Abstract
OBJECTIVE
Increased deposition of extracellular matrix (ECM) within the kidney is driven by profibrotic mediators including transforming growth factor-β (TGF-β) and connective tissue growth factor (CTGF). We investigated whether some of their effects may be mediated through changes in expression of certain microRNAs (miRNAs).
RESEARCH DESIGN AND METHODS
Proximal tubular cells, primary rat mesangial cells, and human podocytes were analyzed for changes in the expression of key genes, ECM proteins, and miRNA after exposure to TGF-β (1–10 ng/μl). Tubular cells were also infected with CTGF-adenovirus. Kidneys from diabetic apoE mice were also analyzed for changes in gene expression and miRNA levels.
RESULTS
TGF-β treatment was associated with morphologic and phenotypic changes typical of epithelial-mesenchymal transition (EMT) including increased fibrogenesis in all renal cell types and decreased E-cadherin expression in tubular cells. TGF-β treatment also modulated the expression of certain miRNAs, including decreased expression of miR-192/215 in tubular cells, mesangial cells, which are also decreased in diabetic kidney. Ectopic expression of miR-192/215 increased E-cadherin levels via repressed translation of ZEB2 mRNA, in the presence and absence of TGF-β, as demonstrated by a ZEB2 3′-untranslated region luciferase reporter assay. However, ectopic expression of miR-192/215 did not affect the expression of matrix proteins or their induction by TGF-β. In contrast, CTGF increased miR-192/215 levels, causing a decrease in ZEB2, and consequently increased E-cadherin mRNA.
CONCLUSIONS
These data demonstrate the linking role of miRNA-192/215 and ZEB2 in TGF-β/CTGF–mediated changes in E-cadherin expression. These changes appear to occur independently of augmentation of matrix protein synthesis, suggesting that a multistep EMT program is not necessary for fibrogenesis to occur.
Diabetic nephropathy is a major microvascular complication and a leading cause of chronic kidney failure in individuals with both type 1 and type 2 diabetes. The risk is dramatically elevated with poor blood glucose control and the greatest rate of progression occurs with elevated blood pressure, confirming that hemodynamic and metabolic factors participate in the development and progression of this disorder. The glomerulus is considered to be the primary site of initial renal injury, but increasing evidence points to the tubulointerstitium also playing a critical role via the process of epithelial-mesenchymal transition (EMT) (1). During this process, epithelial cells acquire features of mesenchymal cells such as myofibroblasts, resulting in loss of E-cadherin expression, the acquisition of mesenchymal markers such as α-smooth muscle actin (α-SMA), and the increased deposition of extracellular matrix (ECM).
In vivo evidence for EMT in diabetic kidney has been controversial. A partial EMT (p-EMT) phenotype was recently described (2,3), identifying cells expressing both epithelial and mesenchymal markers. This phenotype is relevant because the acquisition of mesenchymal markers and expression of ECM proteins that occur early in EMT may be transient, occurring in cells exposed to the diabetic milieu and contributing to fibrosis. We previously demonstrated that connective tissue growth factor (CTGF) induces the p-EMT phenotype in proximal tubular cells that express α-SMA and ECM proteins while maintaining the expression of the epithelial marker, E-cadherin (4). The mechanisms underlying the partial EMT phenotype and the reasons that E-cadherin is spared in this situation have not been clear.
Several profibrotic factors and pathways are known to drive EMT (2,5), primarily by converging on the Snail and ZEB families of transcriptional repressors of E-cadherin that bind to the E-cadherin promoter (6–9) and collagen expression (10). These factors play a role in maintaining the mesenchymal phenotype (11). Recent reports have established a role for microRNAs (miRNAs) in the process of EMT by regulating the expression of ZEB2. The miR-200 family regulates transforming growth factor (TGF)-β–mediated EMT in MDCK cells via targeting of ZEB1/2 (7). Interestingly, ZEB1/2 were found to be negative transcriptional regulators of the miR-200 family (7). This observation has been confirmed by others (12–15) and for the first time demonstrates clear cross-talk between miRNA and TGF-β pathways, establishing a potentially important role for miRNAs in EMT and fibrosis.
Recently, several miRNAs (miR-192, -194, -215, and -216) were identified as preferentially and highly expressed in kidney (16). One of these, miR-192, was increased by TGF-β treatment in mesangial cells and in the kidney in experimental diabetes and was associated with increased expression of col1a2 (10). Also, miR-192 and miR-215 were recently demonstrated to play a role in cell cycle control (17,18). In more recent studies, miR-192 was increased in human kidney in both hypertensive nephrosclerosis (19) and IgA nephropathy but not in glomerulosclerosis (20). In those studies, miR-192 levels did not correlate with markers of EMT or fibrosis, whereas members of the miR-200 family did. The role of miR-192 in human kidney disease therefore appears to be more complex than originally reported.
In this study we investigated the role of the miR-192/215 family as translational regulators of the ZEB2 transcription factor and ECM production in a number of renal cells, including proximal tubular cells, primary mesangial cells, and human conditionally immortalized podocytes. In addition, we examined these miRNAs in the kidney of streptozotocin (STZ)-induced diabetic apoE−/− mice, which is a model of more advanced renal disease with prominent fibrosis and EMT. We have demonstrated that the miR-192/215 family is indeed a regulator of E-cadherin expression but not ECM.
RESEARCH DESIGN AND METHODS
Cell culture.
