Abstract
OBJECTIVE
Glucagon-like peptide 1 (GLP-1) exerts beneficial antidiabetic actions via effects on pancreatic β- and α-cells. Previous studies have focused on the improvements in β-cell function, while the inhibition of α-cell secretion has received less attention. The aim of this research was to quantify the glucagonostatic contribution to the glucose-lowering effect of GLP-1 infusions in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Ten male patients with well-regulated type 2 diabetes (A1C 6.9 ± 0.8%, age 56 ± 10 years, BMI 31 ± 3 kg/m2 [means ± SD]) were subjected to five 120-min glucose clamps at fasting plasma glucose (FPG) levels. On day 1, GLP-1 was infused to stimulate endogenous insulin release and suppress endogenous glucagon. On days 2–5, pancreatic endocrine clamps were performed using somatostatin infusions of somatostatin and/or selective replacement of insulin and glucagon; day 2, GLP-1 plus basal insulin and glucagon (no glucagon suppression or insulin stimulation); day 3, basal insulin only (glucagon deficiency); day 4, basal glucagon and stimulated insulin; and day 5, stimulated insulin. The basal plasma glucagon levels were chosen to simulate portal glucagon levels.
RESULTS
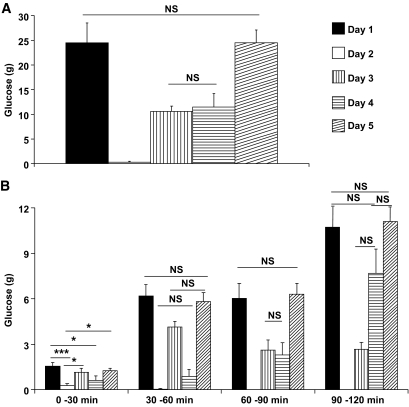
Peptide infusions produced the desired hormone levels. The amount of glucose required to clamp FPG was 24.5 ± 4.1 (day 1), 0.3 ± 0.2 (day 2), 10.6 ± 1.1 (day 3), 11.5 ± 2.7 (day 4), and 24.5 ± 2.6 g (day 5) (day 2 was lower than days 3 and 4, which were both similar and lower than days 1 and 5).
CONCLUSIONS
We concluded that insulin stimulation (day 4) and glucagon inhibition (day 3) contribute equally to the effect of GLP-1 on glucose turnover in patients with type 2 diabetes, and these changes explain the glucose-lowering effect of GLP-1 (day 5 vs. day 1).
The incretin hormone glucagon-like peptide 1 (GLP-1), as well as GLP-1 analogues that are now being used for the treatment of patients with type 2 diabetes, potently suppresses α-cell secretion (1–4). Despite their hyperglycemia, patients with type 2 diabetes tend to have elevated fasting glucagon levels and exaggerated glucagon responses to meal ingestion (5). Since the hyperglucagonemia is thought to contribute to the hyperglycemia of these patients by increasing hepatic glucose production (HGP) (6), it follows that the glucagonostatic effect of GLP-1 may be as important clinically as its insulinotropic effect (7). Glucose-induced inhibition of α-cell secretion may be impaired in type 2 diabetic patients, but pharmacological amounts of GLP-1 have been shown to restore α-cell sensitivity to glucose (8,9), and we recently demonstrated that the glucagon-suppressive effect of GLP-1 was similar in patients with type 2 diabetes and healthy control subjects (10).
Thus GLP-1 potently influences both β- and α-cell secretion in patients with type 2 diabetes, but the relative roles of these two effects in relation to the overall glucose-lowering action of GLP-1 are unclear. In the present studies, we sought to determine the importance of the glucagonostatic effect by measuring its contribution to changes in glucose turnover induced by the infusion of GLP-1 in pharmacological doses. We employed the pancreatic clamp technique (11) using somatostatin to block the endogenous secretion from the islets while substituting insulin and/or glucagon levels by infusions designed to mimic either basal levels and/or responses to a GLP-1 infusion rate (1 pmol/kg/min) known to normalize blood glucose in patients with type 2 diabetes (4). All examinations were done in the fasting state with plasma glucose (PG) clamped at individual fasting PG (FPG) levels. The amount of glucose infused to maintain the clamp was expected to accurately reflect the influence of the endocrine perturbations on glucose turnover (decreased hepatic glucose production and increased peripheral disposal).
