Abstract
OBJECTIVE
Maturity onset diabetes of the young type 3 (MODY3) is a consequence of heterozygous germline mutation in HNF1A. A subtype of hepatocellular adenoma (HCA) is also caused by biallelic somatic HNF1A mutations (H-HCA), and rare HCA may be related to MODY3. To better understand a relationship between the development of MODY3 and HCA, we compared both germline and somatic spectra of HNF1A mutations.
RESEARCH DESIGN AND METHODS
We compared 151 somatic HNF1A mutations in HCA with 364 germline mutations described in MODY3. We searched for genotoxic and oxidative stress features in HCA and surrounding liver tissue.
RESULTS
A spectrum of HNF1A somatic mutations significantly differed from the germline changes in MODY3. In HCA, we identified a specific hot spot at codon 206, nonsense and frameshift mutations mainly in the NH2-terminal part, and almost all amino acid substitutions were restricted to the POU-H domain. The high frequency of G-to-T tranversions, predominantly found on the nontranscribed DNA strand, suggested a genotoxic mechanism. However, no features of oxidative stress were observed in the nontumor liver tissue. Finally, in a few MODY3 patients with HNF1A germline mutation leading to amino acid substitutions outside the POU-H domain, we identified a different subtype of HCA either with a gp130 and/or CTNNB1 activating mutation.
CONCLUSIONS
Germline HNF1A mutations could be associated with different molecular subtypes of HCA. H-HCA showed mutations profoundly inactivating hepatocyte nuclear factor-1α function; they are associated with a genotoxic signature suggesting a specific toxicant exposure that could be associated with genetic predisposition.
Hepatocellular adenoma (HCA) is a rare, benign, liver tumor frequently associated with oral contraception (1,2). HCA usually manifests as a single tumor, but in some cases, several adenomas are detected in the same patient; when >10 nodules are identified in the liver it is called liver adenomatosis (3). Recently, by the analysis of a large series of patients with HCAs, we established a new molecular classification of these tumors. Adenomas were classified according to the genotype of the tumors, such as the finding of mutations in HNF1A, in CTNNB1-activating β-catenin and/or in the interleukin-6 transducer of signal (IL6ST) activating gp130 (4–8). Close relationships were found between the molecular subgroups defined by genotype and the clinical/pathologic findings (4,8). Particularly, HNF1A-mutated HCAs (H-HCAs) represent a homogeneous group of tumors with marked and diffuse steatosis without significant inflammation or cytologic abnormalities (4,8). In these tumors, downregulation of LFABP1 (encoding liver fatty acid-binding protein), a gene positively regulated by HNF1A, may contribute to this phenotype through impaired fatty acid trafficking together with an aberrant promotion of lipogenesis (9).
Heterozygous germline mutations of HNF1A are also the cause of maturity onset diabetes of the young type 3 (MODY3), a monogenic form of noninsulin-dependent diabetes (10). A few cases of familial liver adenomatosis were identified in patients with MODY3 (5,11,12). In these patients, one HNF1A mutation was germline, whereas the other was somatic and occurred only in tumor cells. In most of these families, penetrance of adenomatosis is low, and in one of them, we identified a CYP1B1 heterozygous germline mutation as a genetic event associated with the occurrence of HCA (13). Occurrence of HCA in individuals with MODY3 is rare, and the biologic underpinnings of this phenomenon remain to be explained.
The HNF1A gene (previously called TCF1) codes for the transcription factor hepatocyte nuclear factor (HNF)-1α. HNF1α protein recognizes specific palindromic nucleotide DNA sequences and interacts with DNA as either a homodimer or a heterodimer with HNF1β (14,15). HNF1α plays an important role by regulating the expression of many key liver genes involved in glucidic metabolism, lipidic transport, and detoxication (16–19). HNF1α is a protein composed of three functional domains (Fig. 1A): a dimerization domain (amino acids 1–32), a DNA-binding domain (91–276), and a carboxyl-terminal transactivation domain (281–631). The DNA-binding domain is composed of a POU-S domain (91–181) and an atypical homeodomain POU-H (203–280) formed by 3 α-helices and an insertion of 21 amino acids between Hα2 and Hα3 (20). Most of the HNF1A mutations observed in patients with HCA and MODY3 are predicted to inactivate the protein function (5,8,10,21,22). Correlations have been identified linking the position of the mutations in HNF1A and the age of diabetes onset, a phenomenon that can be explained by the specific isoforms that are expressed in the fetal or adult pancreas (21,22). Based on these data, we can hypothesize that not all mutations demonstrate the same effect on the HNF1α function.
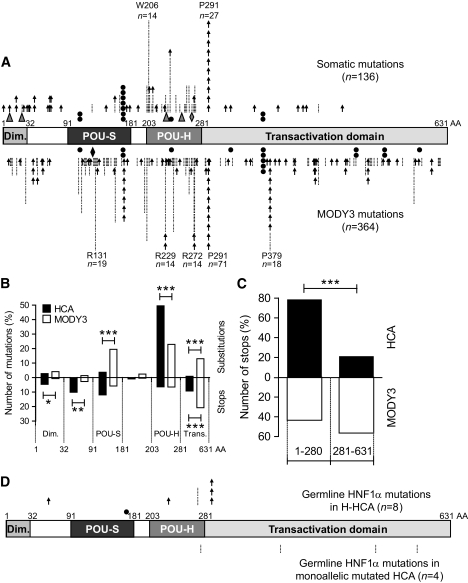
FIG. 1.
