. At least three fields from each group were analyzed by ImageJ (National Institutes of Health, Bethesda, MD) software. The percentage of viable and dead cells was determined by linearly converting ImageJ arbitrary units into percentages.
Mice.
Male 6–8 week old C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-Ins2Akita/+ breeder pairs (female C57BL/6 + male C57BL/6-Ins2Akita/+) were also purchased from The Jackson Laboratory but bred in-house at the Rangos Research Center (Pittsburgh, PA). Male C57BL/6-Ins2Akita/+ mice develop spontaneous diabetes by 4 weeks of age and do not require exogenous insulin or fluids/electrolytes to thrive. This strain is well suited for transplantation studies due to their ease of care, lack of exogenous diabetes induction (STZ), and ability to be rendered indefinitely euglycemic via syngeneic islet transplants (300 C57BL/6 islets), yet reject islet transplants from major histocompatibility complex (MHC)-mismatched donors (300 BALB/c islets) (27).
Systemic treatment with catalytic antioxidant to inhibit STZ-induced diabetes.
C57BL/6 male mice were injected intraperitoneally with 10 mg/kg CA for 7 days. On day 2, all mice were given an intravenous injection of 170 mg/kg STZ. Blood glucose was measured every other day. Two consecutive blood glucose readings >300 mg/dl were indicative of STZ-induced diabetes. Diabetic animals were killed after the second consecutive reading.
Syngeneic transplants.
Eight to twelve week old C57BL/6 male mice were used as donors and recipients. Recipients were injected intraperitoneally with 240 mg/kg STZ (Sigma) on day 1. Animals were tested for diabetes using urine test strips (Bayer) on day 3. All diabetic animals received 1 unit of insulin (Lantus) and 500 μl of Ringer solution on days 3–5. On day 5, islets were isolated from naive donor C57BL/6 mice as described in Bertera et al. (28). The same day, islets were simultaneously picked, counted, and redistributed into Petri dishes containing 175 islets each. Each dish of islets contained islet media (10% heat-inactivated FBS, 2% 1 mmol/l HEPES, 10,000 mg/ml 1% penicillin/streptomycin, 200 mmol/l 1% l-glutamine, and 50 μmol/l 0.1% 2-β-mercaptoethanol in RPMI 1640 sterile-filtered) alone or islet media plus 68 μmol/l catalytic antioxidant. Treated and control islets were incubated for 24 h at 37°C and 5% CO2. Nonfasting blood glucose levels were tested in recipient mice on day 6 by obtaining a small blood sample from the retro-orbital sinus. Only animals with blood glucose ≥400 mg/dl were used as recipients. On day 6, each recipient was transplanted with 100 or 175 syngeneic CA-treated or untreated control islets inserted under the kidney capsule as described in Bertera et al. (28).
Recipients of 100 untreated or catalytic antioxidant-treated syngeneic islets had their blood glucose monitored approximately every other day for 19 days. Recipients of 175 islets were subject to a fasting intraperitoneal glucose tolerance test (IPGTT) on postoperative days 9 and 70). Food was removed from the recipients' cages 18 h before IPGTT with water available ad libitum. Mice were weighed and a baseline (0-min) blood glucose was taken immediately before each recipient was injected with a 10 μl/g body wt dose of sterile-filtered 20% glucose solution in distilled H2O. Blood glucose readings were taken at 30, 60, 90, and 120 min after glucose injection. Nephrectomies were performed on day 77 as described in Bertera et al. (29).
Islet-directed CA treatment to delay allograft rejection.
Male C57BL/6 mice were used as transplant recipients, and male BALB/c mice were used as islet donors. C57BL/6 mice were rendered diabetic and recipients were prepared for transplant as previously described. Islets were incubated in islet media alone or in islet media with 68 μmol/l catalytic antioxidant for 24 h. Three hundred CA-treated or control-treated BALB/c islets were transplanted under the kidney capsule of diabetic C57BL/6 recipients as previously mentioned. All diabetic recipients became euglycemic post-transplant. Blood glucose was checked every other day. Two consecutive blood glucose readings >400 mg/dl were indicative of allograft rejection. Diabetic animals were euthanized after the second consecutive reading.
In vivo human islet experiments: xenotransplants.
Immediately after isolation, human islets were cultured in human islet medium with or without the addition of 68 μmol/l CA for 30–40 h before transplanting 400–500 untreated or CA-treated islets into diabetic C57BL/6 mice in the absence of additional immunosuppression. Two human pancreata were used for these experiments; however, the islets were not pooled together. Each recipient animal only received islets from one donor to add the variable of islet quality disparity among donors to our experiment. All C57BL/6 animals were rendered diabetic and transplanted with islets as previously mentioned. Recipient blood glucose levels were tested daily for 7 days post-transplant and then euthanized.