The rat kidney tubular epithelial cell line (NRK-52E) was obtained from the American Type Culture Collection (Rockville, MD) and maintained in Dulbecco's modified Eagle's medium (DMEM) (4). Primary mesangial cells were isolated and cultured as previously described (21,22). Human conditionally immortalized podocytes were propagated as previously described (23). Experimental treatments for all cell lines were in serum-reduced conditions (2%) with recombinant human TGF-β (R&D Systems, Minneapolis, MN).
RNA extraction and real-time PCR.
Gene expression analysis was by real-time RT-PCR (24) using the TaqMan system based on real-time detection of accumulated fluorescence (ABI Prism 7500; Perkin-Elmer). Gene expression levels were normalized against 18S rRNA (18S rRNA TaqMan Control Reagent Kit, ABI Prism 7500; Perkin-Elmer). Details of primers and TaqMan probes for most genes have been previously reported (4). Triplicate experiments were performed, with six replicates. Results are expressed relative to control, which was arbitrarily assigned a value of 1. Values are shown as means ± SEM unless otherwise specified. P < 0.05 was considered significant (Student t test).
miRNA assays.
For miRNA analysis, cDNA synthesis and real-time PCR were performed using TaqMan miRNA assays per the manufacturer's recommendations (Applied Biosystems, Foster City, CA). Experimental groups were in replicates of six and normalized to RN6UB, Sno135, or U87 for rat, human, and mouse samples, respectively. Values are shown as means ± SEM unless otherwise specified. P < 0.05 was considered significant (t test).
Transfection of miRNA Precursors and Inhibitors.
NRK52E cells were seeded at 3 × 104 cells per well in 12-well plates and transfected the following day in OptiMEM medium (Invitrogen) with premiRNAs (100 nmol/l) or anti-miRNA (100–200 nm) (Applied Biosystems) using Oligofectamine (Invitrogen). Cells were harvested 3 days after transfection.
Western analysis.
Precast gels (4–12%) were used for ZEB2 analyses. ZEB2 antibody was obtained from Santa Cruz. Quantitation of blots was carried out using Quantity One software on the Chemidoc XRS imaging system (Bio-Rad).
Snail and ZEB2 3′untranslated region-luciferase reporter analyses.
The luciferase-3′ untranslated region (UTR) ZEB2 construct contains the conserved region among human, mouse, and rat ZEB2 genes (7). NRK52E cells were cotransfected with RL-reporter plasmids (0.5 μg/ml), cytomegalovirus-galactosidase construct, and miRNA or miRNA inhibitors, using Lipofectamine 2000 (Invitrogen) in OptiMEM medium (Invitrogen). Cells were harvested 48 h after transfection using the Luciferase Reporter Assay System (Promega), and luciferase and galactosidase assays were performed per the manufacturer's recommendations. All experiments were repeated at least twice and performed in triplicate. Values are shown as means ± SEM unless otherwise specified. P < 0.05 was considered significant.
Immunofluorescence.
Cells were grown on coverslips, washed twice with PBS, fixed in 4% paraformaldehyde for 20 min, permeabilized using 1% SDS, and incubated in a blocking buffer (1% BSA, 0.25% Triton X-100 in PBS, pH 7.4). Primary and secondary antibodies were diluted in blocking buffer and incubated with cells overnight at room temperature. Coverslips were then mounted using Prolog Gold antifade reagent with DAPI (Invitrogen), and cells were viewed using an Olympus BX61 fluorescence microscope. Primary antibodies were α-SMA (clone 1A4; Dako, Cupertino, CA), E-cadherin (Transduction Laboratories, Lexington, KY), ZO-1 (Invitrogen), vimentin (Sigma, St. Louis, MO), fibronectin (Sigma), and nephrin (Santa Cruz). Secondary antibodies included Alexa Fluor 488 (mouse anti-rabbit and goat anti-rabbit) and Alexa Fluor 594 (rabbit anti-goat). F-actin was stained with phalloidin (red) (Invitrogen).
In vivo studies.
To explore the relationship between miR-192/215 and the development of fibrotic kidney disease, a study was carried out using apoE knockout mice (n = 8/group) that were rendered diabetic by five daily intraperitoneal STZ injections as previously described (25). Only animals with blood glucose levels >15 mmol/l at 5 days after the induction of diabetes (>90% of injected mice) were included in the study. After 10 weeks, animals were anesthetized and killed, and tissues were collected as previously described (25).
Immunohistochemistry.
Four-micrometer paraffin kidney sections were used for immunohistochemical analyses as previously described (25,26). Primary antibodies were α-SMA (clone 1A4; Dako), collagen IV (Southern Biotechnology, Birmingham, AL), and fibronectin (Dako) and used as previously described (25,26). Finally, sections were counterstained with Mayer's hematoxylin, dehydrated, and mounted and analyzed for staining using light microscopy (Olympus BX-50; Olympus Optical, Tokyo, Japan).
RESULTS
TGF-β induces expression of ECM proteins and markers of EMT in renal cells.
TGF-β is a powerful inducer of ECM protein expression and fibrosis in many cell types, as well as an inducer of EMT in epithelial cells. To determine the effect of TGF-β on the kidney-specific miRNAs (16) and correlate the observed changes with expression of ECM proteins, three renal cell types were studied. These included proximal tubular cells, primary mesangial cells, and human conditionally immortalized podocytes, representing the main kidney cell types potentially contributing to the development of fibrosis in the diabetic kidney.