A: Spectrum of 136 somatic HNF1A mutations observed in 75 HCA samples (top) and in 364 MODY3 individuals (bottom) (data from Ellard and Colclough [25]). Each arrow indicates a point mutation leading to a frameshift or a stop codon. Each bar indicates a point mutation leading to an amino acid substitution. ▲, in-frame deletion; ♦, in-frame duplication; ●, mutation in splicing site. B: Comparison of the number of substitutions and stops in different HNF1α domains. HCA (■) and MODY3 (□) histograms represent the percentage of total mutations observed in each domain; substitutions and stops are represented in the upper part and the lower part, respectively. Significant differences between HCA and MODY3 individuals are indicated: *0.05 > P > 0.01; **0.01 > P > 0.001; ***P < 0.001. C: Comparison of the number of stops in the transactivation domain (281–631) vs. the rest of the protein observed in individuals with HCA (■) and MODY3 (□). ***Significant difference (P < 0.001) between the two populations. D: Spectrum of eight germline HNF1A mutations identified in H-HCA (top) and five germline HNF1A mutations identified in monoallelic mutated HCA (bottom). AA, amino acids.
In this study, we hypothesized that rare occurrence of HCA in MODY3 may be caused by differences in the somatic and germline spectra of mutations, both concerning their origin and their functional consequences. To test this hypothesis, we compared the spectrum of HNF1A mutations identified in HCA with that identified in MODY3. Then, taking advantage of working on human liver tissues with mutations occurring under their endogenous promoter, we evaluated the consequences of several HNF1α mutants at the mRNA expression and protein levels. We also investigated the potential mechanism of HNF1A mutagenesis by 1) comparing our observations with studies of a known genotoxic agent and 2) assessing the level of oxidative stress in the liver.
RESEARCH DESIGN AND METHODS
A group of 14 university hospitals participated in this study and 72 patients were recruited between 1992 and 2006. Individuals with confirmed HNF1A-mutated HCA who underwent curative resection of the tumor(s) were eligible for inclusion in this study. The mean age of the patients was 37.5 years (ranging from 14 to 66 years), and there were only seven male patients. Among the women studied, 71% (34 of 48) had a history of oral contraception use, and 17 patients were missing this data. There were 89 HNF1A-mutated HCA samples from 72 patients, although 44 samples had been reported previously (8). In 10 patients with multiple and sporadic tumors, several nodules were genotyped. For each patient, different somatic HNF1A mutations were observed in the genotyped nodules. For all patients, a representative part of the HCA nodule, as well as of the nontumor liver when it was available, was immediately frozen in liquid nitrogen and stored at −80°C until used for molecular studies. The study was conducted in accordance with French law and institutional ethical guidelines. The Ethical Committee of the Saint-Louis Hospital (Paris, France) approved the overall design of the study.
Mutation screening.
In all 89 tumors, we sequenced the HNF1A gene using direct sequencing of the exons on genomic DNA or RT-PCR to identify mutation, as previously described (5). All identified mutations (Tables 1 and 2) were confirmed by two independent PCR amplifications of genomic DNA from tumor, and corresponding nontumor tissues were systematically sequenced to search for germline mutations. In samples with mutations affecting splicing sites, we characterized the transcripts by sequence analysis of the mRNA transcripts. In 37 H-HCA, the coding exons of MYH (exons 1–16) and OGG1 (exons 1–7) were screened for mutations by direct sequencing of PCR products. Oligonucleotides used for all PCR reactions and experimental conditions are listed in supplementary Tables 1 and 2 available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/db09-1819/DC1. Allelic frequency of an OGG1 polymorphism observed in H-HCA was compared with those obtained in a control group of non-HNF1A-mutated hepatocellular tumors (58 samples) as previously described (13) and presented in supplementary Table 3 (available in an online appendix).
TABLE 1.
