Abstract
OBJECTIVE
Connective tissue growth factor (CTGF) is a major fibrogenic factor. Increased retinal CTGF levels have been implicated to play a role in diabetic retinopathy. SERPINA3K is a serine proteinase inhibitor, and its levels were decreased in retinas with diabetic retinopathy. The purpose of this study was to investigate the role of SERPINA3K in the regulation of CTGF and fibrogenesis and its mechanism of action.
RESEARCH DESIGN AND METHODS
Adenovirus expressing SERPINA3K was injected intravitreally into streptozotocin-induced diabetic rats. CTGF expression was measured using Western blot analysis and real-time RT-PCR. Fibrosis was evaluated by quantifying retinal fibronectin using enzyme-linked immunosorbent assay. Wnt pathway activation was determined by phosphorylation of LDL receptor–related protein 6, a coreceptor of Wnt ligands, and stabilization of β-catenin, an essential effector of the canonical Wnt pathway.
RESULTS
Ad-SERPINA3K attenuated the CTGF and fibronectin overexpression in retinas of diabetic rats. In cultured retinal cells, SERPINA3K blocked the overproduction of CTGF induced by high glucose. Dickkopf-1, a specific Wnt antagonist, also attenuated the high-glucose–induced CTGF overexpression, indicating a role of Wnt signaling in CTGF overexpression in diabetes. Similarly, increased SERPINA3K blocked Wnt pathway activation in diabetic retinas and in cells treated with high glucose. Further, SERPINA3K also attenuated the Wnt3a-induced activation of the canonical Wnt pathway and the overexpression of CTGF.
CONCLUSION
SERPINA3K is an antifibrogenic factor, and its antifibrogenic activity is through blocking the Wnt pathway. Decreased SERPINA3K levels may contribute to the fibrosis in diabetic retinopathy.
SERPINA3K, a serine proteinase inhibitor (serpin), is expressed in the liver, kidney, pancreas, and retina (1–3). SERPINA3K specifically binds to tissue kallikrein to form a covalent complex and inhibits proteolytic activities of tissue kallikrein (3) and is believed to participate in the regulation of vasodilation and local blood flow via interactions with the kallikrein-kinin system (4). Later studies suggest that SERPINA3K has other functions independent of inhibition of tissue kallikrein. For example, SERPINA3K has been found to inhibit retinal neovascularization in ischemia-induced retinopathy, which is not dependent on its interactions with the kallikrein-kinin system (5). Further, in a diabetic rat model, SERPINA3K levels have been shown to decrease in retinas, suggesting that decreased SERPINA3K levels may contribute to diabetic retinopathy (6).
Diabetic retinopathy is one of the leading causes of blindness (7). In advanced stages of diabetic retinopathy, retinal fibrosis occurs and fibrovascular contraction can cause hemorrhages and retinal detachment (7,8). Connective tissue growth factor (CTGF) is a profibrogenic factor that stimulates fibroblast proliferation, cell adhesion, and extracellular matrix production (9,10). The potential role of CTGF in pathological fibrosis has been established (11), and CTGF has been suggested to be an attractive therapeutic target in some fibrotic diseases (12). The protein and mRNA levels of CTGF were found to be elevated in retinas with diabetic retinopathy (13), and the roles of CTGF in fibrovascular proliferation and thickening of capillary basement membrane were also demonstrated in proliferative diabetic retinopathy (13–16). All of these previous findings suggest a therapeutic potential for anti-CTGF therapy in diabetic retinopathy.
Wnts are a group of secreted, cysteine-rich glycoproteins (17). As shown in online appendix Figure S1 (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1056/DC1), in the absence of Wnt ligands, transcription factor β-catenin, a downstream effector of the canonical Wnt pathway, is phosphorylated by a protein complex containing glycogen synthase kinase (GSK)-3 in the cytosol and constantly degraded to prevent its accumulation (18,19). Upon binding of certain Wnt ligands, the Frizzled (Fz) receptor dimerizes with the coreceptor, LDL receptor–related protein (LRP) 5 or 6, forming a receptor/coreceptor complex (17). As a result, the downstream signaling is stimulated, including phosphorylation of LRP5/6 and stabilization of β-catenin (20,21). β-Catenin is subsequently translocated into the nucleus, associates with T-cell factor (TCF) for DNA binding, and regulates expression of target genes including CTGF (17).
The Wnt signaling pathway is involved in multiple physiological and pathological processes. It has been well studied in embryogenesis and carcinogenesis (22). Recent evidence suggests that the Wnt pathway is also important in ocular diseases; for example, mutations in the Fz receptor and LRP coreceptor have been shown to associate with the vascular developmental defects (23). Furthermore, it has been revealed that Wnt signaling is responsible for pathological fibrosis in the lung, suggesting that inhibition of Wnt signaling, such as Wnt antagonists, may represent a therapeutic option (24–27). As a profibrogenic factor, CTGF was also found to be regulated by Wnt signaling in osteoblast differentiation (28,29). However, there is little previous evidence to implicate Wnt signaling in fibrosis in the retina with diabetic retinopathy.
In the present study, we have investigated the inhibitory effect of SERPINA3K on the hyperglycemia-induced CTGF overexpression and Wnt pathway activation and further determined if the beneficial effects of SERPINA3K in diabetic retinopathy are through the Wnt antagonistic activity.
RESEARCH DESIGN AND METHODS
Cell culture.