RESEARCH DESIGN AND METHODS
Ten male patients with type 2 diabetes (age 56 ± 10 years, duration of diabetes 6 ± 3 years, BMI 31 ± 3 kg/m2, waist-to-hip ratio 1.0 ± 0.0, FPG 10.3 ± 2.9 mmol/l [after 1 week of oral antidiabetic drug wash-out], A1C 6.9 ± 0.8% [during treatment] [means ± SD]) were studied. All participants were negative for both islet cell and GAD-65 autoantibodies, were well-treated with regard to plasma lipids and blood pressure, and were without overt diabetic complications. One patient was treated with diet and exercise alone, while the rest were in oral antidiabetic treatment with metformin (n = 4), metformin and sulfonylurea (n = 4), or metformin and sitagliptin (n = 1). All patients had an oral antidiabetic drug wash-out period of 7 days prior to examination, and none of the patients were treated with other drugs known to affect the responses of glucose, insulin, glucagon, or incretin hormones. All subjects agreed to participate after receiving oral and written information. The protocol was approved by the Scientific-Ethical Committee of the Capital Region of Denmark (registration no. H-C-2007-0072), registered with the Danish Data Protection Agency (registration no. 2008-41-1809) and ClinicalTrials.gov (no. 00655603), and performed in accordance with the Helsinki Declaration II.
Experimental protocol.
All patients were investigated in the morning after an overnight fast (from midnight) on five separate days. On each day, subjects received variable infusions for 120 min during which PG was clamped at their individual FPG level. On day 1, GLP-1 (1.0 pmol/kg/min) was infused to stimulate endogenous insulin release and suppress endogenous glucagon. On days 2–5, pancreatic endocrine clamps were performed with the infusion of somatostatin (450 μg/h) and the selective replacement of insulin and/or glucagon as follows: day 2, GLP-1 plus basal insulin and glucagon (no glucagon suppression or insulin stimulation); day 3, basal insulin only (glucagon deficiency); day 4, basal glucagon and stimulated insulin; day 5, stimulated insulin and glucagon deficiency. Peptides were infused using separate infusion pumps from time 0 to 120 min at the following rates: day 2, GLP-1 (1.0 pmol/kg/min), glucagon (1.25-0.6 ng/kg/min), and insulin (0.1 mU/kg/min); day 3, insulin (0.1 mU/kg/min); day 4, glucagon (1.25-0.6 ng/kg/min) and insulin (0.2 mU/kg/min [0–60 min], 0.4 mU/kg/min [60–120 min]); day 5, insulin (0.2 mU/kg/min [0–60 min], 0.4 mU/kg/min [60–120 min]). The glucagon infusion rate was chosen to give rise to glucagon concentrations equivalent to the basal levels in the portal vein, while the insulin infusion rates on days 4 and 5 were designed to mimic the insulin response to GLP-1 on day 1 (Table 1). As the experiments lasted for only 2 h each, we chose not to substitute growth hormone (12). Each peptide was infused using a separate infusion pump. Experiments were randomized and performed within 1 week for each patient. Subjects were investigated in the recumbent position with one cannula inserted into a cubital vein for infusion of glucose (20% [weight/volume]) and hormones, and another cannula inserted into the contralateral cubital vein for the collection of blood samples. This arm was kept in a heated box (50°C) throughout the experiment to arterialize the venous blood (13). For bedside PG analyses, blood was collected into fluoride tubes every 5 min for adjustment of the glucose infusion rate. Additional samples were drawn at time −15, −10, 0, 15, 30, 45, 60, 75, 90, 105, and 120 min into iced tubes containing EDTA (6 mmol/l) plus aprotinin (500 kallikrein inhibitor unit/ml blood) (Trasylol; Bayer, Leverkusen, Germany), and a specific dipeptdyl peptidase (DPP)-4 inhibitor (valine-pyrrolidide; 0.01 pmol/l, final concentration) for hormone analyses. Before and after centrifugation (1,200 g at 4°C for 20 min), tubes were kept on ice; plasma was pipetted to storage tubes and stored at −20°C until analysis. Serum for insulin and C-peptide analysis was collected in Z serum clot activator glasses (Greiner Bio-One, Kremsmünster, Austria) and left to coagulate for 20 min before centrifugation and handling as described above. Serum was stored at −80°C until analysis.