Systemic delivery of CA in pellet form to delay allograft rejection.
Male C57BL/6-Ins2Akita/+ mice were used as transplant recipients, and male BALB/c mice were used as islet donors. This work is based on Matthews et al. (27), and the transplants were performed as previously mentioned, transplanting 300 BALB/c islets into each spontaneously diabetic C57BL/6-Ins2Akita/+ recipient on day 0. Three days before transplant, a placebo or 21-day CA pellet (0.1 mg/day or 5 mg/kg/day) (Innovative Research of America, Sarasota, FL) was inserted into each recipient. An additional pellet was inserted into mice on postoperative day 16, as pellets were administered every 20 days. All diabetic recipients became euglycemic after transplant. Blood glucose was checked every other day. Two consecutive blood glucose readings >400 mg/dl were indicative of allograft rejection. Diabetic animals were euthanized after the second consecutive reading.
Hematoxylin and eosin staining of islet-bearing kidneys.
Mouse kidney samples were fixed in 4% paraformaldehyde for 3 h and then transferred to 30% sucrose. After being imbedded in frozen section medium (Richard-Allan Scientific, Kalamazoo, MI), cryosections (10 μm) were cut using a cryostat (Microm Cryostar HM550 Cryostat, Thermo Fisher Scientific, Waltham, MA) and mounted onto gelatin-coated or precleaned slides. Hematoxylin and eosin staining was performed using a frozen section staining kit (Thermo Electron, Pittsburgh, PA). Images were captured at 40× magnification using a Nikon confocal microscope (D-ECLIPSE C1, Nikon, Tokyo, Japan).
Statistical analysis.
Data are expressed as means ± SEM. The difference between mean values was determined by a Student t test for singular comparisons and by one-way ANOVA for multiple comparisons. The area under the curve was determined using the trapezoidal rule. Kaplan-Meier survival plots were analyzed by the log-rank test. All statistical analysis was performed with the aid of PRISM (GraphPad, San Diego, CA) and JMP statistical software from the SAS Institute using P < 0.05 to achieve significance.
RESULTS
CA protects human islets from STZ-induced cell death.
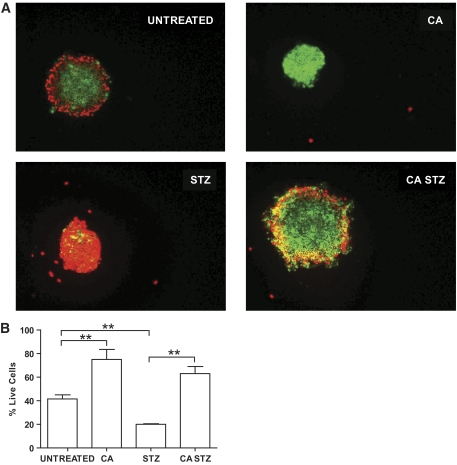
In vitro STZ treatment was used to mimic ischemia-reperfusion injury in human islets to examine the ability of CA treatment to protect from islet cell death. For this experiment, human islets were divided into four groups: 1) media alone (untreated), 2) CA, 3) STZ, or 4) CA + STZ. After an 8-h incubation, a double-fluorescence viability assay was performed using acridine orange, which penetrates the plasma membrane of living cells and stains their nuclei green, and ethidium bromide, which only penetrates dead cells, in which membrane integrity is compromised, to stain their nuclei red. Panels in Fig. 1A are representative fluorescent images from each treatment group correlating to quantitative data analysis graphed in Fig. 1B. A substantial decrease in viability was recorded when islets were treated with STZ. As we hypothesized, islets treated with STZ in the presence of CA demonstrated a significant increase in cell viability versus islets treated with STZ alone (Fig. 1B). These data indicate that the addition of CA protects human islets when used in the media alone and, even more significantly, when islets are treated with CA in the presence of STZ, a model of free-radical induced cell death.
FIG. 1.
Catalytic antioxidant (CA) protects human islets from STZ-induced cell death. Human islets (60 islets/group) were cultured in media alone, 68 μmol/l catalytic antioxidant, 11 mmol/l STZ, or 68 μmol/l catalytic antioxidant + 11 mmol/l STZ. A: Representative images of each group of islets after an 8-h incubation, stained with acridine orange (green/live) and ethidium bromide (red/dead) and then visualized under a fluorescence microscope at 10× magnification. B: The percentages of live islet cells from (A) were assessed by ImageJ software (n ≥ 3). Data are presented as means ± SEM. Significance was tested using one-way ANOVA (**P < 0.05). (A high-quality digital representation of this figure is available in the online issue.)
Systemic treatment with CA inhibits STZ-induced diabetes.