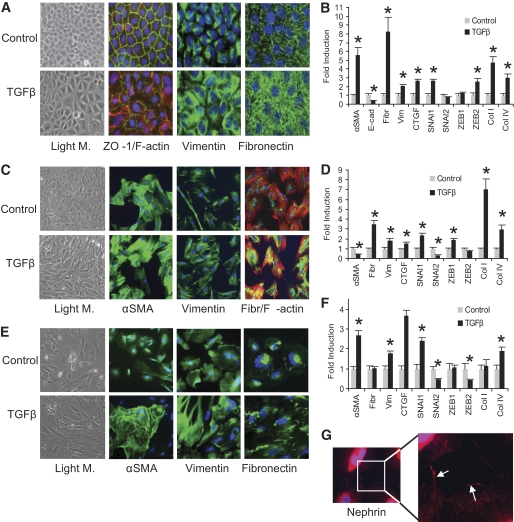
TGF-β induced the classic EMT morphologic changes in NRK52E cells, including the elongation of cells with a loss of apical-basal polarity (Fig. 1A) as previously reported (4). These changes were associated with decreased expression of the epithelial marker ZO-1 and increased staining for F-actin, vimentin, and fibronectin (Fig. 1A). Significant increases were observed in the mRNA levels of α-SMA, vimentin, fibronectin, CTGF, and collagens I and IV, with a decrease of the epithelial marker, E-cadherin (Fig. 1B). Interestingly, the transcriptional repressors of E-cadherin, SNAI1, and ZEB2, were increased twofold (Fig. 1B). In this particular model of EMT, TGF-β had minimal effect on cell proliferation as evidenced by the marginal decrease in PCNA expression (supplementary Fig. 2, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1736/DC1).
FIG. 1.
TGF-β induced changes in renal cell morphology, protein, and gene expression. A: NRK52E cells (DMEM, 25 mmol/l glucose, 2% serum), were treated with TGF-β (10 ng/ml, 3 days). Light microscopy (Light M.) images (×20) demonstrate that TGF-β causes a loss of the typical epithelial morphology to larger and more irregularly-shaped cells typical of the myofibroblast phenotype. TGF-β treatment also caused a dramatic decrease of ZO-1 expression at tight junctions between neighboring cells (green) accompanied with increased F-actin expression (red) (×40). Nuclei were stained with DAPI. Expression of vimentin and fibronectin protein was also dramatically increased with TGF-β treatment (green). B: The expression of several genes was assessed by real-time quantitative PCR in NRK52E cells and the significant changes caused by TGF-β treatment are indicated (*P < 0.05, compared with control). C: Rat mesangial cells (DMEM, 2% serum) were treated with TGF-β (10 ng/ml, 3 days). Changes in morphology are evident by light microscopy (×20) with cells adopting a more spindly appearance after TGFβ treatment. Immunostaining for α-SMA expression was increased with TGF-β, as were vimentin and fibronectin (green). A change in F-actin arrangement (red) was more obvious after TGF-β treatment. D: The expression of a number of genes was assessed by real-time quantitative PCR and significant changes in response to TGF-β are indicated (*P < 0.05, compared with control). E: Human podocytes were seeded (33°C) and subsequently differentiated (37.8°C, 10–14 days), followed by treatment with TGF-β (10 ng/ml, 3 days, RPMI with 2% serum). Light microscopy (×20) reveals a dramatic change in phenotype with loss of the typical arborized morphology in control cells, becoming hypertrophic, elongated, and in close proximity with neighboring cells after TGF-β treatment. Immunostaining of podocytes revealed increased α-SMA (green) with TGF-β treatment. Vimentin staining (green) was also increased and altered with rearrangement from fine fibers radiating from the nucleus to the periphery to very strong staining and thicker fibers with TGF-β. Fibronectin was also significantly increased (green) from being mainly associated with the nucleus to a more patchy cytoplasmic localization after treatment with TGF-β. All error bars represent ± SEM. F: Gene expression analysis by real-time quantitative PCR revealed significant changes after treatment with TGF-β as indicated (*P < 0.05, compared with control). G: Mature and differentiated podocytes were characterized by punctuated nephrin expression (red) at the periphery of untreated cells as distinct spots, confirming the identity of these cells as podocytes (enlarged inset).
TGF-β also induced morphologic changes in primary rat mesangial cells (Fig. 1C), with increased expression of αSMA, vimentin, and fibronectin, compared with control cells. Significant increases in vimentin, fibronectin, collagens I and IV, CTGF, SNAI1, and ZEB1 mRNA were observed (Fig. 1D). Interestingly, despite a modest increase in α-SMA protein (Fig. 1C), TGF-β caused a decrease in α-SMA mRNA (Fig. 1D), suggesting that the regulation of αSMA protein expression is more complex in mesangial cells than in proximal tubular epithelial cells.
The effect of TGF-β on human conditionally immortalized podocytes was also investigated. Upon differentiation, these cells expressed the podocyte-specific slit-pore protein, nephrin, which was punctuated and present at the cell periphery (Fig. 1G) as previously described (23). Podocyte morphology was also dramatically altered in TGF-β–treated cells with cells losing their arborized irregular flat shape and becoming more dense and elongated (Fig. 1E). Increased staining for α-SMA, vimentin, and fibronectin was also observed after treatment with TGF-β with the appearance and organization altered by TGF-β in each case (Fig. 1E). TGF-β treatment significantly increased α-SMA, vimentin, CTGF, collagen IV, and SNAI1 RNA (Fig. 1F). In contrast, levels of SNAI2 and ZEB2 were significantly decreased and ZEB1 remained unchanged. These results are consistent with the recently described changes described in murine podocytes after TGF-β treatment (27) leading to podocyte dysfunction.