A cell line derived from rat retinal Müller cells (rMCs) (rMC-1; a kind gift from Dr. Vijay Sarthy at Northwestern University), were cultured in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) containing 10% FBS (Invitrogen, Carlsbad, CA) (30). Human telomerase reverse transcriptase (HTERT)-immortalized retinal pigment epithelial (RPE) cell line (HTERT-RPE), a cell line derived from human RPE cells, was purchased from the American Type Culture Collection (ATCC) (Manassas, VA) and cultured in DMEM containing 10% FBS following ATCC recommendations. L-cells and L-cells stably expressing Wnt3A (l-Wnt3a) were purchased from the ATCC and cultured in DMEM containing 10% FBS and 0.4 mg/ml G-418 (Invitrogen). The cells and conditioned media (1 g/l glucose, 1% FBS) were harvested following the procedure recommended by the ATCC. The cultured cells were starved in 1 g/l glucose (5 mmol/l) DMEM containing 1% FBS overnight before treatment. For the high-glucose treatment, the cells were exposed to 30 mmol/l d-glucose (Sigma, St. Louis, MO), and the low-glucose control included 5 mmol/l d-glucose and 25 mmol/l l-glucose (Sigma) in the culture medium.
Experimental animals.
Brown Norway (BN) rats were purchased from Charles River Laboratories (Wilmington, MA). Care, use, and treatment of all animals in this study were in strict agreement with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines in the care and use of laboratory animals set forth by the University of Oklahoma.
Induction of experimental diabetes.
Experimental diabetes was induced as described previously (31). Briefly, BN rats (8 week of age) were given a single intraperitoneal injection of streptozotocin (STZ; 50 mg/kg in 10 mmol/l citrate buffer, pH 4.5) after an overnight fast. Serum glucose levels were monitored 48 h after the STZ injection and every 2 weeks thereafter, and only the animals with blood glucose levels >350 mg/dl were used as STZ-induced diabetic rats.
Recombinant proteins, adenovirus, plasmids, transfection, and reporter Assay.
The SERPINA3K cDNA was cloned into the pET28 vector (Novagen, Madison, WI), and the construct was transformed into E. coli strain BL-21/DE3 (Novagen). The expression and purification followed the protocol described previously (5). Endotoxin levels were measured using a limulus amebocyte kit (Biowhittaker, Walkersville, MD). BSA (Sigma) was used as protein control for SERPINA3K. Recombinant Dickkopf (DKK)-1 protein was purchased from R&D Systems (Minneapolis, MN). To clone the SERPINA3K cDNA, total RNA was extracted from the liver and was reverse transcribed to cDNA. The full-length sequence of SERPINA3K containing the signal peptide was cloned into the shuttle vector. The adenoviral vector used in the study is human adenovirus serotype 5 (Ad5). Adenoviruses expressing SERPINA3K and LacZ were generated using AdEasy systems from Qbiogene (Irvine, CA) following manufacturer's protocol. These adenoviruses were purified using Adeno-X Virus Purification Kits from BD Biosciences (San Jose, CA). TOPFLASH vector was constructed as described (32). Fugene 6 (Roche Applied Science, Indianapolis, IN) was used for transfection following manufacturer's protocol. Luciferase reporter assays were performed in 12-well plates. The TOPFLASH construct and renilla luciferase pRL-TK vector were cotransfected into the cells. TOPFLASH activity was measured using the dual-luciferase reporter system (Promega, Madison, WI) and normalized by renilla luciferase activity.
Real-time RT-PCR.
Total RNA was isolated using an RNeasy Mini Kit (Qiagen Sciences, Germantown, MD). mRNA was reverse transcribed to cDNA using a TaqMan kit from Roche. This cDNA was then used for specific real-time PCR. The specific primers for CTGF (5′-AAGACCTGTGGGATGGGC-3′ and 5′-TGGTGCAGCCAGAAAGCTC-3′) were synthesized from Integrated DNA Technologies (San Diego, CA). To normalize the variation of the amount of mRNA in each reaction, 18S rRNA (primers: 5′-TTTGTTGGTTTTCGGAACTGA-3′ and 5′-CGTTTATGGTCGGAACTACGA-3′) was simultaneously processed in the same sample as an internal control. iQ SYBR Green Supermix from BioRad (Hercules, CA) was used for real-time PCR following the manufacturer's procedure.
Standard curves for CTGF primers and 18S primers were constructed using serial 1–10 dilutions of the cDNA product from RT reaction (online appendix Fig. S6). The efficiency of CTGF primers was 99.46% and the efficiency of 18S primers was 97.43%. Dilutions of 1:1,000, 1:100, and 1:10 were used in the assay, and all of the samples were diluted by 1:100 for the real-time PCR. To calculate relative changes in gene expression, we used the δ-δ method following the BioRad's introduction.
Western blot analysis.
The same amounts of proteins from the cytosolic fraction, total cell lysates, and retinal homogenates were resolved by SDS-PAGE (8 or 10%) and analyzed by Western blotting using specific antibodies. For cytosolic β-catenin measurement, cells were lysed by three freeze/thaw cycles in PBS with a protease inhibitor cocktail (Roche Applied Science) followed by centrifugation, and the supernatants were isolated for Western blot analysis. For total cell lysates, harvested cells were sonicated in radioimmunoprecipitation assay buffer (Cell Signaling Technology, Danvers, MA) containing 1% SDS. For retinal homogenate preparation, retinas were homogenized in PBS with a protease inhibitor cocktail using a soft-tissue pestle (Fisher Scientific, Pittsburgh, PA). Antibodies for CTGF, LRP6, and β-catenin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at 1:400, 1:500, and 1:2,500 dilutions, respectively. Antibody for β-actin (1:3,000) was purchased from Invitrogen. Antibodies for phosphorylated LRP6 (S1490), phosphorylated β-catenin (S33/37/T41), phosphorylated GSK3β (S9), GSK3β, and histone H3 were purchased from Cell Signaling Technology and used at 1:500 dilution for the first two antibodies and 1:2,500 dilution for the last three antibodies. The monoclonal antibody for SERPINA3K (1:1,000) was generated using the recombinant SERPINA3K through contracted service with Proteintech Group (Chicago, IL).