Biallelic HNF1Α mutations identified in HCA
| No. | Tumor tissue |
|
|---|---|---|
| Allele 1 | Allele 2 | |
| Somatic origin | ||
| 154* | 872–873insC, P291fs† | 749A>C, Q250P |
| 196* | 379 A>T, N127Y | 495G>T, W165C |
| 357*‡ | IVS2 + 1G>A, W165X† | 618G>T, W206C |
| 358*‡ | 617G>T, W206L | 872–884del, P291fs |
| 368*‡ | 710A>G, N237S | LOH |
| 369*‡ | 436–437delC, Q146fs | LOH |
| 370*‡ | 872–873insC, P291fs† | 803T>G, F268C |
| 371*‡ | 617G>T, W206L | 730A>G, R244G |
| 373*‡ | 82C>T, Q28X† | LOH |
| 380‡ | 196G>T, E66X | 779C>T, T260M† |
| 383‡ | 493T>A, W165R | 1340C>T, P447L† |
| 384* | 17–35insT, 7–12del | 26–32del, Q9fs |
| 385*‡ | 817A>G, K273E | LOH |
| 461‡ | 872–873insC, P291fs† | 872–873delC, P291fs† |
| 462‡ | 632A>C, Q211P | 670C>G, P224A |
| 463 | 632A>C, Q211P | 617G>T, W206L |
| 464‡ | 71–82del, A25-Q28del | 747–764del, Q250-G255del |
| 474‡ | 617G>T, W206L | LOH |
| 476‡ | 232–245dup, T81fs | 1,288–1289delG, G430fs |
| 496 | 872–873insC, P291fs† | 796–798dup, N266dup |
| 508‡ | 77T>A, L26Q | 872–873delC, P291fs† |
| 516‡ | 185–194del, N62fs | 788G>A, R263H† |
| 532‡ | 198–202del, T67fs | 618G>T, W206C |
| 535‡ | 617G>T, W206L | 872–873insC, P291fs† |
| 539‡ | 686G>A, R229Q† | 775G>C, V259L |
| 540‡ | 618G>T, W206C | LOH |
| 546‡ | 1 A>G, M1X | 620G>A, G207D† |
| 575‡ | IVS2 + 1 del13 | 956–957delG, G319fs |
| 578 | 788 G>T, R263L† | IVS5 + 1 G>T |
| 579 | 132–156del, D45fs | 872–873insC, P291fs† |
| 583‡ | 811–818del, R271fs | 815G>A, R272H† |
| 584‡ | 607C>T, R203C† | 710A>G, N237S |
| 591‡ | 787C>T, R263S | LOH |
| 592‡ | 526C>T, Q176X† | IVS5–2 A>G† |
| 621 | 711 T>G, N237K | 872–873insC, P291fs† |
| 633 | 872–873insC, P291fs† | 650–654del, A217fs |
| 635 | 618G>C, W206C | 872–873insC, P291fs† |
| 682‡ | 197–198insA, T67fs | 872–873insC, P291fs† |
| 683 | 695–697del, V233del | 613 A>C, K205Q† |
| 687‡ | 814C>A, R272S† | LOH |
| 688 | 710A>G, N237S | LOH |
| 689 | IVS1 + 2delTA | 56 C>A, S19X |
| 690 | IVS1–2 A>T | 872–873insC, P291fs† |
| 694‡ | 682G>T, E228X | IVS2–2 A>G |
| 695 | 105–144delinsTTC, P35fs | IVS2–2 A>G |
| 696‡ | 872–873insC, P291fs† | 1168G>T, E390X |
| 699‡ | 685C>G, R229G | 710–711insA, N237fs |
| 705‡ | 618G>T, W206C | 631C>T, Q211X |
| 749 | 618G>T, W206C | LOH |
| 759 | IVS2 + 1 G>A† | 608G>T, R203L |
| 761 | 617G>T, W206L | 618–628del, W206fs |
| 762 | IVS2 + 1 G>A† | LOH |
| 763 | 872–873insC, P291fs† | IVS2 + 1 G>A† |
| 778 | 1,067–1073del7, L356fs | 1441C>T, Q481X |
| 785 | 108C>A, Y36X | 570–577del, T191fs |
| 806 | 618G>T, W206C | 872–873insC, P291fs† |
| 814 | 476G>A, R159Q† | 872–873insC, P291fs† |
| 815 | 779C>T, T260M† | 872–873insC, P291fs† |
| 816 | 872–873insC, P291fs† | IVS3 + 8, insAGT, dup637-IVS3 + 7 |
| 817 | 196G>T, E66X | 711T>A, N237K |
| 818 | 710A>G, N237S | 872–873delC, P291fs† |
| 829 | 695T>A, L232Q | 931–932delGinsACCTA, A311fs |
| 831 | 815G>A, R272H† | 872–873insC, P291fs† |
| 833 | 607C>T, R203C† | LOH |
| 850 | 872–873insC, P291fs† | 133–149del, D45fs |
| 851 | 872–873delC, P291fs† | LOH |
| 856 | 872–873delC, P291fs† | 770A>C, N257T |
| 957 | 730A>G, R244G | 1,274–1275delC, T425fs |
| 964 | 629C>T, S210F | LOH |
| 971 | 620G>A, G207D† | 872–873delC, P291fs† |
| 972 | 618G>T, W206C | 1,249–1250insT, G417fs |
| 1,025 | 97–103del, P33fs | 872–873insC, P291fs† |
| Germline origin (allele 1) | ||
| 340*‡ | 685C>T, R229X† | LOH |
| 487‡ | 814 C>A, R272S† | LOH |
| 509‡ | 391C>T, R131W† | 872–873delC, P291fs† |
| 514‡ | 829–837del, F277-H279 | 872–873insC, P291fs† |
| 518‡ | 164–168del, G55fs† | LOH |
| 523‡ | 872–873insC, P291fs† | LOH |
| 590‡ | 257–258delT, L86fs | IVS2 + 1 G>T |
| 965 | 252–258del, I85fs | 815G>A, R272H† |
| Undetermined origin | ||
| 479‡ | 476–479del, R159Pfs | 811C>T, R271W† |
| 482‡ | 653 A>G, Y218C† | LOH |
| 489‡ | IVS1–2 A>T | 1,072–1073delCins11, P358fs |
| 548 | IVS1–1 G>T | 1,072–1073delCins11, P358fs |
| 951 | 534–535insA, H179fs | 779C>T, T260M† |
Cases of HCA were previously described in *Bluteau et al. (5) or ‡Zucman-Rossi et al. (8). MODY3 mutations were previously described in †Ellard and Colclough (25) or Bellanné-Chantelot et al. (21). Case 535 has a third somatic mutation, 51–60delins6, S19fs. Boldface indicates allele 1. del, deletion; fs, frameshift; IVS, intervening sequence; LOH, loss of heterozygosity; ins, insertion; Nm, nonmutated.