From these experiments, it was evident that all three renal cell types increased expression of ECM proteins in response to TGF-β, confirming that each cell type may potentially contribute to the deposition of ECM and, hence, fibrosis in diabetic nephropathy. The underlying mechanism is likely to involve part of the EMT program in epithelial cells such as proximal tubular cells, but also occurs in the absence of EMT in mesangial cells and podocytes. Changes in the mRNA levels of the transcriptional repressors of E-cadherin, SNAI1/2, and ZEB1/2, which have been previously implicated in EMT in epithelial cells, were also observed in proximal tubular cells; however, these changes did not appear to be consistent with ECM production in the other cell types.
TGF-β alters the expression of “kidney-specific” miRNAs.
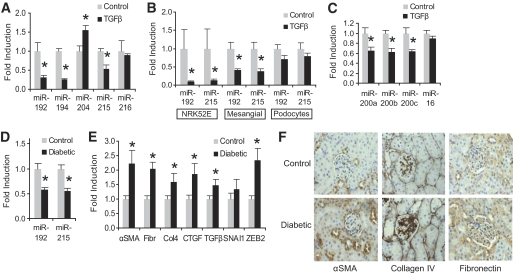
Given that a number of miRNAs (miRNA-192, -194, -204, -215, and -216) are highly expressed in kidney tissue (16), we examined whether TGF-β could alter their expression in proximal tubular cells. Analysis of miRNA levels in NRK52E cells adapted to TGF-β identified significant decreases in miR-192, -194, and -215 (−68, −74, and −46%, respectively) compared with control cells (Fig. 2A). We also observed that miR-204 was significantly increased (+54%) and miR-216 remained unchanged. Interestingly, both miR-192 and miR-215, which belong to the same family and share the same seed sequence (Fig. 3A), were both downregulated by TGF-β. These observations demonstrate that the expression of certain miRNAs is altered in tubular cells in response to TGF-β, consistent with the possibility that miRNAs may mediate some of the profibrotic effects of TGF-β in the kidney.
FIG. 2.
Changes in kidney-specific miRNAs in renal cells after TGF-β treatment, as well as miRNA and gene expression changes in diabetic mouse kidney. A: NRK52E cells were cultured in the presence of TGF-β (10 ng/ml, 10 days) before miRNA expression levels were assessed by real-time quantitative PCR. A significant increase was observed in miR-204, whereas miR-192, miR-194, and miR-215 were significantly decreased (*P < 0.05, compared with control). B: The experiment was repeated with a shorter exposure to TGF-β (10 ng/ml, 3 days), and levels of miR-192 and miR-215 were assessed in proximal tubular cells (NRK52E), primary rat mesangial cells, and human podocytes. Both miRNAs were significantly decreased with TGF-β in proximal tubular and mesangial cells (*P < 0.05, compared with control). C: TGF-β (10 ng/ml, 3 days) also decreased the expression of some members of the miR-200 family in NRK52E cells (* P < 0.05, compared with control), whereas expression of miR-16 remained unaltered. D: RNA extracted from the renal cortex of control and 10-week diabetic apoE mice (n = 10 per group) displayed a significant decrease for both miR-192 and miR-215 in diabetic mice (*P < 0.05, compared with control). E: Gene expression analysis of control and 10-week diabetic kidney was assessed by real-time quantitative PCR for a number of genes. Significant increases were observed in α-SMA, fibronectin, collagen 4, CTGF, TGF-β, and ZEB2 (*P < 0.05, compared with control). F: Immunohistochemical analysis demonstrates increased staining for α-SMA, collagen IV, and fibronectin in the kidneys of 10-week diabetic apoE knockout mice compared with control. All error bars represent ± SEM.
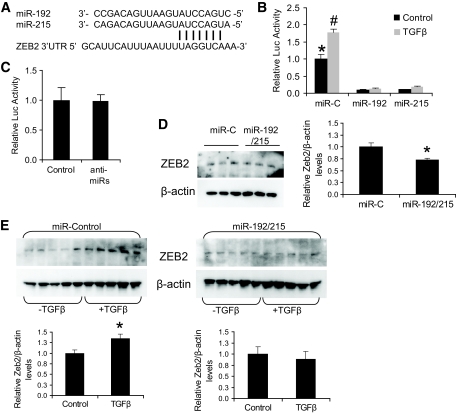
FIG. 3.
Regulation of the ZEB2 3′UTR by miR-192/215. A: Alignment of the miR-192/215 sequences and the targeted area of the 3′UTR of ZEB2 (http://www.targetscan.org). The seed sequence at the 5′ end of the miRNA-192/215 and the targeted region in the ZEB2 3′UTR at nucleotides 955–961 downstream of the stop codon are shown. B: NRK52E cells were transfected with ZEB2 3′UTR luciferase reporter plasmid (1 μg), β-galactosidase plasmid (0.2 μg), and either miR-control (miR-C), miR-192, or miR-215 (100 nmol/l). TGF-β (10 ng/ml) was added 4 h after transfection, and cells were analyzed for β-galactosidase and luciferase (Luc) activity 3 days later. TGF-β significantly increased luciferase activity (P < 0.05 compared with control). MiR-192/215 significantly reduced luciferase activity from the ZEB2 3′UTR in the absence (*P < 0.05) or presence (#P < 0.001) of TGF-β, compared with control. C: Anti-miR-192/215 (anti-miRs) had no effect on the luciferase activity of the ZEB2 3′UTR construct compared with control. D: Western analysis and quantitation for ZEB2 expression in NRK52E cells transfected as in B with miR-control and miR-192/215. miR-192/215 significantly reduced ZEB2 protein expression (*P < 0.05, compared with control). E: Western analyses and quantitation of ZEB2 expression in NRK52E cells transfected either with miR-conrtol or miR-192/215 in the presence or absence of TGFβ. miR-control had no effect on the increased expression of ZEB2 induced by TGF-β; however, miR-192/215 prevented this induction (*P < 0.05, compared with control). All error bars represent ± SEM.
miR-192/215 expression in renal cells is downregulated by TGF-β.