Because of the posttranslational modifications, CTGF can display multiple bands with different molecular weights, which has been reported in the literature (33). Typically, a 36- or 38-kD band, or double bands at these molecular weights, can be detected, dependent on the tissue, cell type, and treatment. In the present study, CTGF showed double bands (36/38 kD) in the Western blotting data using the HTERT–RPE-1 cell lysate.
Enzyme-linked immunosorbent assay for fibronectin.
Fibronectin concentrations in the retina homogenates were measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from Assaypro (Winfield, MO), according to manufacturer's instruction. The working range of the fibronectin ELISA kit used here is 4–1,000 ng/ml. All of the samples were measured in the linear part of the working range. The assay coefficients of variation are <2%. The total protein concentration was measured by Bradford protein assay.
Statistical analysis.
Student t test (two tailed) was used for comparisons between two groups. ANOVA was used for comparisons between groups in Table 1. Statistical significance was accepted when the P value was <0.05.
TABLE 1.
Physiological parameters of diabetic rats
| No Ad-IVT |
Diabetes with Ad-IVT |
|||||
|---|---|---|---|---|---|---|
| Control | Diabetes | P value | LacZ | SA3K | P value | |
| Blood glucose (mg/dl) | ||||||
| Time after STZ injection | ||||||
| −1 day | 97 ± 8 | 99 ± 6 | 0.955 | 107 ± 11 | 103 ± 12 | 0.789 |
| 3 days | — | 544 ± 55 | — | 515 ± 79 | 528 ± 59 | 0.916 |
| 1 month | 94 ± 8 | 524 ± 59 | <0.001 | 531 ± 66 | 530 ± 72 | 1.000 |
| 2 months | 98 ± 4 | 521 ± 67 | <0.001 | 533 ± 88 | 517 ± 77 | 0.965 |
| 3 months | 108 ± 22 | 543 ± 37 | <0.001 | 534 ± 61 | 530 ± 50 | 0.992 |
| Body weight (g) | ||||||
| Time after STZ injection | ||||||
| −1 day | 151 ± 13 | 145 ± 9 | 0.790 | 148 ± 15 | 150 ± 9 | 0.993 |
| 3 days | — | 143 ± 12 | — | 146 ± 15 | 145 ± 9 | 0.979 |
| 1 month | 176 ± 15 | 146 ± 13 | 0.002 | 152 ± 17 | 149 ± 13 | 0.983 |
| 2 months | 183 ± 11 | 147 ± 17 | <0.001 | 150 ± 19 | 151 ± 15 | 1.000 |
| 3 months | 187 ± 16 | 149 ± 16 | 0.001 | 149 ± 20 | 152 ± 17 | 0.976 |
Data are means ± SD. n = 8–10.
RESULTS
Clinical characteristics of diabetic animals.
Two months after the induction of diabetes, the ATZ-induced diabetic rats received an intravitreal injection of adenovirus expressing SERPINA3K (Ad-SA3K, 5 × 107 pfu/eye), and the same titer of adenovirus expressing LacZ (Ad-LacZ) was injected for control. The rats were separated into four groups: normal rats without STZ-induced diabetes (control group); rats with STZ-induced diabetes (DM group); STZ-induced diabetic rats with injection of Ad-SA3K (DM-SA3K group), and STZ-induced diabetic rats with injection of Ad-LacZ (DM-LacZ group). There were 8–10 rats in each group. Before the STZ injection, all of these rats had similar blood glucose levels (∼100 mg/dl) and similar body weights (∼150 g). Three months after STZ injection, average body weight of the DM group (149 ± 16 g) was significantly lower than in nondiabetic control group (187 ± 16 g) (P < 0.05). At each time point, there was no significant blood glucose or body weight difference between the DM group, the DM-LacZ group, and the DM-SA3K group (Table 1), suggesting that the ocular adenovirus injection had no systemic effect in the diabetic animals.
A novel antifibrogenic activity of SERPINA3K in the retina with diabetic retinopathy.
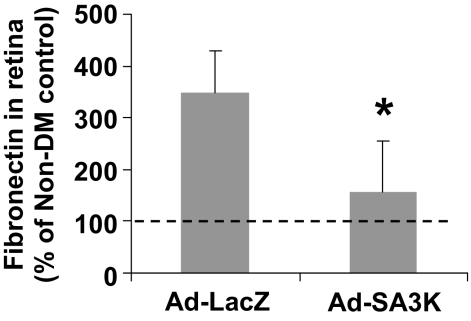
To investigate the effect of SERPINA3K on fibrosis in the retina with diabetic retinopathy, we measured the retinal levels of fibronectin in the STZ-induced diabetic rats. Fibronectin is an extracellular matrix protein, and overproduction of fibronectin has been shown to contribute to capillary basement membrane thickening in diabetic retinopathy (34). Consistent with previous studies, our ELISA results showed that fibronectin levels were significantly higher in the retinas of the diabetic rats compared with that in nondiabetic control subjects (Fig. 1). Ad-SA3K blocked retinal fibronectin overexpression in diabetic rats (Fig. 1), suggesting an antifibrogenic activity of SERPINA3K.