TABLE 2.
Germline HNF1Α mutations in monoallelic mutated HCA
Quantitative RT-PCR.
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) from all frozen samples of available HCA and nontumor liver tissues. RNA isolated from 16 H-HCA and 24 nontumor tissue samples was deemed to be of acceptable quality for quantitative RT-PCR experiments (23). Specific assays for MYH (Hs01014866_g1), OGG1 (Hs00213454m1), APE1 (Hs00172396_m1), POLβ (Hs00160263_m1), FABP1 (Hs00155026_m1), UGT2B7 (Hs00426592_m1), and R18S (rRNA, Hs99999901_s1) were obtained from Applied Biosystems (Foster City, CA). The relative amount of target gene mRNAs was determined using the 2−ΔΔCT method (24). The values obtained for FABP1 and UGT2B7 were expressed as the n-fold ratio of the gene expression in a tested sample as compared with the mean of 11 nontumor tissues. Only six nontumor tissues from patients with a H-HCA were used with the MYH, OGG1, APE1, and POLβ assays. The values obtained were compared with the mean of seven nontumor tissues from patients with a non-HNF1A–mutated HCA.
Western blotting.
Western blot analyses were performed as described (9) using two primary goat polyclonal anti-HNF1α antibodies (Santa Cruz Biotechnology), one detecting the amino terminus, and the other detecting the carboxy terminus of the protein (SC-6548 and SC-6547, respectively), used at the dilution 1:500.
Determination of reduced and total glutathione levels.
Approximatively 50 mg of frozen nontumor liver tissues were homogenized in 5% 5-sulfosalicylic acid. After centrifugation at 8,000g for 10 min, the supernatant was assessed for reduced and total glutathione content with an ApoGSH Glutathione Colorimetric Assay Kit (BioVision, Mountain View, CA), following the manufacturer's protocol.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded sections (5 μm) were mounted on glass slides. Sections were deparaffinized in xylene, rehydrated in a series of graded alcohol concentrations, and placed in PBS with 0.1% Tween 20. Immunostaining was performed using a DAKO EnVision System horseradish peroxidase (Dako Cytomation, Carpinteria, CA) kit using primary antibody (1:2,000 dilution in PBS containing 1% BSA, incubated overnight at 4°C) against 4-hydroxynonenal protein adducts (Alpha Diagnostics, San Antonio, TX). Slides were counterstained with hematoxylin and mounted with glass coverslips.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software (version 4, GraphPad Software, San Diego, CA). Allelic frequencies of the OGG1 polymorphism in H-HCA and the group control were compared using contingency tables with a Fisher exact test.
RESULTS
Spectrum of HNF1A mutations in HCA differs from that in MODY3.
Among 89 HCA samples with HNF1A alteration, mutations and deletion were biallelic in 84 (94%) samples. To further analyze the spectrum of HNF1A mutations, we included 151 HNF1A alterations with a firm somatic origin identified in 75 different HCAs; in contrast, all samples with a proven or highly suspected germline mutation were excluded from this first analysis (Fig. 1A).
Among 151 HNF1A somatic alterations, 90% were point mutations, and the remaining 10% (15 of 151) were gene deletions as evidenced by the loss of heterozygosity at a HNF1A single nucleotide polymorphism(s) compared with the corresponding nontumor tissues. Among the mutations, 57 were missense, 62 were frameshift and nonsense, and 12 were mutations affecting a splicing site. The five remaining mutations were small in-frame deletions or duplication (Fig. 1A, Table 1).