TABLE 1.
Study design
| Basal insulin | Stimulated insulin | Glucagon inhibition | Basal (portal) glucagon | GLP-1 | Somatostatin infusion | Glucose demand (g) | |
|---|---|---|---|---|---|---|---|
| Day 1 | + | + | + | 24.5 ± 4.1 | |||
| Day 2 | + | + | + | + | 0.3 ± 0.2 | ||
| Day 3 | + | + | + | 10.6 ± 1.1 | |||
| Day 4 | + | + | + | 11.5 ± 2.7 | |||
| Day 5 | + | + | + | 24.5 ± 2.6 |
Data shown are means ± SE. Study design and glucose demands for each day.
Peptides.
Synthetic GLP-1 (7–36) amide (Good Manufacturing Practice quality, product no. 40061) (PolyPeptide Laboratories, Limhamn, Sweden) and synthetic somatostatin (GMP quality, product code FP-0467) (PolyPeptide Laboratories, Torrance, CA) were dissolved in sterilized water containing 2% human serum albumin and subjected to sterile filtration. The GLP-1 was demonstrated to be >97% pure and somatostatin >98% pure, and both peptides were shown to be identical to their natural human counterparts by high-performance liquid chromatography, mass, and sequence analysis. Appropriate amounts of each peptide for each experimental subject were dispensed into separate glass ampoules and stored frozen (−20°C) under sterile conditions until the day of the experiment when the solution was further diluted with saline containing 1% human albumin (Human Albumin; CSL Behring, Marburg, Germany) (viral safety achieved by procedures specified in the European Pharmacopoeia). Doses of all hormones were calculated according to body weight (except for somatostatin, which was given as a fixed dose [450 μg/h]). Insulin (Actrapid; Novo Nordisk, Bagsværd, Denmark) and glucagon (GlucaGen; Novo Nordisk, Bagsværd, Denmark) were diluted in saline containing 1% human serum albumin (Human Albumin; CSL Behring, Marburg, Germany) in saline.
Analyses.
PG concentrations were measured during the experiments using a glucose oxidase method glucose analyzer (Yellow Springs Instrument Model YSI 2300 STAT plus analyzer; Yellow Springs, OH). Serum insulin and C-peptide concentrations were measured using two-site assays (electrochemiluminescense immunoassay, Roche/Hitachi Modular analytics, Roche Diagnostic, Mannheim, Germany). The detection limit for each assay is <2 pmol/l, and intraassay coefficient of variation are 1.9% (insulin) and 4.6% (C-peptide) (14). Glucagon and GLP-1 concentrations were measured after extraction of plasma with 70% ethanol (volume/volume, final concentration). The glucagon radioimmunoassay is directed against the C-terminus of the glucagon molecule (antibody code no. 4305) and therefore mainly measures glucagon of pancreatic origin. The sensitivity is ∼1 pmol/l (15). Total GLP-1 concentrations were determined using antiserum code no. 89390 directed against the amidated C-terminus of GLP-1. The assay reacts equally with intact GLP-1 and with GLP-1 (9–36) amide, the primary metabolite (16).
Statistical analyses and calculations.
All results are presented as means ± SEM unless otherwise stated. Area under the curve (AUC) was calculated using the trapezoidal rule with subtraction of baseline values when calculating incremental/decremental AUCs. Comparisons between the five different days were made using two-way ANOVA and Bonferroni post hoc tests. Comparisons between specific days were made using repeated-measures ANOVA with Tukey post hoc test. P values <0.05 were considered statistically significant. Glucose demands were calculated as the amount of glucose (20% [weight/volume]) infused (administered by adjustable pump).
RESULTS
Glucose.
FPG levels were similar on all five experimental days. Mean values for the 120-min clamp period were 10.3 ± 0.05 (day 1), 11.5 ± 0.25 (day 2), 10.2 ± 0.06 (day 3), 10.8 ± 0.11 (day 4), and 10.0 ± 0.1 mmol/l (day 5) with two-way ANOVA disclosing a slight but significant increase in PG on day 2 compared with days 1, 3, and 5 (Fig. 2).
FIG. 2.