FIG. 1.
SERPINA3K attenuated overexpression of fibronectin in the retinas with diabetic retinopathy. Ad-SA3K and Ad-LacZ were injected separately into the vitreous of diabetic rats, and the retinas were dissected 4 weeks following the injection. Retinal fibronectin levels were measured by ELISA and expressed as percentage of the retinal fibronectin level in the nondiabetic rats (means ± SD, n = 6). *P < 0.05.
Retinal endothelial cells and pericytes are two major vascular cell types that are profoundly affected in diabetic retinopathy, and their dysfunctions contribute to the blood-retinal barrier breakdown in diabetic retinopathy (35–37). Since these cells are also the sources of fibronectin production responsible for thickening of the basement membrane, we measured the concentration of fibronectin in primary retinal pericytes and endothelial cells by ELISA. The high-glucose–induced overproduction of fibronectin was attenuated by SERPINA3K in both of the cell types (Fig. S2).
Inhibitory effects of SERPINA3K on CTGF overexpression.
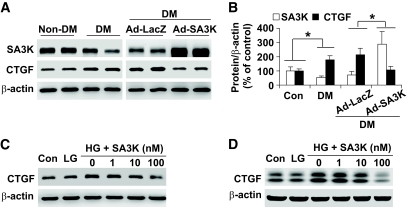
The inhibitory effect of SERPINA3K on CTGF was evaluated in the STZ-induced diabetic rats. Four weeks after the injection of Ad-SA3K or Ad-LacZ, the retinas were dissected following a thorough perfusion to remove the blood in the retinal vasculature. As measured by Western blot analysis, retinal levels of SERPINA3K were decreased in the untreated diabetic rats, compared with that in nondiabetic rats (Fig. 2A and B). The Ad-SA3K injection resulted in an increase of SERPINA3K levels in the retinas of the diabetic rats (Fig. 2A and B). Retinal levels of CTGF were significantly increased in the retinas of untreated diabetic rats, compared with nondiabetic rats at the same age. The injection of Ad-SA3K mitigated the overexpression of CTGF in the retinas compared with the control Ad-LacZ virus (Fig. 2A and B).
FIG. 2.
SERPINA3K attenuated overexpression of CTGF in the retinas with diabetic retinopathy and in retinal cells treated with high glucose. A: STZ-induced diabetic rats (DM) received an intravitreal injection of Ad-SA3K, with Ad-LacZ as control. Four weeks after the injection, retinal levels of SERPINA3K and CTGF were measured by Western blot analysis using 100 μg of retinal proteins from each rat. B: Retinal levels of SERPINA3K and CTGF were quantified by densitometry from three independent experiments and normalized by β-actin levels (*P < 0.05, means ± SD, n = 6). C and D: rMC-1 cells (C) and HTERT RPE-1 cells (D) were exposed to low-glucose (LG, 5 mmol/l d-glucose + 25 mmol/l l-glucose) and high-glucose (HG, 30 mmol/l d-glucose) media with various concentrations of SERPINA3K for 24 h. Cellular CTGF levels were measured by Western blot analysis and normalized by β-actin levels.
It has been shown that RPE cells and rMCs are two major cell types expressing CTGF in the proliferative vitreoretinopathy (38). The diabetes-induced blood-retinal barrier breakdown occurs predominantly at the level of the retinal blood vessels (39). However, the failure of the RPE barrier also occurs at an lower level, suggesting that the pathological change of the RPE is involved in diabetes (39). To evaluate the direct effect of SERPINA3K on hyperglycemia-mediated CTGF overexpression in vitro, HTERT–RPE-1 cells (human RPE cell line) and rMC-1 (rat rMC line) were exposed to media containing 30 mmol/l d-glucose (high glucose). CTGF expression was significantly elevated by high glucose exposure, when compared with low glucose control (5 mmol/l d-glucose and 25 mmol/l l-glucose, low glucose). SERPINA3K blocked the high-glucose–induced CTGF overexpression in a dose-dependent manner in both of the cell lines (Fig. 2C and D).
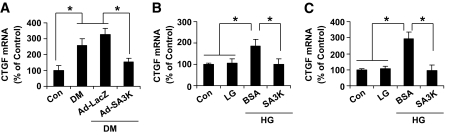
To further study the mechanism for the regulation of CTGF by SERPINA3K, real-time RT-PCR was performed to measure mRNA levels of CTGF in the retinas and cultured retinal cells. The mRNA levels of CTGF were increased in retinas with diabetic retinopathy and decreased by Ad-SA3K (Fig. 3A). Similarly, exposure to high-glucose media for 16 h significantly elevated CTGF mRNA levels in both HTERT–RPE-1 cells and rMC-1 cells. In a time course experiment, the cells were exposed to high-glucose medium for 0–24 h. The high-glucose treatment continuously increased the CTGF mRNA level after 4 h of exposure (online appendix Fig. S3). The high-glucose–induced CTGF mRNA overexpression was attenuated by 100 nmol/l SERPINA3K (Fig. 3B and C). Taken together, these results indicate that SERPINA3K blocks the hyperglycemia-induced CTGF expression at the transcription level.
FIG. 3.