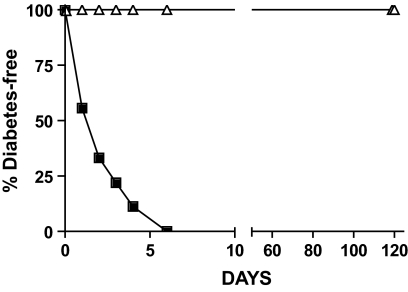
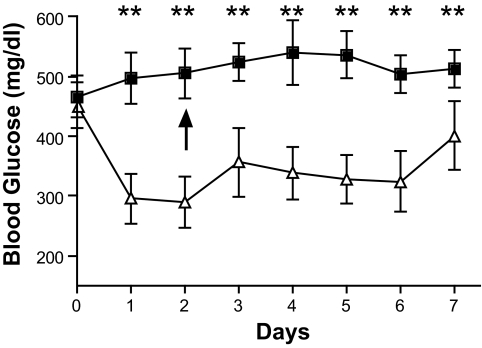
STZ induces diabetes through a nitric oxide free radical mechanism resulting in DNA damage and islet cell death (30). Because CA can inhibit free radical damage (20,24,25,31), we wanted to determine whether systemic catalytic antioxidant treatment could protect islets from STZ-induced diabetes. Five C57BL/6 mice were injected with 10 mg/kg CA daily for 7 days, and nine C57BL/6 mice were used as untreated controls. On day 2, all mice were injected with STZ. As shown in Fig. 2, untreated mice (n = 9) developed diabetes (2.3 ± 0.6 days), whereas none of the CA-treated mice (n = 5) developed diabetes (>120 days) (log-rank test, P = 0.005). These results demonstrate that systemic treatment with CA protects islets from free radical damage to prevent STZ-induced diabetes.
FIG. 2.
Systemic catalytic antioxidant (CA) treatment inhibits STZ-induced diabetes. Mice received CA treatment on days 1–7, and STZ was given on day 2. ■, untreated (n = 9); ▵, CA treated (n = 5). Significance was tested using the log-rank test (P = 0.0005).
Islets incubated with CA before syngeneic transplant exhibit increased function.
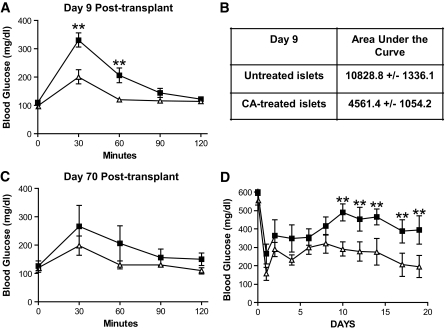
On the basis of previously published data demonstrating improved islet mass, viability, and function when human islets are treated with CA treatment during or after isolation (15,16), we wanted to determine whether islet-directed CA could improve graft function in syngeneic transplant models. Diabetic C57BL/6 recipients were transplanted with 175 syngeneic islets, which were previously incubated in the presence (n = 5) or absence (n = 5) of 68 μmol/l CA for 24 h. All animals were rendered euglycemic after transplant and sustained long-term euglycemia. After nephrectomy, all transplant recipients reverted to diabetes (data not shown). Fasting IPGTT were performed on post-operative days 9 and 70.
Recipients of CA-treated islets demonstrated significantly improved glycemic control at 30 and 60 min time points compared with untreated controls (Fig. 3A). Specifically, the area under the curve for recipients of CA-treated islets was less than one-half (4,561.4 ± 1,054.2 min · mg−1 · dl−1) of the area under the curve for recipients of untreated islets (10,828.8 ± 1,336.1 min · mg−1 · dl−1) (Fig. 3B). To observe any long-lasting effect of the CA incubation on islet function, mice from each group were monitored long-term and another IPGTT was performed on day 70. Although the 70-day IPGTT did not show statistical significance, the trend of improved glycemic control in recipients of CA-treated islets, namely, at 30 and 60 min, was maintained (Fig. 3C).
FIG. 3.
Pretreating islets with catalytic antioxidant (CA) before syngeneic transplant improves islet function. Islets were incubated with CA or remained untreated for 24 h. Syngeneic recipients were transplanted with 175 untreated or CA-treated islets (A–C). For suboptimal syngeneic experiments 100 untreated or 100 CA-treated islets were transplanted into syngeneic recipients (D). A: Fasting IPGTT performed on postoperative day 9. ■, untreated islets (n = 5); ▵, CA-treated islets (n = 5). B: Area under the curve calculation for (A). Significance was tested using a Student t test (**P < 0.05). C: Fasting IPGTT performed on postoperative day 70. ■, untreated islets (n = 3); ▵, CA–treated islets (n = 3). D: Blood glucose readings from recipients of 100 CA-treated or untreated islets within 3 weeks of suboptimal syngeneic transplant. ■, untreated islets (n = 5); ▵, CA–treated islets (n = 5). Significance was tested using one-way ANOVA (**P < 0.05).