Glucose demand (g) for each day: day 1: GLP-1(insulin stimulation, glucagon inhibition); day 2: GLP-1, somatostatin, basal insulin, basal glucagon; day 3: somatostatin, basal insulin; day 4: somatostatin, “stimulated” insulin (mimicking day 1-insulin response), basal glucagon; day 5: somatostatin, “stimulated” insulin (mimicking day 1-insulin response). Data shown are means ± SEM. A: Glucose demands on each day shown in grams infused (g); means ± SEM. B: Glucose demand calculated from each interval of 30 min on each day. Data shown in grams (g); means ± SEM. NS indicates P > 0.5, *P < 0.5, ***P < 0.001.
AUCs for PG are given in Table 2. The amounts of glucose infused in order to maintain the clamp (Table 1 and Fig. 2) were similar on days 1 and 5 (P = not significant [NS]). On day 2, three of the ten patients required a minimal amount of glucose early in the clamp (0–30 min) (Fig. 2B). Similar amounts of glucose were needed on day 3 (representing isolated glucagon inhibition) and day 4 (representing insulin stimulation) (P = NS), which, however, were significantly (P < 0.5) less than the amounts required on day 1 or day 5.
TABLE 2.
AUC values
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Glucose (mol/l · 120 min) | 1.4 ± 0.1*2 | 1.6 ± 0.1*1,3,5 | 1.4 ± 0.1*2 | 1.5 ± 0.1 | 1.4 ± 0.1*2 |
| GLP-1 (nmol/l · 120 min) | 20 ± 2 | 18 ± 1 | |||
| Glucagon (nmol/l · 120 min) | −0.9 ± 0.1*2,4 | 2 ± 0.5*1,3,5 | −1.4 ± 0.1*2,4 | 2 ± 0.6*1,3,5 | −1.2 ± 0.1*2,4 |
| Insulin (nmol/l · 120 min) | 37 ± 11*2,3 | 1.1 ± 1*1,4,5 | −1.1 ± 1*1,4,5 | 25 ± 2*2,3 | 24 ± 2*2,3 |
| C-peptide (nmol/l · 120 min) | 215 ± 39*2,3,4,5 | −56 ± 5*1 | −80 ± 7*1 | −76 ± 6*1 | −82 ± 8*1 |
Data shown are means ± SE. Differences between the five days were evaluated for significance (P < 0.05) using repeated-measures ANOVA with Tukey post hoc analysis. Glucose is shown as total AUC; GLP-1, glucagon, insulin, and C-peptide as incremental/decremental AUCs. Significant differences are indicated by asterisks (*) followed by the number(s) of the group(s) compared with. Day 1: GLP-1 (insulin stimulation, glucagon inhibition); day 2: GLP-1, somatostatin, basal insulin, basal glucagon; day 3: somatostatin, basal insulin; day 4: somatostatin, “stimulated” insulin (mimicking day 1-insulin response), basal glucagon; and day 5: somatostatin, “stimulated” insulin (mimicking Day 1-insulin response).
GLP-1.
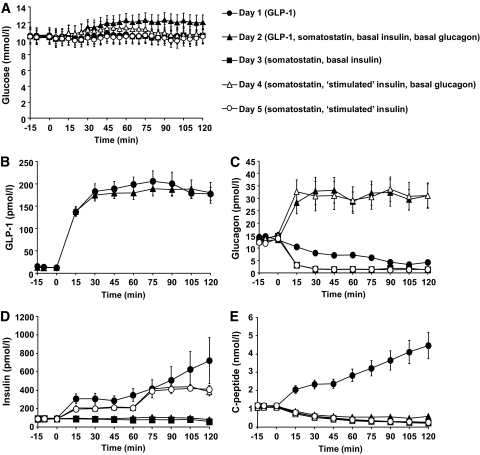
On days 1 and 2, the GLP-1 infusions resulted in similar AUCs (Table 2), and no difference between the curves was observed using two-way ANOVA (P = NS). Plateau levels of 190 ± 11 and 183 ± 14 pmol/l were reached within 30 min of starting each infusion (P = NS) (Fig. 2).
Glucagon.