SERPINA3K decreased CTGF mRNA levels in the diabetic retinas and in high-glucose–treated cells. A: Ad-SA3K and Ad-LacZ were injected intravitreally into the eyes of diabetic rats, and the retinas were dissected 4 weeks after the injection. B and C: rMC-1 cells (B) and HTERT RPE-1 cells (C) were exposed to low-glucose and high-glucose media for 16 h, with and without 100 nmol/l SERPINA3K or BSA. Real-time RT-PCR was performed to quantify relative mRNA levels of CTGF in the retinas (A), rMC-1 cells (B), and RPE cells (C). Values are means ± SD, n = 3. *P < 0.05.
The role of the high-glucose–induced Wnt/β-catenin signaling in CTGF overexpression.
To explore the signaling pathway mediating the inhibitory effect of SERPINA3K on CTGF transcription, we first investigated cell signaling responsible for CTGF overexpression induced by high glucose. As the β-catenin–TCF/LEF-binding (TBE) site was identified in the promoter region of the CTGF gene, and CTGF has been shown to be a target gene of β-catenin-TCF/LEF transcription factor (33), a downstream effector of the canonical Wnt pathway, we investigated the role of the Wnt pathway in the CTGF overexpression in diabetes. A number of Wnt ligands and Fz receptors and both LRP5 and -6 were found to express in the cultured HTERT–RPE-1 cells by RT-PCR, suggesting that this cell line is a suitable model for Wnt signaling studies (online appendix Fig. S4). As phosphorylation of LRP6 is a critical step in the canonical Wnt pathway activation (40), we measured phosphorylated LRP6 (p-LRP6) levels. As shown by Western blot analysis using an antibody specific for p-LRP6, phosphorylation of the endogenous LRP6 was increased in the RPE cells exposed to 30 mmol/l glucose, compared with that in control cells exposed to the low-glucose medium (Fig. 4A). In the same cell line, cytosolic β-catenin levels were also elevated by the high-glucose medium in an exposure time-dependent manner (Fig. 4A). Further, under high-glucose conditions, phosphorylated β-catenin levels were decreased, compared with that in low-glucose control (online appendix Fig. S5A), while nuclear β-catenin levels were elevated (online appendix Fig. S5B). Consistently, GSK3β phosphorylation (inactive form) was increased (online appendix Fig. S5A). In vivo, cytosolic β-catenin levels in the retina homogenates were also significantly elevated in the diabetic rats, indicating an activation of the canonical Wnt pathway in retinas with diabetic retinopathy (Fig. 4C and D). Further, when the Wnt pathway was blocked by DKK-1, a specific inhibitor of the canonical Wnt pathway, the high-glucose–induced CTGF overexpression was attenuated (Fig. 4B), suggesting that the Wnt pathway activation induced by high glucose and diabetes is responsible for the CTGF overexpression.
FIG. 4.
Wnt/β-catenin signaling was responsible for the high-glucose–induced CTGF overexpression. A: HTERT RPE-1 cells were exposed to high glucose for different durations as indicated. p-LRP6 levels were measured by Western blot analysis using 100 μg of total proteins with an antibody specific for p-LRPE6, and cytosolic β-catenin levels were determined by Western blot analysis using 20 μg cytosolic proteins. B: HTERT RPE-1 cells were exposed to low glucose and high glucose media with 100 nmol/l DKK1 or BSA. After 24-h treatment of the cells, CTGF levels were measured by Western blot analysis. C and D: The retinas were dissected from nondiabetic rats and diabetic rats. β-Catenin levels in the retinal homogenates were measured by Western blot analysis (C) and quantified by densitometry (D) (*P < 0.05, means ± SD, n = 6).
Inhibitory effects of SERPINA3K on Wnt/β-catenin signaling in diabetic retinopathy and high-glucose–treated retinal cells.
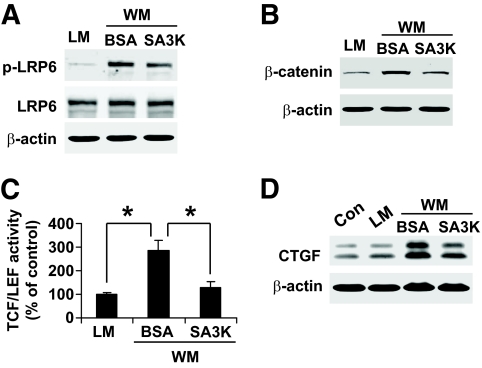
To further investigate whether SERPINA3K inhibits the Wnt pathway in high-glucose–treated cells and in diabetic retinas, SERPINA3K was delivered into the RPE cell culture medium containing high glucose and into the vitreous humor of diabetic rats. After a 6-h exposure, SERPINA3K decreased the high-glucose–induced phosphorylation of LRP6 and accumulation of β-catenin in a concentration-dependent manner (Fig. 5A). SERPINA3K at 100 nmol/l decreased p-LRP6 and cytosolic β-catenin to levels similar to that in the low-glucose control (Fig. 5A). Similarly, 100 nmol/l SERPINA3K completely reversed the high-glucose–induced changes of phosphorylated β-catenin levels, nuclear β-catenin levels, and phosphorylated GSK3β levels (online appendix Fig. S5A and B). In STZ-induced diabetic rats, the injection of Ad-SA3K also blocked the accumulation of cytosolic β-catenin in the retina, compared with the control virus Ad-LacZ, suggesting an inhibitory effect of SERPINA3K on the canonical Wnt pathway in diabetes (Fig. 5B and C).
FIG. 5.