Our finding that both miR-192 and miR-215 were decreased in proximal tubular cells by TGF-β contrasts with previous work (10) in which miR-192 was increased in mouse mesangial cells and correlated with increased col1a2. In that work, serum starvation caused a significant decrease in miR-192 and TGF-β treatment and restored expression to that observed in cells grown in the presence of serum. In the same study, TGF-β decreased miR-215, which was consistent with our observations. We therefore examined miR-192/215 levels after a short exposure (3 days) to TGF-β and observed significantly decreased miR-192/215 levels in NRK52E cells and mesangial cells (Fig. 2B). Human podocytes displayed the same trend; however, the differences did not reach significance. The importance of these miRNAs to the TGF-β response of podocytes remains to be determined. These data demonstrate that the three renal cell populations studied all respond to TGF-β by increasing expression of ECM proteins, but also by reducing the expression of miR-192/215, suggesting that these miRNA changes may possibly contribute to fibrotic changes in the diabetic kidney. In all cell types, miR-192 was more abundantly expressed than miR-215 (supplementary Fig. 1, available in an online appendix). We also assessed the expression of some members of the miR-200 family as these have been previously shown to be downregulated with TGF-β in other cell lines and are drivers of the EMT phenotype in epithelial cells (7). As shown in Fig. 2C, miR-200a, -200b, and -200c were all significantly decreased with TGF-β in NRK52E cells, consistent with the earlier reports. However, miR-16 is considered to be relatively inert and did not alter with TGF-β treatment.
Decreased expression of miR-192/215 in apoE-knockout diabetic mouse kidney.
Changes in miRNA levels in in vitro models are well documented. To determine the relationship between miR-192/215 and diabetic kidney disease, we examined kidney cortex from STZ-induced diabetic apoE knockout mice. As observed in our in vitro experiments, miR-192/215 were significantly decreased (−40 and −43%, respectively) in diabetic kidney compared with control (Fig. 2D). These data are in contrast to the work of Kato et al. (10) who reported an increase in miR-192, albeit in different models, the STZ-induced diabetic C57BL/6 mouse kidney (7-week) and in the kidneys of 10-week db/db mice, but more consistent with human glomerulosclerosis and diabetes (20,28). The decrease in miR-192/215 was associated with increased ZEB2 RNA (Fig. 2E) and protein (see later Fig. 3D), consistent with our observations in NRK52E cells. Levels of α-SMA, fibronectin, CTGF, collagen IV, and TGF-β mRNA were significantly increased in diabetic kidney (Fig. 2E), as were protein levels of αSMA, collagen IV, and fibronectin (Fig. 2F). These in vivo observations are consistent with the in vitro data and suggest that decreased levels of miR-192/215 are associated with enhanced renal fibrosis in diabetes. These findings are consistent with the progression of renal fibrosis, occurring at least in part via a mechanism that involves dysregulation of ZEB2 and, consequently, E-cadherin.
miR-192 and miR-215 target the ZEB2 3′UTR.
miR-192 and miR-215 share the same seed sequence (Fig. 3A) and are expressed in renal cells (Fig. 2B). The ZEB2 3′UTR contains several sites for the miR-200 family (7), including a single site for miR-192/215 (Fig. 3A). Because ZEB2 mRNA (Fig. 1B) and protein (Fig. 3E) are increased by TGF-β in NRK52E cells, we investigated whether these genes might be targeted by miR-192/215 for translational repression. NRK52E cells cotransfected with the ZEB2 3′UTR-luciferase reporter construct and the miR-control had significantly increased luciferase activity after TGF-β treatment (+78%; Fig. 3B). In the same experiment, miR-192 and miR-215 totally abolished the TGF-β–induced increase in luciferase activity (90 and 88%, respectively), even lower than that observed in non-TGF-β–treated cells (Fig. 3B). The above data confirm that both miR-192 and miR-215 independently act as specific and potent translational repressors of ZEB2 by targeting the 3′UTR of this gene. This is in contrast with the earlier report in which miR-215 was not shown to have any effect on ZEB2 (10). In similar experiments we tested whether the translational repression of the ZEB2 3′UTR by endogenous miR-192/215 could be relieved by anti-miR-192/215, however no increase in luciferase activity was observed (Fig. 3C). This was not surprising since a plethora of miRNAs target the 3′UTR of ZEB2, including the members of the miR-200 family (Fig. 2C) which bind at several sites (7). In the absence of TGF-β, all of these miRNAs would have to be inhibited to relieve translational repression acting on the 3′UTR of ZEB2.
Western analysis of NRK52E cells transfected with miR-192/215 revealed a small but significant reduction in ZEB2 protein levels (−27%, P < 0.05; Fig. 3D) compared with control. This is consistent with ZEB2 expression being tightly regulated in resting cells. TGF-β treatment resulted in a significant increase of ZEB2 protein in miR-control–transfected cells compared with the control (+36%, P < 0.05; Fig. 3E, left panels). This TGF-β-induced increase in ZEB2 protein, however, was abrogated when cells were transfected with miR-192/215 (Fig. 3E, right panels), confirming our 3′UTR data (Fig. 3B) that miR-192/215 regulate ZEB2 translation and, therefore, protein levels.