We also performed suboptimal syngeneic transplants where diabetic C57BL/6 mice received 100 islets treated in the presence (n = 5) or absence (n = 5) of 68 μmol/l CA for 24 h. We monitored the blood glucose levels of both groups for ∼3 weeks post-transplant and observed significantly decreased blood glucose levels in the recipients of 100 CA-treated islets compared with recipients of untreated islets (Fig. 3D). Specifically, the most significant divergence of islet function occurred 10–20 days post-transplant, during islet engraftment under the kidney capsule (32). The protective effects of redox modulation are further evidenced by an IPGTT performed 4 weeks post-transplant, which demonstrates improved glycemic control for recipients of a suboptimal number of CA-treated islets (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0588/DC1). These results corroborate that islet-directed CA treatment is able to suppress antigen-independent ischemia-reperfusion injury and perhaps promote islet engraftment as demonstrated by improved islet graft function.
Islet-directed CA treatment delays allograft rejection in an MHC-mismatched islet transplant model.
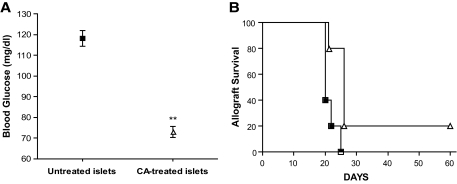
Next, we tested the ability of islet-directed redox modulation to improve islet allograft survival. Because islet-directed CA treatment can improve islet function against antigen-independent ischemia-reperfusion injury in a syngeneic transplant model (Fig. 3) and in human islet isolation (15,16), we wanted to determine whether islet-directed redox modulation using catalytic antioxidant could delay antigen-dependent allograft rejection. These experiments were performed using BALB/c (H-2d) donor islets incubated in the presence or absence of 68 μmol/l catalytic antioxidant for 24 h. Three hundred CA-treated or untreated islets were transplanted into diabetic C57BL/6 (H-2b) recipients.
Post-transplant, recipients of CA-treated islets (73.0 ± 2.6) normalized (n = 5) to significantly lower blood glucose levels compared with recipients (n = 5) of control islets (118.2 ± 3.7) (Fig. 4A), indicative of an increase in early islet graft survival. Although all allograft recipients were euglycemic for >2 weeks (range of 65–175 mg/dl), recipients of CA-treated islets retained euglycemia, and thus a functioning allograft, significantly (log-rank test, P = 0.0132) longer (31.8 ± 7.1 days) than the recipients of untreated islets (21.4 ± 1.0 days) (Fig. 4B). Taken together, these data indicate that islet-directed CA treatment alone can delay allograft rejection.
FIG. 4.
Pretreating islets with catalytic antioxidant (CA) before allogeneic transplant improves islet function. Islets were incubated with CA for 24 h, and then 300 untreated or CA-treated islets were transplanted into allogeneic recipients. A: Normalization blood glucose of recipients within 2 postoperative days. Significance was tested using a Student t test (**P < 0.05). B: Allograft survival of recipients. ■, untreated islets (n = 5); ▵, CA–treated islets (n = 5). Significance was tested using a log-rank test (P = 0.0132).
Islet-directed CA treatment improves islet function in xenogeneic transplantation.
To more stringently analyze islet-directed redox modulation in ischemia-reperfusion injury, we performed xenogeneic transplants. Human islets were incubated in the presence or absence of 68 μmol/l CA for 30–40 h post-isolation, and then 400–500 untreated or CA-treated islets were transplanted into diabetic C57BL/6 recipients under the cover of no additional immunosuppression to fully assess the efficacy of CA to inhibit innate immune-derived primary graft dysfunction in a xenograft model. One control animal died between days 2 and 3 (black arrow) before the completion of the experiment, whereas the average blood glucose of recipients of catalytic antioxidant-treated islets was significantly lower compared with recipients of untreated islets (Fig. 5). We suspect that our data would reflect even higher statistical significance if the glucometers were not constrained by a maximum reading of 600 mg/dl, as all glucometer readings of “HIGH” were recorded as 600 mg/dl. Four of five recipients of untreated islets had postrejection blood glucose readings of HIGH. Comparatively, all recipients of CA-treated islets remained <500 mg/dl throughout the experiment, indicative of enhanced islet function for recipients of islets pretreated with CA. These results indicate that redox modulation would be an effective islet preservation strategy if/when the limited availability of allogeneic islets is overcome by using xenogeneic islets.