SERPINA3K inhibited the high-glucose–induced Wnt/β-catenin signaling. A: HTERT RPE-1 cells were exposed to low glucose and high glucose media for 6 h with various concentrations of SERPINA3K. Levels of p-LRP6 and cytosolic β-catenin were determined by Western blot analysis. B and C: The retinas were dissected from the diabetic rats 4 weeks after the intravitreal injection of Ad-LacZ or Ad-SA3K. β-Catenin levels in the retinal homogenates were measured by Western blot analysis (B) and quantified by densitometry (C) (mean ± SD, n = 6, *P < 0.05).
The Wnt inhibitory effect of SERPINA3K was responsible for CTGF regulation.
To determine whether SERPINA3K also blocks CTGF expression induced by Wnt signaling, the RPE cells were exposed to a 50% Wnt3a conditioned medium, with the 50% L-cell medium as control. The Wnt3a-conditioned medium elevated p-LRP6 and cytosolic β-catenin levels in the RPE cells (Fig. 6A and B). SERPINA3K at 100 nmol/l blocked the increase of p-LRP6 and β-catenin levels induced by Wnt3a (Fig. 6A and B). TOPFLASH is a luciferase reporter construct under the control of a promoter containing the TCF/LEF-binding sites. To measure β-catenin–dependent reporter gene transcription, TOPFLASH activity assay was performed to further confirm the inhibitory effect of SERPINA3K on Wnt signaling. The RPE cells were transfected with the TOPFLASH construct and then incubated with the 50% Wnt3a-conditioned medium. TOPFLASH assay showed that Wnt3a induced an approximately threefold increase in TOPFLASH reporter (TCF/LEF) activity, which was attenuated by SERPINA3K (Fig. 6C). These results indicated that SERPINA3K is a Wnt inhibitor.
FIG. 6.
SERPINA3K blocked the Wnt ligand-induced CTGF overexpression. A and B: HTERT RPE-1 cells were exposed to 50% control L-cell medium (LM) or 50% Wnt3a conditioned medium (WM) for 1 h (A) or 2 h (B), with 100 nmol/l SERPINA3K or BSA. The same amounts of total cellular proteins (100 μg) (A) or cytosolic proteins (20 μg) (B) were blotted separately with antibodies specific for p-LRP6 and total LRP6 (A), or for β-catenin (B). C: The cells were transfected with the TOPFLASH vector, followed by exposure to the LM or WM containing 1,000 nmol/l BSA or SERPINA3K for 24 h. The TOPFLASH activity was measured using luciferase assay (means ± SD, n = 3, *P < 0.05). D: HTERT RPE-1 cells were exposed to LM and WM media with 100 nmol/l SERPINA3K or BSA. After culture for 24 h, CTGF levels were measured by Western blot analysis.
To determine the effect of SERPINA3K on the Wnt-induced CTGF expression, the 50% Wnt3a-conditioned medium was used to treat the cultured RPE cells. Wnt3a, as well as high glucose, induced CTGF overexpression in the RPE cells (Fig. 6D). Correlating with its inhibitory effect on Wnt signaling, SERPINA3K also inhibited the Wnt3a-induced CTGF upregulation, similar to that in the high-glucose model (Fig. 6D).
DISCUSSION
SERPINA3K is an extracellular serpin and has been found to function as an antiangiogenic factor (5) and an anti-inflammatory factor (41). Our previous studies showed that SERPINA3K levels are decreased in the retina of diabetic rats (6). The present study revealed a novel antifibrogenic activity of this serpin, as it inhibits CTGF overexpression and reduces the production of extracellular matrix in retina with diabetic retinopathy. These findings suggest that decreased SERPINA3K levels in the diabetic retina may contribute to pathological fibrosis in diabetic retinopathy. Further, our results demonstrate that the inhibitory effect of SERPINA3K on CTGF overexpression is through mitigating the Wnt signaling activation induced by diabetes.
Fibrosis is an important pathological feature of diabetic retinopathy (7,8). At early stages of diabetic retinopathy, increased production of extracellular matrix has been shown to contribute to the capillary basement membrane thickening (42). At advanced stages of diabetic retinopathy, fibrosis can result in contraction and retinal detachment, a major cause of blindness in diabetic retinopathy (7,8). The molecular mechanism for retinal fibrosis in diabetic retinopathy is not clear. CTGF is a major fibrogenic factor, and its overexpression has been found to play a key role in the basement membrane thickening in diabetic retinopathy models (14,16). CTGF upregulates production of extracellular matrix proteins such as fibronectin (43,44). Therefore, CTGF is considered a promising target for treating retinal fibrosis in diabetic retinopathy. The present study identified SERPINA3K as an endogenous inhibitor of CTGF and, thus, an antifibrogenic factor in the retina. On the other hand, CTGF has been reported to bind to Wnt receptor/coreceptor (45). However, the function of CTGF in canonical Wnt signaling is still controversial (33,45). Despite the possible feedback regulation by CTGF, blockade of Wnt signaling by SERPINA3K should result in a decrease of CTGF levels and inhibition of fibrogenesis, which supports our conclusion (i.e., the antifibrogenic effect of SERPINA3K is mediated, at least in part, through the Wnt pathway).
Our previous studies have shown that retinal levels of SERPINA3K are decreased in STZ-induced diabetic rats after 1, 2, and 4 months of diabetes (6). In the same animal model, CTGF overexpression was found in the diabetic rat retinas 3 months after STZ injection (13), correlating with the decrease of SERPINA3K. The disturbed balance between profibrogenic factor CTGF and anti-fibrogenic factor SERPINA3K may represent a new pathogenic mechanism for the basement membrane thickening in diabetic retinopathy.