Although the direct targeting of the ZEB2 3′UTR by miR-192/215 for translational repression is clear, the mechanism behind the observed TGF-β–induced changes in ZEB2 mRNA in the different renal cells is more complex (Fig. 1). This may relate in part to the feedback loop between the miR-200 family and the translation/transcription of ZEB1/2 in some TGF-β–treated cells (29), as well as the ability of miRNAs to alter the stability of their target mRNAs as recently described by Hendrickson et al. (30).
miR-192/215 regulate the transcription of E-cadherin but not ECM proteins.
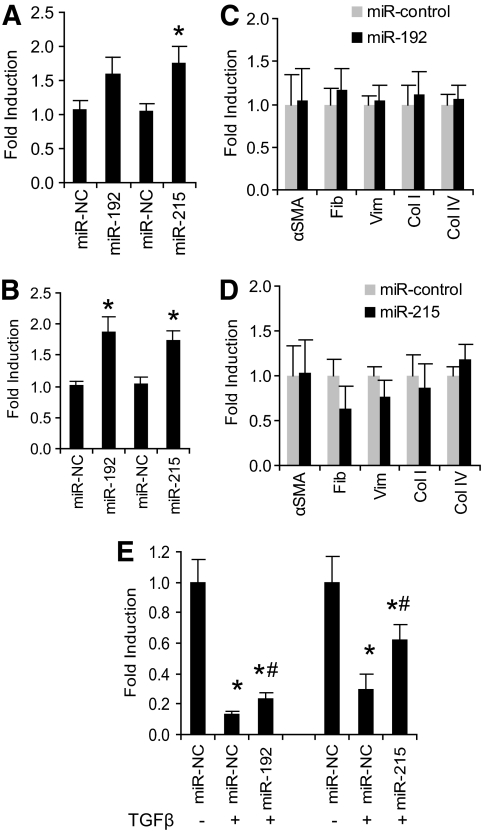
ZEB2 is a well-known regulator of E-cadherin transcription. Based on the above observations, we hypothesized that the miR-192/215 family regulated E-cadherin expression through ZEB2 and possibly ECM accumulation via modulation of the EMT phenotype. Downregulation of mir-192/215 may be a contributing factor to how TGF-β drives EMT and the development of renal fibrosis. To test this hypothesis, NRK52E cells were transfected with miR-192, miR-215, or miR-control in the absence of TGF-β. Expression analysis demonstrated that miR-192 and miR-215 independently elevated E-cadherin mRNA levels compared with miR-control; however, only the miR-215-mediated increase was significant (+76%, P < 0.05; Fig. 4A). We performed a separate experiment in TGF-β–adapted NRK52E cells, which typically have reduced E-cadherin (Fig. 1B) and found that E-cadherin mRNA levels were significantly increased (Fig. 4B) by both miR-192 and miR-215 (+85 and +75%, respectively, P < 0.05) compared with miR-control–transfected cells. Interestingly, the expression of α-SMA, fibronectin, vimentin, and collagen I and IV was not altered with miR-192/215 in the absence (Fig. 4C) or presence of TGF-β (Fig. 4D). These experiments demonstrate that although miR-192/215 alter the expression of E-cadherin via ZEB2, they do not directly alter ECM protein expression in proximal tubular cells.
FIG. 4.
Effect of miR-192/215 on E-cadherin expression in proximal tubular cells. A: Proximal tubular cells were transfected with either miR-control (miR-NC), miR-192, or miR-215 (100 nmol/l), and E-cadherin expression was assessed by real-time quantitative PCR. Both miR-192 and miR-215 resulted in an increase in E-cadherin mRNA, but only the change with miR-215 was significant (*P < 0.05, compared with control). B: To test the effect of miR-192/215 in the context of TGF-β, NRK52E cells that were previously adapted to TGF-β for >10 days were transfected with miR-control, miR-192, or miR-215, and expression of E-cadherin was assessed by real-time quantitative PCR 3 days later. Both miR-192 and miR-215 significantly increased E-cadherin mRNA (*P < 0.01, compared with control). C: Similar transfection experiments using miR-192 did not alter mRNA levels of ECM genes or vimentin, compared with miR-control. D: Transfection with miR-215 did not alter the expression of ECM genes or vimentin compared with miR-control. E: NRK52E cells that had not been previously exposed to TGF-β were transfected with miR-control, miR-192, or miR-215, and 4 h later treated with TGF-β (10 ng/ml). Cells were harvested 3 days later, and expression of E-cadherin was assessed by real-time quantitative PCR. TGF-β treatment dramatically reduced E-cadherin mRNA levels in cells transfected with miR-control (*P < 0.001, compared with no TGF-β treatment). The reduction in E-cadherin mRNA was partially but significantly reversed by both miR-192 and miR-215 (#P < 0.05, compared with miR-control with TGF-β treatment). All error bars represent ± SEM.
We further wished to establish whether ectopic expression of miR-192/215 could prevent the TGF-β–induced downregulation of E-cadherin. NRK52E cells transfected with miR-control had significantly decreased E-cadherin levels after TGF-β treatment (P < 0.001; Fig. 4E), which was partially but significantly reversed by both miR-192 (+1.7-fold) and miR-215 (+2.1-fold) (P < 0.05; Fig. 4E). Total reversal may not be possible as several TGF-β–responsive factors may act to regulate E-cadherin levels apart from miR-192/215. These data confirm that miR-192/215 can partially reverse the downregulation of E-cadherin by TGF-β in proximal tubular cells and potentially play a role in regulating the epithelial cell phenotype.