FIG. 5.
Pretreating islets with catalytic antioxidant (CA) before xenogeneic transplant improves islet function. Human islets were incubated in the presence or absence of CA for 30–40 h. Then, 400–500 untreated or CA–treated human islets were transplanted into xenogeneic diabetic C57BL/6 recipients. ■, untreated islets (n = 5); ▵, CA–treated islets (n = 5). The black arrow represents the death of a transplant recipient in the untreated islet group. For the remainder of the study, n = 4 for the untreated islet group. Significance was tested using the Student t test (**P < 0.05).
Systemic delivery of CA prolongs allograft function in an MHC-mismatched islet transplant model.
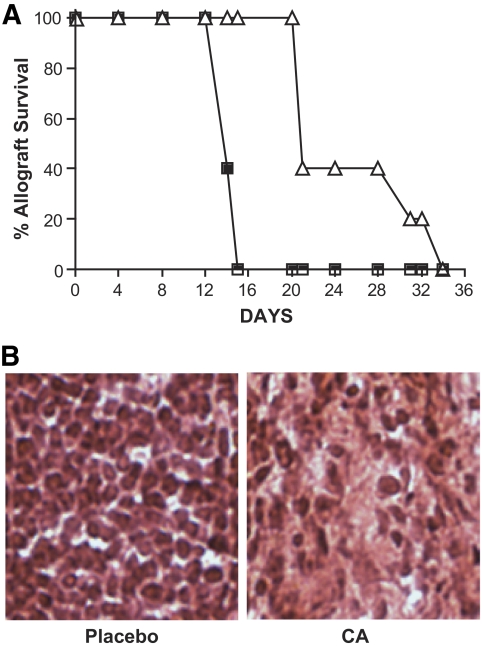
To determine whether systemic administration of CA would equate to a more substantial delay in allograft rejection, we transplanted 300 BALB/c islets into spontaneously diabetic C57BL/6-Ins2Akita/+ mice (27). In our colony, 7- to 8-week-old diabetic C57BL/6-Ins2Akita/+ males have a blood glucose level of 580 ± 19 mg/dl, which is comparable to the average blood glucose level (544 ± 11 mg/dl) for the same mice housed at The Jackson Laboratory. Three days before transplant, a catalytic antioxidant (21-day, 5 mg/kg/day) or placebo pellet was inserted into diabetic recipients. All 10 recipient mice normalized posttransplant (125 ± 16.94 mg/dl). CA-treated recipients (n = 5) displayed a significant increase (log-rank test, P = 0.0023) in graft function (25.6 ± 2.9 days) compared with untreated recipients (n = 5) (14.4 ± 0.2 days) (Fig. 6A). Hematoxylin and eosin sections of islet-bearing kidneys postrejection demonstrate a pronounced infiltrate in untreated recipients and a sparse infiltrate in CA-treated recipients (Fig. 6B), suggesting the delay in graft rejection with CA treatment is associated with decreased migration of immune cells to the site of the graft. These results demonstrate that systemic delivery of CA can delay graft rejection by depressing the cytotoxic free radical and inflammatory damage generated by the innate (antigen-independent) immune response and possibly by impacting the T-cell-mediated adaptive (antigen-dependent) immune response (20,25) to hinder allograft rejection.
FIG. 6.
Systemic catalytic antioxidant (CA) treatment delays allograft rejection. Recipients were treated with a placebo or CA pellet 3 days before transplant and then 300 allogeneic islets were transplanted into all recipients. A: Allograft survival of recipients. ■, placebo pellet (n = 5); ▵, CA pellet (n = 5). Significance was tested using a log-rank test (P = 0.0023). B: Post-rejection hematoxylin and eosin staining representative of untreated and CA-treated recipients. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Allograft acceptance can be achieved using immunosuppressive drugs, although most drugs that inhibit T-cell–mediated graft destruction have the unfortunate side effect of islet toxicity and do not significantly protect islets from ischemia-reperfusion insults (12–14), leading to primary nonfunction and β-cell death. Current literature in islet transplantation expresses a need for improved therapeutic agents that control rejection while preserving islet function through cytoprotection (11,13,15–18,33,34). Because redox modulation affects the innate and the adaptive immune responses (20,24,25) and demonstrates cytoprotection during human islet isolation (15,16), we hypothesized that it may be a useful approach in islet transplantation. Because only a fraction of transplanted islets survive ischemia-reperfusion injury (3,15,16), our current study used the well-described redox modulator, CA (FBC-007), to determine whether islet graft survival and function could be improved by inhibiting free radical and inflammatory damage.