No significant differences were found in the fasting glucagon levels between the five days (day 1, 14.1 ± 0.8; day 2, 13.5 ± 1.2; day 3, 14.0 ± 1.2; day 4, 13.8 ± 0.9; and day 5, 12.7 ± 0.9 pmol/l, P = NS).
A glucagon infusion rate of 1.25 ng/kg/min (n = 4) was calculated to give rise to plasma levels mimicking those in the portal vein (7) (see below). However, this infusion rate was individually adjusted in an attempt to maintain PG at FPG-/clamp-level, thus resulting in actual infusion rates of 1.25 (n = 4), 1.0 (n = 3), and 0.6 ng/kg/min (n = 3). Despite this, PG increased by 1–2 mmol/l in eight out of ten patients, therefore the results were combined (Fig. 1C, Table 2). Assuming minimal hepatic and splanchic degradation, the ratio between portal and peripheral glucagon concentrations (Cportal/Cperipheral) is 1.9 (17). Therefore we aimed to achieve peripheral plasma glucagon concentrations of 26 pmol/l (fasting glucagon concentration × 1.9). This level was reasonably matched during the glucagon infusions with glucagon plateau levels on the two days (days 2 and 4) of 31 ± 4.7 and 31 ± 5.1 pmol/l (P = NS).
FIG. 1.
Plasma levels of glucose (A), GLP-1 (B), glucagon (C), insulin (D), and C-peptide (E) during day 1 (●), day 2 (▲), day 3 (■), day 4 (△), and day 5 (○). Data shown are means ± SEM. NS indicates P > 0.5.
Somatostatin proved very efficient in blocking α-cell secretion on both day 3 and day 5 where plasma glucagon decreased to around 1–2 mmol/l (Fig. 1). A significant inhibition of glucagon was also seen when GLP-1 was infused (P < 0.0001) (Table 2). Toward the end of the GLP-1 infusion, endogenous glucagon concentrations fell to levels equal to those on days 3 and 5 (Fig. 1C).
Insulin and C-peptide.
As illustrated in Fig. 1D, GLP-1 proved to be highly insulinotropic in all patients resulting in a mean plasma insulin plateau of 312 ± 57 pmol/l and a mean plasma C-peptide plateau of 2.4 ± 0.1 nmol/l during the first 60 min of GLP-1 infusion (15–60 min) followed by a steady increase in β-cell secretion to maximum values of 722 ± 251 pmol/l (insulin) and 4.5 ± 0.7 nmol/l (C-peptide) (Fig. 1D and E). On days 2 and 3, insulin levels remained at basal levels throughout the 120 min clamps (NS vs. day 1). On days 4 and 5, the insulin infusions successfully mimicked the response to GLP-1 (day 1) with no significant differences according to time (two-way ANOVA) or AUC levels (Table 2). Endogenous β-cell secretion was strongly inhibited by somatostatin as evidenced from the C-peptide levels decreasing equally on days 2–5 (Fig. 1E).
Side effects.
Infusion of somatostatin induced a mild, transient nausea in some of the patients, typically in combination with glucagon infusion. No other side effects were observed.
DISCUSSION
GLP-1 administration has a glucose-lowering effect in patients with type 2 diabetes (4). The mechanism behind this antidiabetic effect is dual as GLP-1 both inhibits α-cell secretion and thereby HGP (3) and stimulates β-cell secretion leading to insulin-mediated glucose disposal in peripheral tissues and additional (insulin-mediated) inhibition of HGP (3). However in clinical studies it is difficult to distinguish between the glucose-lowering effect of decreased glucagon on one side and the effect of increased insulin on the other. We decided, therefore, to quantify the individual contributions of these two glucose-lowering components by clamping plasma glucose at the preinfusion level (thereby eliminating glucose-induced effects on glucagon and insulin secretion) and then mimicking the GLP-1–induced changes in islet hormone secretion using exogenous insulin and glucagon infusions while simultaneously blocking endogenous α- and β-cell secretion with somatostatin. Consequently, the amount of glucose required to maintain the clamp reflects the sum of changes in HGP (although HGP itself was not measured) and peripheral disposal of glucose and, thereby represents the net effect of the endocrine perturbations. Furthermore by substituting each of the pancreatic hormones separately, it was possible to determine the individual contributions of these hormones. Thus, day 1 represented the effects of GLP-1 alone. On day 2, GLP-1 was again infused but changes in pancreatic hormone levels were prevented by means of somatostatin, while basal pancreatic hormone levels were substituted using insulin and glucagon replacement infusions. On day 3, we examined the effects of inhibition of glucagon alone, while insulin was kept at basal levels, and on day 4, glucagon was maintained at basal (portal) levels, while GLP-1–induced insulin secretion was mimicked by infusion. On day 5, we studied the effect of mimicking the changes in stimulated insulin and suppressed glucagon seen in response to GLP-1, but brought about by somatostatin and insulin infusion (as on day 4) rather than GLP-1; this served as a control for the accuracy of the infusion protocols (Figs. 1 and 2).