CTGF results in increased levels of miR-192/215 and increased E-cadherin mRNA.
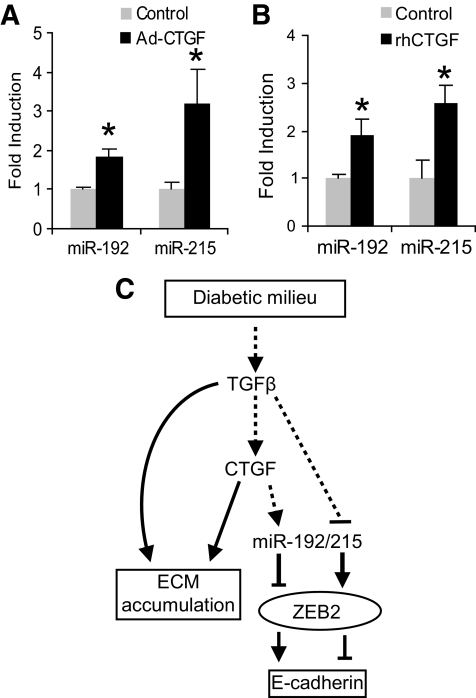
We previously demonstrated that CTGF causes NRK52E cells to undergo partial EMT (4) independently of TGF-β. This phenotype is characterized by the increased expression of ECM proteins while maintaining expression of E-cadherin and has been observed by others (3,31,32). NRK52E cells were infected with CTGF containing adenovirus (Ad-CTGF) or treated with recombinant human CTGF (rhCTGF) and the expression of ECM proteins was assessed at both mRNA and the protein level (4). Interestingly, despite an increase in α-SMA and ECM proteins, these cells maintained a relatively high level of E-cadherin mRNA (4). Because TGF-β resulted in decreased E-cadherin expression (Fig. 1B) and CTGF resulted in increased E-cadherin mRNA levels, we wanted to know the effect of CTGF on miR-192/215. We hypothesized that miR-192/215 would be increased in cells expressing more E-cadherin as these cells would express less ZEB2, the transcriptional repressor of E-cadherin. To test this hypothesis, we obtained RNA from our well-characterized study (4) and observed that miR-192 and miR-215 were both significantly elevated in cells infected with Ad-CTGF or treated with rhCTGF (Fig. 5A and B). These data were consistent with our other observations that miR-192/215 regulate E-cadherin levels via ZEB2. CTGF is thought to be downstream of TGF-β, and both factors are considered profibrotic with overlapping functions. Our data clearly demonstrate that CTGF and TGF-β both result in increased ECM production in proximal tubular cells but also have distinct functions in that they regulate miR-192/215 levels and, consequently, E-cadherin in opposite directions.
FIG. 5.
CTGF increases miR-192/215 expression. A: NRK52E cells were infected with Ad-CTGF or Ad-vector, and miR-192/215 levels were assessed 6 days after infection (4). At this time point, ECM production and E-cadherin RNA levels are increased as previously reported (4). miR-192/215 levels were significantly increased by CTGF (*P < 0.01 and *P < 0.05, respectively, compared with empty vector control). B: NRK52E cells were treated with rhCTGF (250 ng/ml) for 6 days (4), and miR-192/215 levels were assessed and were both found to be significantly increased (*P < 0.05) compared with control. C: Schema showing the mechanism by which the profibrotic factors TGF-β and CTGF regulate E-cadherin expression and ECM accumulation. However, they also regulate the expression of miR-192/215 in opposite directions and, consequently, the expression of ZEB2, the transcriptional regulator of E-cadherin, and E-cadherin. Error bars represent ± SEM.
DISCUSSION
In summary, we have demonstrated that TGF-β regulates the expression of some of the kidney-specific miRNAs in renal cell lines. We clearly demonstrated that miR-192 and miR-215 were both decreased in the kidneys of diabetic animals, and this decrease was associated with an increased expression of ECM proteins. These results confirm that the diabetic milieu appears to alter miRNA expression in the kidney, consistent with the effect of TGF-β in our in vitro experiments. We further demonstrated that TGF-β results in increased levels of ZEB2 mRNA and protein (Fig. 3B, D, and E), the transcriptional repressor of E-cadherin, and consequently resulted in decreased E-cadherin transcription in proximal tubular cells. The mechanism is likely to involve miR-192/215, which target to the 3′UTR of ZEB2 to repress translation of this gene (Fig. 3E). Importantly, we did not observe any direct effect of miR-192/215 on the expression of collagens, α-SMA, vimentin, or fibronectin in proximal tubular cells. This finding is interesting because it suggests that the miR-192/215 family, in contrast to the miR-200 family (7), can regulate the expression of E-cadherin through ZEB2 independently of any changes in ECM protein expression.