In this study, CA protects islets from STZ-induced free radical damage and antigen-independent inflammatory ischemic damage in syngeneic, allogeneic, and xenogeneic transplantation and delays rejection in allogeneic transplantation. Specifically, we used STZ-induced diabetes as a model of islet cell death and found that systemic delivery of CA protected all mice from STZ-induced diabetes (Fig. 2), probably by increasing antioxidant defenses in islets to render them more resistant to STZ-induced free radical damage. To isolate antigen-independent ischemia-reperfusion injury in a transplant setting, we used syngeneic transplant models to analyze the effects of pretreating islets with catalytic antioxidant. These data indicate that islet-directed CA is protective of islet function (Fig. 3A–D).
Antigen-independent injury by the innate immune system plays a larger role in allograft rejection than previously thought, correlating to the activation state of the powerful redox-dependent transcription factor, NF-κB. NF-κB shapes the innate and adaptive immune responses (35–40) by controlling a myriad of proinflammatory and proapoptotic genes in multiple cell types, including β-cells (41–43). Our previously published work indicates that redox modulation using CA can hinder apoptotic and necrotic pathways by inhibiting NF-κB-DNA binding, poly(ADP-ribose) polymerases activation, and the production of chemokines and cytokines in human islets (16). In this study, we expanded our previous work with human islets (15,16) using STZ treatment in the presence or absence of redox modulation by catalytic antioxidant to mimic islet cell damage prevalent in ischemia-reperfusion injury. This data demonstrated redox modulation is protective in a robust setting of ischemia-reperfusion injury (Fig. 1), probably by hindering the previously mentioned apoptotic and necrotic pathways. Other studies have also sighted NF-κB in islet death, demonstrating that manipulating components of the NF-κB pathway to hinder its activation and imminent inflammatory damage, can protect islets from apoptosis in autoimmunity and islet transplantation (43–45). Because redox modulation using CA can enter mitochondria in vivo to impart antioxidant protection (31), preservation of mitochondrial function and, therefore, ATP is a potential mechanism by which CA protects from β-cell death (25). Using redox modulation to treat the recipient (Figs. 2 and 6) and/or the donor islets (Figs. 1, 3, and 5) would limit β-cell exposure to cytokine and nitric oxide damage, thus hindering the hypoxic and inflammatory onslaught endured by islets during isolation and post-transplantation.
Moreover, Contreras et al. (46) noted that increasing islet yield post-isolation is paramount to propelling the success of clinical islet transplantation, as the Edmonton protocol requires one to three pancreata per recipient. Currently, the demand for islets far outweighs the supply (46), especially because more than one islet infusion is frequently required to eliminate the need for exogenous insulin for any time period (47). Because CA-treatment increased islet viability in human islets in vitro (Fig. 1) (15,16) and in murine in vivo (Figs. 1–5) models of syngeneic, allogeneic, and xenogeneic (human) ischemia-reperfusion injury, CA treatment may allow a reduced number of islets to normalize a recipient. The ability to decrease the number of human pancreata/islet infusions required to achieve long-term insulin independence would overcome a major hurdle in islet transplantation (46–48). Additionally, it has been reported that rodent islets have decreased protection from oxidative stress compared with human islets (49). This evidence suggests that our favorable results using redox modulation in syngeneic and allogeneic murine models of transplantation may translate successfully to allogeneic clinical transplantation.
In addition to reducing primary islet nonfunction in syngeneic (Fig. 3), allogeneic (Fig. 4), and xenogeneic models (Fig. 5), islet-directed CA treatment also delayed antigen-dependent allogeneic islet transplantation (Fig. 4). Comparatively, systemic delivery of CA (Fig. 6) extends allograft rejection beyond islet-directed catalytic antioxidant treatment to support islet engraftment, viability, and function because CA can also act as an immunomodulatory agent by inhibiting antigen-presenting cell activation (24), CD4 (20) and CD8 T-cell effector function (25), and cytotoxic lymphocyte target cell lysis by decreasing cytolytic effector molecule production in transgenic and alloreactive models (25). However, in this study, we are not certain if the delay in allograft rejection is due to increased cytoprotection and preservation of islets by catalytic antioxidant during ischemia-reperfusion injury (Figs. 3 and 4) or as a result of immune modulation correlating to decreased alloimmune-related inflammation and a subsequent decrease in migration of immune cells to the sight of the graft in CA-treated recipients (Fig. 6). Most likely, the delay in allograft rejection is a combination of the two.