The infusion protocols appeared successful. Thus somatostatin suppressed glucagon levels to similar low levels as during GLP-1 infusion, although the suppression occurred somewhat earlier during somatostatin infusion. Insulin responses were also well matched (days 4 and 5 vs. day 1) with similar AUCs (Table 1). For replacement of basal glucagon levels during somatostatin infusion, we had to make a choice: either maintain peripheral plasma concentrations or try to mimic portal levels, which in the fasting state have been calculated to exceed peripheral levels by a factor 1.9 (17), representing the dilution of the splanchnic circulation into the systemic circulation. Since in humans the target of glucagon action is the liver (18,19), which is exposed to portal glucagon levels, we chose the portal levels; these were successfully reached (in peripheral plasma) during the glucagon infusions. However, the infusion rate designed for this (1.25 ng/kg/min [7]) resulted in small and variable increases in PG when given together with basal insulin replacement. This indicates a slight overmatching of portal glucagon-to-insulin ratio, presumably reflecting that normally portal insulin levels are also higher than peripheral levels. However because of the prominent actions of insulin on glucose disposal in peripheral tissues, we decided not to attempt to mimic portal insulin levels. Therefore instead of adjusting insulin infusions, glucagon infusion rates were varied in an attempt to maintain stable glucose levels. It is clear, however, that the portal insulin levels are under-matched, both because of the dilution of the portal into the systemic circulation and because of the portal insulin uptake. As mentioned, appropriately matched infusions would have significant effects on peripheral glucose disposal and furthermore would have resulted in even higher glucose requirements. This would indicate the even stronger influence of glucagon on glucose turnover. In order to resolve this problem however further experiments with higher insulin infusion rates and preferably direct measurements of HGP should be carried out. The identical glucose requirements do show that the changes in glucose turnover were similar, and increasing insulin infusion rates to expected portal levels during GLP-1 infusion therefore must not result in further glucose requirements. There are two possible explanations for this conflict 1) either the hepatic glucose production is already minimal during days 1 and 5 so that further increases in insulin would not result in significant further decreases in HGP (disregarding the effects on peripheral glucose disposal), or 2) the slower suppression of glucagon secretion on day 1 compared to day 5 makes up for the under-matching of portal insulin, resulting in identical glucose requirements. Possibly, both contribute. From the present results, we conclude that both inhibition of glucagon and stimulation of insulin secretion are important for the blood glucose lowering effects of GLP-1, with each contributing about half of the total effect.
Our findings suggest that the inhibition of glucagon secretion is a very important part of the antidiabetic, glucose-lowering effect of GLP-1. In most clinical studies of type 2 diabetes treatment with GLP-1 or its analogues where glucagon levels have been reported, there have been significant reductions both in the fasting state and after (e.g., meal stimulation), whereas in general, insulin levels have been less affected (20–24). This should be considered, however, in light of the often pronounced reductions in PG levels observed concomitantly. Therefore, regarding insulin secretion, the effect of GLP-1 seems to consist of the enhancement of glucose-induced insulin secretion, resulting in rather unchanged absolute secretion rates, whereas glucagon secretion, which would have been expected to increase with falling PG levels, actually decreases. In many patients with type 2 diabetes, both fasting and postprandial glucagon levels are abnormally elevated (25–28), possibly due to a decreased ability of glucose to inhibit glucagon secretion appropriately. The effects of GLP-1 may, in fact, be viewed as restoration of the ability of the α-cells to react to glucose as was demonstrated in hyperglycemic clamp studies (8,29). Together, these results support that the inhibition of glucagon secretion by GLP-1 constitutes a very important part of the glucose-lowering effect of GLP-1, also in the clinical setting.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
This study has been submitted in abstract form for presentation at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
We are grateful to the participating patients and to the technical assistance from J. Purtoft, N. Kjeldsen, L. Albæk, and S. Pilgaard.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Clinical trial reg. no. NCT00655603, clinicaltrials.gov.