Our findings in rat proximal tubular and mesangial cells are in contrast with those previously reported (10) in mouse mesangial cells. However cell culture conditions were different in the experiments of Kato et al. (10), in which serum-deprived conditions were used to establish a baseline from which to measure the effect of TGF-β. They observed a 70% drop with serum deprivation, which was essentially reversed by TGF-β. They also observed very little miR-215 in their mesangial cells. In our experiments, under reduced serum conditions, TGF-β resulted in decreased miR-192/215 levels, and our cells expressed both miR-192 and miR-215. Kato et al. (10) also reported an increase in miR-192 in diabetic kidney, in contrast to our studies. The observed differences are likely to be due to the different models and time points that were used. The role and regulation of miR-192 may, in fact, be far more complex, since Wang et al. (19,20) demonstrated increased glomerular filtration rate correlated with miR-192 levels in IgA nephropathy but not in control patients or patients with glomerulosclerosis in whom slightly decreased levels were observed. They demonstrated a clear correlation between the miR-200 family and markers of fibrosis; however, no correlation was found between markers of EMT or fibrosis with miR-192 levels, consistent with our data. More recently, Krupa et al. (28) demonstrated decreased miR-192 expression in human diabetic kidney samples. Our data are consistent with these recent studies (19,20), but further demonstrate that miR-192/215 do not directly affect the expression of fibrotic markers and ECM proteins.
In this work we have also demonstrated that both TGF-β and CTGF increase ECM expression independently of E-cadherin. TGF-β lowers E-cadherin, whereas CTGF, which is a driver of the partial EMT phenotype, increases E-cadherin expression (4). The mechanism by which these factors control E-cadherin expression is likely to involve miR-192/215, which regulate ZEB2 independently of ECM protein expression as demonstrated in our experiments (Fig. 4). Our experiments also suggest that the entire “classic EMT” program, which is observed in vitro with the associated decrease in E-cadherin expression, might not be critical for the development and progression of renal fibrosis (Fig. 5C). Similar observations have been made with the Id1 gene, which decreased E-cadherin expression without altering the expression of ECM proteins (32).
Our work demonstrates a clear link between miRNA-192/215 and ZEB2 in TGF-β/CTGF-mediated changes in E-cadherin expression. Furthermore, we demonstrate that these changes can occur independently of matrix protein synthesis, suggesting that the classical multistep EMT program may not be necessary for fibrogenesis to occur.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a Centre Grant from the Juvenile Diabetes Research Foundation and by the National Health and Medical Research Council of Australia (NHMRC367620 and NHMRC526663).
No potential conflicts of interest relevant to this article were reported.
Author contributions: B.W., M.H.-E., P.K., W.B., K.J.-D., and A.W. researched data. M.S., G.J.G., S.M.T., and M.E.C. reviewed and edited the manuscript. P.K. researched data and wrote the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kalluri R, Neilson EG: Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JM, Dedhar S, Kalluri R, Thompson EW: The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006;172:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroy P, Mostov KE: Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. Mol Biol Cell 2007;18:1943–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P: Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 2006;17:2484–2494 [DOI] [PubMed] [Google Scholar]
- 5.Burns WC, Kantharidis P, Thomas MC: The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 2007;185:222–231 [DOI] [PubMed] [Google Scholar]
- 6.Nieto MA: The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002;3:155–166 [DOI] [PubMed] [Google Scholar]
- 7.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ: The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601 [DOI] [PubMed] [Google Scholar]
- 8.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F: The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 2001;7:1267–1278 [DOI] [PubMed] [Google Scholar]
- 9.Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, Mikulits W, Beug H, Foisner R: beta-Catenin and TGFbeta signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene 2004;23:2672–2680 [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 2007;104:3432–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peinado H, Olmeda D, Cano A: Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415–428 [DOI] [PubMed] [Google Scholar]
- 12.Christoffersen NR, Silahtaroglu A, Orom UA, Kauppinen S, Lund AH: miR-200b mediates post-transcriptional repression of ZFHX1B. RNA 2007;13:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurteau GJ, Carlson JA, Spivack SD, Brock GJ: Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res 2007;67:7972–7976 [DOI] [PubMed] [Google Scholar]
- 14.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T: A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 2008;9:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korpal M, Lee ES, Hu G, Kang Y: The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008;283:14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ: Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acid Res 2004;32:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Ørntoft TF, Andersen CL, Dobbelstein M: p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res 2008;68:10094–10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georges SA, Biery MC, Kim SY, Schelter JM, Guo J, Chang AN, Jackson AL, Carleton MO, Linsley PS, Cleary MA, Chau BN: Coordinated regulation of cell cycle transcripts by p53-Inducible microRNAs, miR-192 and miR-215. Cancer Res 2008;68:10105–10112 [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC: Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens 2010;23:78–84 [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC: Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest 2010;90:98–103 [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB: Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 1998;275:F962–971 [DOI] [PubMed] [Google Scholar]
- 22.Schulze-Lohoff E, Brand K, Fees H, Netzker R, Sterzel RB: Role of ornithine decarboxylase for proliferation of mesangial cells in culture. Kidney Int 1991;40:684–690 [DOI] [PubMed] [Google Scholar]
- 23.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002;13:630–638 [DOI] [PubMed] [Google Scholar]
- 24.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME: Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 2001;108:1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassila M, Jandeleit-Dahm K, Seah KK, Smith CM, Calkin AC, Allen TJ, Cooper ME: Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol 2005;16:363–373 [DOI] [PubMed] [Google Scholar]
- 26.Lassila M, Seah KK, Allen TJ, Thallas V, Thomas MC, Candido R, Burns WC, Forbes JM, Calkin AC, Cooper ME, Jandeleit-Dahm KA: Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: role of advanced glycation end products. J Am Soc Nephrol 2004;15:2125–2138 [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y: Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 2008;172:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D: Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 2010;21:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ: A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008;68:7846–7854 [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson DG, Hogan DJ, McCullough HL, Myers JW, Herschlag D, Ferrell JE, Brown PO: Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol 2009;7:e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR: Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 2008;135:642–659 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Yang J, Luo JH, Dedhar S, Liu Y: Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol 2007;18:449–460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.