CA is known to promote islet (15,16) and cell survival while inhibiting effector function (20,25); however, studies examining the effects of redox modulation on β-cell proliferation and genes that regulate proliferation are yet to be performed. Because CA inhibits primary immunogenic proliferation (20,25) and sustains disruption of effector function, but does not significantly inhibit secondary expansion (20), redox modulation could be of benefit when coupled to noncalcineurin or mammalian target of rapamycin targeted antiproliferative drugs, like mycophenolate mofetil in syngeneic, allogeneic, and xenogeneic transplants of vascularized or nonvascularized tissue. Additionally, if islets are treated with CA before transplant, they may be afforded increased protection from toxic immunosuppressive agents currently used in transplant protocols, thereby increasing transplant success by limiting graft loss. Redox modulation may also allow for the dose and duration of immunosuppressants to be weaned over a transplant recipient's lifetime because CA protects from the inflammatory damage associated with islet isolation and transplantation.
Taken together, redox modulation is a favorable therapeutic agent to add to the currently administered immunosuppressives because CA is a nontoxic, nonimmunogenic small-molecule compound that offers the flexibility of systemic and/or tissue-specific treatment, ease of application, and a history of reproducible outcomes (15,16,20,24–26,31). The reported benefits of CA treatment for islets, autoimmunity, and alloimmunity suggest redox modulation as a clinical approach, especially for diabetic recipients of islet allografts.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Cochrane-Weber research award by the Children's Hospital of Pittsburgh Foundation (to M.M.S.), a Research Advisory Council award by Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center Health System (to M.M.S.), the Juvenile Diabetes Research Foundation (grant 76-01 to J.D.P.), and the American Diabetes Association CDA 7.07 CD16 (to J.D.P.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC: Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996;45:1161–1167 [DOI] [PubMed] [Google Scholar]
- 2.Crutchlow MF, Yu M, Bae YS, Deng S, Stoffers DA: Exendin-4 does not promote Beta-cell proliferation or survival during the early post-islet transplant period in mice. Transplant Proc 2008;40:1650–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaña E, Bonner-Weir S, Weir GC: Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 1993;91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vajkoczy P, Menger MD, Simpson E, Messmer K: Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation 1995;60:123–127 [PubMed] [Google Scholar]
- 5.Gandy SE, Buse MG, Crouch RK: Protective role of superoxide dismutase against diabetogenic drugs. J Clin Invest 1982;70:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kröncke KD, Brenner HH, Rodriguez ML, Etzkorn K, Noack EA, Kolb H, Kolb-Bachofen V: Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. Biochim Biophys Acta 1993;1182:221–229 [DOI] [PubMed] [Google Scholar]
- 7.Eizirik DL, Delaney CA, Green MH, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerström C, Green IC: Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol 1996;118:71–83 [DOI] [PubMed] [Google Scholar]
- 8.Grankvist K, Marklund SL, Täljedal IB: CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J 1981;199:393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenzen S, Drinkgern J, Tiedge M: Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 10.Tiedge M, Lortz S, Drinkgern J, Lenzen S: Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 11.Kenmochi T, Miyamoto M, Mullen Y: Protection of mouse islet isografts from nonspecific inflammatory damage by recipient treatment with nicotinamide and 15-deoxyspergualin. Cell Transplant 1996;5:41–47 [DOI] [PubMed] [Google Scholar]
- 12.Lohmann T, List C, Lamesch P, Kohlhaw K, Wenzke M, Schwarz C, Richter O, Hauss J, Seissler J: Diabetes mellitus and islet cell specific autoimmunity as adverse effects of immunsuppressive therapy by FK506/tacrolimus. Exp Clin Endocrinol Diabetes 2000;108:347–352 [DOI] [PubMed] [Google Scholar]
- 13.Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C: Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006;32:231–243 [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH: Sirolimus is associated with reduced islet engraftment and impaired β-cell function. Diabetes 2006;55:2429–2336 [DOI] [PubMed] [Google Scholar]
- 15.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD: Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes 2002;51:2561–2567 [DOI] [PubMed] [Google Scholar]
- 16.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD: Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004;53:2559–2568 [DOI] [PubMed] [Google Scholar]
- 17.Obhrai J, Goldstein DR: The role of Toll-like receptors in solid organ transplantation. Transplantation 2006;81:497–502 [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga Y, Sutherland DE, Harmon JV, Papas KK: Pancreas preservation for pancreas and islet transplantation. Curr Opin Organ Transplant 2008;13:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contreras JL, Bilbao G, Smyth CA, Jiang XL, Eckhoff DE, Jenkins SM, Thomas FT, Curiel DT, Thomas JM: Cytoprotection of pancreatic islets before and soon after transplantation by gene transfer of the anti-apoptotic Bcl-2 gene. Transplantation 2001;71:1015–1023 [DOI] [PubMed] [Google Scholar]