See accompanying commentary, p. 1572.
REFERENCES
- 1.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA: Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I (7–36) amide in type I diabetic patients. Diabetes Care 1996;19:580–586 [DOI] [PubMed] [Google Scholar]
- 2.de Heer J, Rasmussen C, Coy DH, Holst JJ: Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 2008;51:2263–2270 [DOI] [PubMed] [Google Scholar]
- 3.Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ: Effect of glucagon-like peptide-1 (proglucagon 78–107 amide) on hepatic glucose production in healthy man. Metabolism 1994;43:104–108 [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W: Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744 [DOI] [PubMed] [Google Scholar]
- 5.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ: Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 6.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA: Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000;85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 7.Shah P, Basu A, Basu R, Rizza R: Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B, Larsson H, Holst JJ: Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:473–478 [DOI] [PubMed] [Google Scholar]
- 9.Vilsboll T, Krarup T, Madsbad S, Holst JJ: Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia 2002;45:1111–1119 [DOI] [PubMed] [Google Scholar]
- 10.Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsbøll T: Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab 2009;94:4679–4687 [DOI] [PubMed] [Google Scholar]
- 11.Orskov L, Holst JJ, Møller J, Orskov C, Møller N, Alberti KG, Schmitz O: GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 1996;39:1227–1232 [DOI] [PubMed] [Google Scholar]
- 12.De Feo P, Perriello G, Torlone E, Ventura MM, Santeusanio F, Brunetti P, Gerich JE, Bolli GB: Demonstration of a role for growth hormone in glucose counterregulation. Am J Physiol 1989;256:E835–E843 [DOI] [PubMed] [Google Scholar]
- 13.Nauck MA, Blietz RW, Qualmann C: Comparison of hyperinsulinaemic clamp experiments using venous, ‘arterialized’ venous or capillary euglycaemia. Clin Physiol 1996;16:589–602 [DOI] [PubMed] [Google Scholar]
- 14.Bablok W, Passing H, Bender R, Schneider B: A general regression procedure for method transformation. Application of linear regression procedures for method comparison studies in clinical chemistry, Part III. J Clin Chem Clin Biochem 1988;26:783–790 [DOI] [PubMed] [Google Scholar]
- 15.Holst JJ: Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem J 1982;207:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ: Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994;43:535–539 [DOI] [PubMed] [Google Scholar]
- 17.Holst JJ, Burcharth F, Kühl C: Pancreatic glucoregulatory hormones in cirrhosis of the liver: portal vein concentrations during intravenous glucose tolerance test and in response to a meal. Diabete Metab 1980;6:117–127 [PubMed] [Google Scholar]
- 18.Alford FP, Bloom SR, Nabarro JD, Hall R, Besser GM, Coy DH, Kastin AJ, Schally AV: Glucagon control of fasting glucose in man. Lancet 1974;2:974–977 [DOI] [PubMed] [Google Scholar]
- 19.Jiang G, Zhang BB: Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E671–E678 [DOI] [PubMed] [Google Scholar]
- 20.Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A: Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89:2078–2084 [DOI] [PubMed] [Google Scholar]
- 21.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde LLEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 22.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O: One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004;53:1187–1194 [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter LDURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 24.Zander M, Madsbad S, Madsen JL, Holst JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
- 25.Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T: Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007;50:797–805 [DOI] [PubMed] [Google Scholar]
- 26.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH: Abnormal alpha-cell function in diabetes: response to carbohydrate and protein ingestion. N Engl J Med 1970;283:109–115 [DOI] [PubMed] [Google Scholar]
- 27.Toft-Nielsen MB, Madsbad S, Holst JJ: Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 1999;22:1137–1143 [DOI] [PubMed] [Google Scholar]
- 28.Unger RH, Orci L: The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–16 [DOI] [PubMed] [Google Scholar]
- 29.Vilsbøll T, Krarup T, Madsbad S, Holst JJ: Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept 2003;114:115–121 [DOI] [PubMed] [Google Scholar]