Wnt signaling is involved in ocular diseases, such as vasculature disorders in the retina (23). Our recent study showed that activation of the Wnt pathway also plays a pathogenic role in subretinal neovascularization in an animal model of wet age-related macular degeneration (AMD) (46). The role of Wnt signaling in pathological fibrosis has been revealed in some tissues (e.g., the lung) (24). However, in most ocular diseases such as diabetic retinopathy, the role of Wnt signaling, especially the association between Wnt signaling and retinal fibrosis remains obscure. Here, our results showed that cytosolic β-catenin, an essential effector of the canonical Wnt pathway, is accumulated in both diabetic retinas and in the high-glucose–treated retinal cells. Phosphorylation of LRP6 is an early, yet essential, step in activation of the canonical Wnt pathway, as the phosphorylation sites in the LRP6 intracellular domain are known to create inducible docking sites for Axin, leading to stabilization of β-catenin in the cytosol and transduction of the extracellular Wnt signal into intracellular compartments (40,47). Our results showed that phosphorylated LRP6 levels were increased under high-glucose conditions. In the hyperglycemia models, the induction of Wnt signaling activity was accompanied by CTGF overexpression. Same as the high-glucose exposure, Wnt3a ligand also induced Wnt signaling activation and upregulated CTGF expression, while DKK1, a specific inhibitor of the Wnt pathway, blocked the Wnt pathway activation. Therefore, our results revealed that Wnt signaling is activated in diabetic retinopathy, which may be responsible for CTGF overexpression and retinal fibrosis.
Since DKK-1 is a commonly accepted, specific inhibitor of the canonical Wnt pathway, DKK-1 was used to specifically attenuate the Wnt signaling activation and further to reveal the role of Wnt signaling in high-glucose–induced CTGF overexpression. DKK-1 has been reported to induce the internalization of LRP6 (48). To investigate the mechanism for the SERPINA3K effect on the Wnt pathway, we have measured several components of the Wnt pathway at different levels of the cascade. Further, we measured the cell surface level of LRP6 to determine the internalization of LRP6 using extracellular biotin labeling. Similar to DKK-1, preincubation of the cells with SERPINA3K decreased the cell surface level of LRP6 (online appendix Fig. S5C). This result suggests that induction of LRP6 internalization may represent a mechanism responsible, at least partially, for the inhibition of LRP6 phosphorylation by SERPINA3K.
There are 19 Wnt ligands, many of which function as agonists of the Fz/LRP5/6 receptor complex and, thus, activate Wnt/β-catenin signaling (49). On the other hand, some natural inhibitors of the Wnt pathway such as DKK family members and IGFbinding protein-4 have been identified (50–52). Here, we showed that SERPINA3K blocks the Wnt pathway activation induced by high glucose and by Wnt ligands, suggesting that SERPINA3K is a novel endogenous inhibitor of the Wnt pathway. SERPINA3K is known to specifically bind to tissue kallikrein, forming a covalent complex and inhibiting proteolytic activities of tissue kallikrein (3). Through interactions with the kallikrein-kinin system, SERPINA3K participates in the regulation of blood pressure and local blood flow (4). However, the kallikrein-binding activity cannot explain the broad functions of SERPINA3K, such as antiangiogenic and anti-inflammatory activities (5,41). In addition, some of these functions have proven to be independent of interactions with tissue kallikrein (5). The present study demonstrates that SERPINA3K has a potent Wnt antagonist activity. Since the Wnt pathway regulates multiple pathological processes, including angiogenesis, inflammation, and fibrosis, the Wnt antagonizing activity of SERPINA3K may represent a unifying mechanism responsible for the broad beneficial effects of this serpin. As an endogenous inhibitor of the Wnt pathway, this serpin molecule may have therapeutic potential.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants EY012231, EY018659, EY019390, and P20RR024215 from the National Center for Research Resources and grants from the Oklahoma Center for the Advancement of Science and Technology and the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gettins PG: Serpin structure, mechanism, and function. Chem Rev 2002;102:4751–4804 [DOI] [PubMed] [Google Scholar]
- 2.Chao J, Tillman DM, Wang MY, Margolius HS, Chao L: Identification of a new tissue-kallikrein-binding protein. Biochem J 1986;239:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, Wang LX, Lu HS, Chao L: Tissue kallikrein-binding protein is a serpin: I. purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem 1990;265:16394–16401 [PubMed] [Google Scholar]
- 4.Ma JX, Yang Z, Chao J, Chao L: Intramuscular delivery of rat kallikrein-binding protein gene reverses hypotension in transgenic mice expressing human tissue kallikrein. J Biol Chem 1995;270:451–455 [DOI] [PubMed] [Google Scholar]
- 5.Gao G, Shao C, Zhang SX, Dudley A, Fant J, Ma JX: Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia 2003;46:689–698 [DOI] [PubMed] [Google Scholar]
- 6.Hatcher HC, Ma JX, Chao J, Chao L, Ottlecz A: Kallikrein-binding protein levels are reduced in the retinas of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 1997;38:658–664 [PubMed] [Google Scholar]
- 7.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R: Diabetic retinopathy. Diabetes Care 1998;21:143–156 [DOI] [PubMed] [Google Scholar]
- 8.Friedlander M: Fibrosis and diseases of the eye. J Clin Invest 2007;117:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR: Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 1996;107:404–411 [DOI] [PubMed] [Google Scholar]
- 10.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF: Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 1997;233:63–77 [DOI] [PubMed] [Google Scholar]
- 11.Leask A: Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med 2004;53:74–77 [DOI] [PubMed] [Google Scholar]
- 12.Crean JK, Lappin D, Godson C, Brady HR: Connective tissue growth factor: an attractive therapeutic target in fibrotic renal disease. Expert Opin Ther Targets 2001;5:519–530 [DOI] [PubMed] [Google Scholar]