- 20.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD: Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol 2007;178:908–917 [DOI] [PubMed] [Google Scholar]
- 21.Curtsinger JM, Lins DC, Mescher MF: Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 2003;197:1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF: Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 1999;162:3256–3262 [PubMed] [Google Scholar]
- 23.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004;351:2715–2729 [DOI] [PubMed] [Google Scholar]
- 24.Tse HM, Milton MJ, Piganelli JD: Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med 2004;36:233–247 [DOI] [PubMed] [Google Scholar]
- 25.Sklavos MM, Tse HM, Piganelli JD: Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med 2008;45:1477–1486 [DOI] [PubMed] [Google Scholar]
- 26.Piganelli JD, Flores SC, Cruz C, Koepp J, Batinic-Haberle I, Crapo J, Day B, Kachadourian R, Young R, Bradley B, Haskins K: A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 2002;51:347–355 [DOI] [PubMed] [Google Scholar]
- 27.Mathews CE, Langley SH, Leiter EH: New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002;73:1333–1336 [DOI] [PubMed] [Google Scholar]
- 28.Bertera S, Crawford ML, Alexander AM, Papworth GD, Watkins SC, Robbins PD, Trucco M: Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes 2003;52:387–393 [DOI] [PubMed] [Google Scholar]
- 29.Bertera S, Alexander AM, Crawford ML, Papworth G, Watkins SC, Robbins PD, Trucco M: Gene combination transfer to block autoimmune damage in transplanted islets of Langerhans. Exp Diabesity Res 2004;5:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szkudelski T: The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001;50:537–546 [PubMed] [Google Scholar]
- 31.Spasojević I, Chen Y, Noel TJ, Yu Y, Cole MP, Zhang L, Zhao Y, St Clair DK, Batinić-Haberle I: Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic Biol Med 2007;42:1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morini S, Brown ML, Cicalese L, Elias G, Carotti S, Gaudio E, Rastellini C: Revascularization and remodelling of pancreatic islets grafted under the kidney capsule. J Anat 2007;210:565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z, Chen M, Nadler JL: Lisofylline: a potential lead for the treatment of diabetes. Biochem Pharmacol 2005;69:1–5 [DOI] [PubMed] [Google Scholar]
- 34.Stegall MD, Lafferty KJ, Kam I, Gill RG: Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation 1996;61:1272–1274 [DOI] [PubMed] [Google Scholar]
- 35.Clark R, Kupper T: Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol 2005;125:629–637 [DOI] [PubMed] [Google Scholar]
- 36.Hernandez A, Burger M, Blomberg BB, Ross WA, Gaynor JJ, Lindner I, Cirocco R, Mathew JM, Carreno M, Jin Y, Lee KP, Esquenazi V, Miller J: Inhibition of NF-κB during human dendritic cell differentiation generates anergy and regulatory T-cell activity for one but not two human leukocyte antigen DR mismatches. Hum Immunol 2007;68:715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N: Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol 2004;173:4331–4441 [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M: Effective antigen presentation by dendritic cells is NF-κB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol 2001;13:675–683 [DOI] [PubMed] [Google Scholar]
- 39.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA: In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 2001;19:23–45 [DOI] [PubMed] [Google Scholar]
- 40.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK: Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol 1998;160:4719–4729 [PubMed] [Google Scholar]
- 41.Eizirik DL, Darville MI: β-Cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes 2001;50(Suppl 1):S64–S69 [DOI] [PubMed] [Google Scholar]
- 42.Cardozo AK, Kruhøffer M, Leeman R, Orntoft T, Eizirik DL: Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes 2001;50:909–920 [DOI] [PubMed] [Google Scholar]
- 43.Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL: Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes 2001;50:2219–2224 [DOI] [PubMed] [Google Scholar]
- 44.Giannoukakis N, Rudert WA, Trucco M, Robbins PD: Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Iκ B repressor. J Biol Chem 2000;275:36509–36513 [DOI] [PubMed] [Google Scholar]
- 45.Kim EK, Kwon KB, Koo BS, Han MJ, Song MY, Song EK, Han MK, Park JW, Ryu DG, Park BH: Activation of peroxisome proliferator-activated receptor-γ protects pancreatic β-cells from cytokine-induced cytotoxicity via NF κB pathway. Int J Biochem Cell Biol 2007;39:1260–1275 [DOI] [PubMed] [Google Scholar]
- 46.Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson JA, Chaudry IH, Eckhoff DE: Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 2003;52:2935–2942 [DOI] [PubMed] [Google Scholar]
- 47.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM: Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 48.Frank A, Deng S, Huang X, Velidedeoglu E, Bae YS, Liu C, Abt P, Stephenson R, Mohiuddin M, Thambipillai T, Markmann E, Palanjian M, Sellers M, Naji A, Barker CF, Markmann JF: Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg 2004;240:631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodström M, Mello MA, Andersson A, Pipeleers DG, Hellerström C: Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1995;1:806–820 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.