Next, we compared the spectrum of somatic HNF1A mutations identified in HCA with 364 germline mutations previously described in individuals with MODY3 without HCA (Fig. 1A, lower panel, supplementary Table 4, available in an online appendix) and by Ellard and Colclough (25). In both HCA and MODY3, a hotspot mutation at codon 291 was present, whereas a hotspot mutation at codon 206 was specifically identified in HCA (Fig. 1A). As previously reported, codon 291 mutation is located in a polyC-8 tract, whereas codon 206 mutation is not located in a repeated sequence motif. Because codon 291 mutations represented 20% of the mutations in both spectra, we did not include them in the following analysis. There was no significant difference (P = 0.16) in the proportion of missense and truncating (frameshift, nonsense, and mutations affecting a splicing site) mutations in MODY3 and HCA spectra. Thus, there were 63 and 55% of missense mutations in MODY3 and HCA, respectively, and 37 and 45% of truncating mutations in MODY3 and HCA, respectively. In contrast, the distribution of the mutations was significantly different in HCA compared with the MODY3 spectrum. Although almost all of the missense mutations were restricted to the POU-H domain (P < 0.001) in HCA, they were distributed in all HNF1α domains in individuals with MODY3 (Fig. 1B). Also, in HCA, truncating mutations were localized predominantly in the first 280 amino acids (P < 0.001), whereas they were distributed equally between the first 280 amino acids and the transactivation domain in the individuals with MODY3 (Fig. 1C).
Germline HNF1A mutations are found in H-HCA or in other subtypes of adenomas.
In a second step, we analyzed the distribution of the 13 germline HNF1A mutations identified in patients with adenomas. In eight patients, we identified a mutation of the second HNF1A allele in the corresponding tumor leading to a biallelic inactivation of HNF1α (Table 1). The corresponding germline mutations fit the distribution of the somatic HNF1A mutations: six of them were nonsense or frameshift occurring before codon 292, and the remaining two mutations were missense affecting codon 272, an amino acid found to be mutated by a somatic event (Fig. 1D). In contrast, in four HCA samples [372 (R583Q), 769 (T525S), 966 (L389V), and 998 (R278Q)], we observed a germline HNF1A mutation without alteration of the second HNF1A allele. All of these germline mutations were missense; three of them localized in the transactivation domain, and the last one was at the end of the POU-H domain. None of these mutations were described in the spectrum of somatic HNF1A mutations; however, three of them (R583Q, T525S, and L389V) have been previously reported in individuals with MODY3 (21,25). Thus, these four germline mutations did not fit the characteristic distribution of the somatic one. Moreover, the corresponding adenomas were classified in different molecular subgroups of HCA: one of them was β-catenin activated (case 372), two were inflammatory adenomas with or without a gp130 activating mutation (cases 769 and 966), and the last one remained unclassified (case 998).
Functional consequences of HNF1A mutations in HCA.
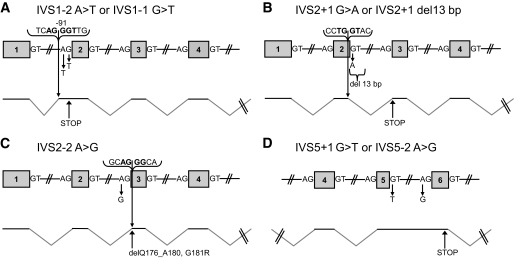
Most of the somatic mutations found in HCA are predicted to inactivate the protein because they are almost all biallelic and frequently nonsense or missense affecting the POU-H domain that is essential for DNA binding. We also analyzed the consequence of seven mutations that affected different splice sites. All of these mutations resulted in aberrant splicing, the outcome of which is either an aberrant protein with an early stop (Fig. 2A, B, and D), or a protein with an amino acid replacement and a deletion of five amino acids in the DNA-binding domain (Fig. 2C). Then we assessed the protein level of mutant HNF1α in HCA and compared it with that in nontumor tissue. Samples with mutations leading to an early stop contained little detectable HNF1α protein (supplementary Fig. 1, available in an online appendix; samples identified with arrows and data on gradient migration, not shown). Additionally, some samples harboring missense mutations exhibited a dramatic increase in the expression of HNF1α compared with normal liver (supplementary Fig. 1).
FIG. 2.
Consequences of the mutations at different splice sites in HNF1A. A: IVS1–2 A>T or IVS1–1 G>T mutations. B: IVS2 + 1 G>A or IVS2 + 1 del 13 bp mutations. C: IVS2–2 A>G mutation. D: IVS5 + 1 G>T or IVS5–2 A>G mutations. del, deletion; ins, insertion; IVS, intervening sequence.
The HNF1α transcription factor regulates expression of several key liver genes, including UGT2B7 and FABP1 (16,18). Indeed, a shutdown of mRNA expression for these target genes was observed in all patients with HCAs tested with biallelic HNF1A mutations, as previously reported (4). An effect of monoallelic HNF1α mutants on expression of UGT2B7 and FABP1 has not been previously examined in liver tissues. Our data show that monoallelic mutations did not affect expression of FABP1 and UGT2B7, which is not in favor of a dominant-negative effect (Fig. 3). However, these results cannot completely exclude a subtle dosage effect or a low dominant effect.
FIG. 3.
Expression of FABP1 and UGT2B7 is abrogated in adenomas with HNF1A biallelic mutations. Mean levels of expression in each group is represented by a horizontal line.
HNF1A mutations in HCA are likely to arise due to a genotoxic event.