- 13.Tikellis C, Cooper ME, Twigg SM, Burns WC, Tolcos M: Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology 2004;145:860–866 [DOI] [PubMed] [Google Scholar]
- 14.Kuiper EJ, de Smet MD, van Meurs JC, Tan HS, Tanck MW, Oliver N, van Nieuwenhoven FA, Goldschmeding R, Schlingemann RO: Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol 2006;124:1457–1462 [DOI] [PubMed] [Google Scholar]
- 15.Hughes JM, Kuiper EJ, Klaassen I, Canning P, Stitt AW, Van Bezu J, Schalkwijk CG, Van Noorden CJ, Schlingemann RO: Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia 2007;50:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper EJ, van Zijderveld R, Roestenberg P, Lyons KM, Goldschmeding R, Klaassen I, Van Noorden CJ, Schlingemann RO: Connective tissue growth factor is necessary for retinal capillary basal lamina thickening in diabetic mice. J Histochem Cytochem 2008;56:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Semenov M, Tamai K, Zeng X: LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 2004;131:1663–1677 [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X: Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108:837–847 [DOI] [PubMed] [Google Scholar]
- 19.Polakis P: Casein kinase 1: a Wnt'er of disconnect. Curr Biol 2002;12:R499–R501 [DOI] [PubMed] [Google Scholar]
- 20.Orsulic S, Peifer M: Cell-cell signalling: wingless lands at last. Curr Biol 1996;6:1363–1367 [DOI] [PubMed] [Google Scholar]
- 21.Dale TC: Signal transduction by the Wnt family of ligands. Biochem J 1998;329:209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clevers H: Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–480 [DOI] [PubMed] [Google Scholar]
- 23.Masckauchan TN, Kitajewski J: Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188 [DOI] [PubMed] [Google Scholar]
- 24.Morrisey EE: Wnt signaling and pulmonary fibrosis. Am J Pathol 2003;162:1393–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O: Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008;3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 2009;20:765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H: Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2008;294:G39–G49 [DOI] [PubMed] [Google Scholar]
- 28.Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC: CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol 2006;26:2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC: Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 2004;279:55958–55968 [DOI] [PubMed] [Google Scholar]
- 30.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW: Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci 1998;39:212–216 [PubMed] [Google Scholar]
- 31.Zhang SX, Ma JX, Sima J, Chen Y, Hu MS, Ottlecz A, Lambrou GN: Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol 2005;166:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenov M, Tamai K, He X: SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 2005;280:26770–26775 [DOI] [PubMed] [Google Scholar]
- 33.Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, Tong XJ, Xie D: Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem 2007;282:36571–36581 [DOI] [PubMed] [Google Scholar]
- 34.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC: Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996;44:1469–1479 [DOI] [PubMed] [Google Scholar]
- 35.Cunha-Vaz J, Bernardes R: Nonproliferative retinopathy in diabetes type 2: initial stages and characterization of phenotypes. Prog Retin Eye Res 2005;24:355–377 [DOI] [PubMed] [Google Scholar]
- 36.Cunha-Vaz J, Faria de Abreu JR, Campos AJ: Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol 1975;59:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha-Vaz JG: Pathophysiology of diabetic retinopathy. Br J Ophthalmol 1978;62:351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui JZ, Chiu A, Maberley D, Ma P, Samad A, Matsubara JA: Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye 2007;21:200–208 [DOI] [PubMed] [Google Scholar]
- 39.Do carmo A, Ramos P, Reis A, Proenca R, Cunha-vaz JG: Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res 1998;67:569–575 [DOI] [PubMed] [Google Scholar]
- 40.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X: Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 2008;135:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Hu Y, Ma JX: Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci 2009;50:3943–3952 [DOI] [PubMed] [Google Scholar]
- 42.Evans T, Deng DX, Chen S, Chakrabarti S: Endothelin receptor blockade prevents augmented extracellular matrix component mRNA expression and capillary basement membrane thickening in the retina of diabetic and galactose-fed rats. Diabetes 2000;49:662–666 [DOI] [PubMed] [Google Scholar]
- 43.Arnott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN: Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. J Cell Physiol 2007;210:843–852 [DOI] [PubMed] [Google Scholar]
- 44.Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR: The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem 2002;277:44187–44194 [DOI] [PubMed] [Google Scholar]
- 45.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC: Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development 2004;131:2137–2147 [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Hu Y, Lu K, Flannery JG, Ma JX: Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem 2007;282:34420–34428 [DOI] [PubMed] [Google Scholar]
- 47.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X: A mechanism for Wnt coreceptor activation. Mol Cell 2004;13:149–156 [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A: Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell 2008;15:37–48 [DOI] [PubMed] [Google Scholar]
- 49.Nusse R: Wnt signaling in disease and in development. Cell Res 2005;15:28–32 [DOI] [PubMed] [Google Scholar]
- 50.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C: LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001;411:321–325 [DOI] [PubMed] [Google Scholar]
- 51.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X: Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001;11:951–961 [DOI] [PubMed] [Google Scholar]
- 52.Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I: IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 2008;454:345–349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.