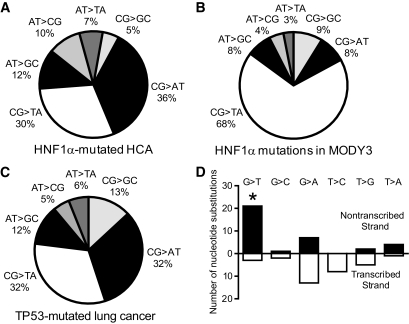
In contrast to other solid tumors, point mutations were much more frequent in HCA than were chromosome deletions. The latter were found in only 10% of the patients with HCAs. Among the point mutations in HCA, we analyzed the proportion of different HNF1A nucleotide changes and found that G-to-T transversions, accounting for 36% of the cases, were the most common type (Fig. 4A). Moreover, the distribution of nucleotide substitution subtypes in H-HCA was strikingly different from that in MODY3 (Fig. 4B) but very similar to a spectrum of mutations known to be induced by genotoxic events, that is, in TP53-mutated lung cancers in smokers (Fig. 4C) (26). In addition, when the HNF1A nucleotide substitutions were partitioned between the two DNA strands, we observed that G-to-T transversions were significantly more frequent on a nontranscribed strand (P = 0.01) (Fig. 4D). Taking these results together, we suggest that HNF1A somatic mutations showed a typical spectrum of mutations induced by the genotoxic exposure targeting a specific nucleotide sequence.
FIG. 4.
Comparison of the mutation profiles in H-HCA (67 transversions and transitions, A), MODY3 (229 transversions and transitions, B), and TP53-mutated lung cancers (311 transversions and transitions, C) and smokers (26) (D). Repartitioning of transition and transversion mutations between the transcribed and the nontranscribed strands in H-HCA. Black and white histograms indicate the number of nucleotide substitutions on the nontranscribed and the transcribed strand, respectively. *Significant (P = 0.01) elevation in G-to-T transversions on the nontranscribed strand.
HNF1A spectrum of mutation is not related to measurable increase in oxidative stress in liver.
Because both direct DNA damage by an environmental agent and oxidative stress are known to result in G-to-T transversion mutations (27,28), we also assessed oxidative stress markers in nontumor liver from six patients with H-HCA and seven patients with non-HNF1A–mutated HCA (supplementary Fig. 2, available in an online appendix). We observed no difference in total or reduced glutathione content, expression of four base excision DNA repair genes, markers of oxidative DNA damage (29), or 4-hydroxynonenal protein adducts, a marker of lipid peroxidation (supplementary Fig. 3), between these two groups. These data showed a lack of evidence for elevated oxidative stress in livers of patients with H-HCA as a potential source of DNA mutations.
In addition, it is possible that a large number of G-to-T transversions could be caused by inefficient repair of DNA damage due to mutations or polymorphisms in MYH and OGG1, genes involved in the repair of 8-hydroxyguanine (30–32). Thus, we sequenced MYH and OGG1 DNA in 37 subjects with an HNF1A-mutated HCA, and no mutations were detected in either gene. A known functional polymorphism in OGG1 (S326C) was detected in HNF1A-mutated HCA subjects, albeit there was no difference in the frequency of this polymorphism compared with that in control subjects (supplementary Table 3, available in an online appendix).
DISCUSSION
In this study we analyzed the spectrum of HNF1A mutations in HCA and showed a significant difference in pattern in comparison with individuals with MODY3, both at the nucleotide and amino acid levels. In HCA, location of the mutations is very restricted because almost all of the truncating mutations led to the loss of the transactivation domain and the missense mutations altered mainly the POU-H domain. When we take into account the two largest series of HNF1A screening in MODY3 (21,25), only 48% of the germline mutations (117 of 720 are missense mutations in POU-H, and 227 of 720 are truncating mutations localized in the first 291 amino acids) fit the features of the HNF1A somatic mutations. This observation suggests that only a part of HNF1A mutations that are associated with diabetes could predispose to the development of a H-HCA.
Previous analysis of the MODY3 mutations showed that the age at onset of diabetes is modulated according to the position of the mutation relative to the HNF1A isoforms: missense mutations located in exons 7 to 10 that affect only A or B isoforms of HNF1A are associated with the late onset of diabetes (21,22). Interestingly, these mutations are predicted to have a less severe effect on the HNF1α function and are not found in HCA. Similarly, we can hypothesize that frequent MODY3 missense mutations located outside the POU-H domain and mutations truncating HNF1α after codon 291 are possibly less inactivating than the mutations found in HCA. In contrast to MODY3, in HCA the lack of mutations leading to amino acid substitution in POU-S suggests that the POU-S and POU-H domains are functionally different. Chi et al. (20) found that the POU-S domain interacts with the POU-H domain in the recognition and binding on promoter sequences; however, the POU-S domain is not conserved in homeobox transcription factors outside the POU family. Thus, based on the profile of the HNF1A mutations that occur in HCA, it may be of interest to reanalyze clinical severity of the diabetes and putative associated phenotypes in patients with MODY3, taking into the account only a premature stop before codon 291, missense restricted at POU-H, and mutations affecting splicing sites from exon 1 to 7.
Interestingly, among the five HCAs with a monoallelic germline HNF1A mutation, three of them were inflammatory. We previously noted that inflammatory adenomas are associated with obesity (4), and the present observation raised the question of an association with MODY3 and possibly with other subtypes of adenomas. We can hypothesize that according to the nature of their HNF1A germline mutation, patients with MODY3 could be at risk of different subtypes of HCA: mutations leading to a severe impaired HNF1α function predispose to the development of H-HCA, whereas germline mutation with a mild functional consequence could predispose to inflammatory or ß-catenin–activated HCAs without a familial context. In consideration of the high risk of malignant transformation of the β-catenin-activated HCA, this observation could have important clinical consequences.
Our analysis of the expression of mutant HNF1α proteins in liver tissue did not reveal a detectable level of truncated proteins, and most of the mutated proteins were not expressed. Moreover, mutations of both HNF1A alleles are required to observe a downregulation of the genes physiologically regulated by HNF1α. These observations suggest that if a dominant-negative effect exists, as suggested by the in vitro analyses for particular HNF1A mutants (15), it is not sufficient to shut down activity of HNF1α in vivo. Accordingly, other researchers (33,34), including Harries et al. (35) showed that HNF1A transcripts harboring a premature termination codon (PTC) were less expressed in lymphoblastoid cells than the normal allele resulting from a “nonsense-mediated mRNA decay,” which detects and degrades the transcripts with a PTC to prevent the synthesis of truncated proteins. In the present study, we observed a decrease of mRNA expression for 16 of 21 different mutants leading to PTC (data not shown). Altogether, in hepatocytes, a dominant-negative effect of an HNF1α mutant is unlikely in vivo.
This study not only confirms, in a large series of adenomas, that the spectrum of HNF1A mutations in HCA is different from that in individuals with MODY3, it also supports the hypothesis that HNF1A mutations in HCA arise because of a genotoxic mechanism. First, we observed a consistent hotspot mutation at the codon 206 of HNF1A in HCA. Hotspots of transversion identified in tumors without a repeated nucleotide sequence context were previously suggested to be a hallmark of an exposure to a genotoxic compound, such as an R249S mutation in the TP53 gene in hepatocellular carcinoma (HCC) associated with an exposure to the aflatoxin B1 mycotoxin (36,37).
Second, Hainaut and Pfeifer (26) showed that there is a significant difference in the pattern of TP53 mutations in lung cancers between smokers and nonsmokers, with a 30% frequency of G-to-T transversions in smoking-related lung cancer vs. 10% in non-smoking–related lung cancer. The overabundance of G-to-T transversions in smoking-related lung cancers was consistent with the studies of DNA damage and mutations due to benzo[a]pyrene and other cigarette smoke-derived carcinogens and their metabolites (38,39). Our observation of an uneven distribution among HNF1A nucleotide substitutions in HCA and high frequency of G-to-T transversions is in many ways similar to that in lung cancer caused by tobacco (Fig. 4A).
Third, Denissenko et al. (40) showed that the repair of benzo[a]pyrene diol epoxide adducts in the nontranscribed strand was slower than that in the transcribed strand and may explain the strand bias of transversions in cancer. Similarly, we observed that G-to-T transversions were significantly localized to the nontranscribed strand in H-HCA, an additional argument in favor of a genotoxic effect and, possibly, a presence of bulky DNA adducts.
Finally, the origin of such a genotoxic signature remains to be elucidated. We can hypothesize that it could be related to a particular genetic susceptibility and/or to an exposure to a specific toxic. According to the first hypothesis, we searched for mutations in OGG1 and MYH, both genes specifically involved in the repair of 8-hydroxyguanine, a product of oxidative DNA damage that can also lead to G-to-T transversions (30–32). However, we did not observe mutations in these genes in patients with HCAs. In addition, we did not observe a higher level of oxidative stress in the livers of patients with a H-HCA. These results strongly suggest that exposure to an environmental agent appears to be the most likely event to explain the elevated rate of G-to-T transversions in H-HCA. Because HCA occurrence is highly associated with oral contraception, we can hypothesize that estrogen exposure could play a role in this mechanism. This last hypothesis is supported by the facts that 1) the genotoxic activity of estrogen metabolites has been shown, particularly for catechol estrogens that may be oxidized to a reactive quinone capable of direct formation of DNA adducts (41,42); and 2) germline heterozygous mutations of CYP1B1, a key metabolism enzyme responsible for the formation of hydroxylated and genotoxic metabolites of estrogen, contribute to the development of H-HCA in women using hormonal contraception (13).
In conclusion, when analyzing a large series of individuals with HCAs, we observed a significant differences between HNF1A somatic mutations identified in those with HCAs and the germline mutations identified in individuals with MODY3. Somatic mutations in HCA predict a more inactivating profile, resulting in a complete loss of function of the protein when biallelic. Moreover, we showed that the origin of the mutations might be the result of DNA damage caused by a genotoxic agent, possibly resulting from the metabolism of estrogen that could also be associated with a genetic susceptibility that remains to be identified.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Institut National de la Santé et de la Recherche Médicale (INSERM) (Réseau de Recherche Clinique et en santé des populations), the Association pour la Recherche sur le Cancer (ARC), and the National Institutes of Health (R01-AA-16258 and R01-ES-15241). E.J. was supported by an ARC doctoral fellowship and J.Z.-R. by a “contrat d'interface” between INSERM and CHU Bordeaux.
No potential conflicts of interest relevant to this article were reported.
We thank all the participants of the Groupe d'étude Génétique des Tumeurs Hépatiques network. We are grateful to Sandra Rebouissou for helpful scientific discussion and critical reading of the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baum JK, Bookstein JJ, Holtz F, Klein EW: Possible association between benign hepatomas and oral contraceptives. Lancet 1973;2:926–929 [DOI] [PubMed] [Google Scholar]
- 2.Edmondson HA, Reynolds TB, Henderson B, Benton B: Regression of liver cell adenomas associated with oral contraceptives. Ann Intern Med 1977;86:180–182 [DOI] [PubMed] [Google Scholar]
- 3.Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP: Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology 1985;89:1132–1138 [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, Balabaud C, Zucman-Rossi J: Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007;46:740–748 [DOI] [PubMed] [Google Scholar]
- 5.Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P, Zucman-Rossi J: Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet 2002;32:312–315 [DOI] [PubMed] [Google Scholar]
- 6.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J: Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 2009;457:200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torbenson M, Lee JH, Choti M, Gage W, Abraham SC, Montgomery E, Boitnott J, Wu TT: Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol 2002;15:189–196 [DOI] [PubMed] [Google Scholar]
- 8.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P: Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006;43:515–524 [DOI] [PubMed] [Google Scholar]
- 9.Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Tercé F, Auffray C, Bioulac-Sage P, Zucman-Rossi J: HNF1α inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem 2007;282:14437–14446 [DOI] [PubMed] [Google Scholar]
- 10.Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Turner RC, Velho G, Chèvre JC, Froguel P, Bell GI: Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 1996;384:455–458 [DOI] [PubMed] [Google Scholar]
- 11.Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, Laurent C, Bourlier P, Pariente D, de Muret A, Fabre M, Bioulac-Sage P, Zucman-Rossi J: Familial liver adenomatosis associated with hepatocyte nuclear factor 1α inactivation. Gastroenterology 2003;125:1470–1475 [DOI] [PubMed] [Google Scholar]
- 12.Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P, Fabre M, Oberti F, Fatome A, Zucman-Rossi J, Bellanne-Chantelot C: Hepatocyte nuclear factor-1α gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab 2004;89:1476–1480 [DOI] [PubMed] [Google Scholar]
- 13.Jeannot E, Poussin K, Chiche L, Bacq Y, Sturm N, Scoazec JY, Buffet C, Van Nhieu JT, Bellanné-Chantelot C, de Toma C, Laurent-Puig P, Bioulac-Sage P, Zucman-Rossi J: Association of CYP1B1 germ line mutations with hepatocyte nuclear factor 1α-mutated hepatocellular adenoma. Cancer Res 2007;67:2611–2616 [DOI] [PubMed] [Google Scholar]
- 14.Mendel DB, Hansen LP, Graves MK, Conley PB, Crabtree GR: HNF-1α and HNF-1β (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev 1991;5:1042–1056 [DOI] [PubMed] [Google Scholar]
- 15.Vaxillaire M, Abderrahmani A, Boutin P, Bailleul B, Froguel P, Yaniv M, Pontoglio M: Anatomy of a homeoprotein revealed by the analysis of human MODY3 mutations. J Biol Chem 1999;274:35639–35646 [DOI] [PubMed] [Google Scholar]
- 16.Akiyama TE, Ward JM, Gonzalez FJ: Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1α (HNF1α). Alterations in fatty acid homeostasis in HNF1α-deficient mice. J Biol Chem 2000;275:27117–27122 [DOI] [PubMed] [Google Scholar]
- 17.Hiraiwa H, Pan CJ, Lin B, Akiyama TE, Gonzalez FJ, Chou JY: A molecular link between the common phenotypes of type 1 glycogen storage disease and HNF1α-null mice. J Biol Chem 2001;276:7963–7967 [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Hansen AJ, Mackenzie PI: Octamer transcription factor-1 enhances hepatic nuclear factor-1alpha-mediated activation of the human UDP glucuronosyltransferase 2B7 promoter. Mol Pharmacol 2000;57:940–947 [PubMed] [Google Scholar]
- 19.Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL, Stoffel M: Hepatocyte nuclear factor-1α is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 2001;27:375–382 [DOI] [PubMed] [Google Scholar]
- 20.Chi YI, Frantz JD, Oh BC, Hansen L, Dhe-Paganon S, Shoelson SE: Diabetes mutations delineate an atypical POU domain in HNF-1α. Mol Cell 2002;10:1129–1137 [DOI] [PubMed] [Google Scholar]
- 21.Bellanné-Chantelot C, Carette C, Riveline JP, Valéro R, Gautier JF, Larger E, Reznik Y, Ducluzeau PH, Sola A, Hartemann-Heurtier A, Lecomte P, Chaillous L, Laloi-Michelin M, Wilhem JM, Cuny P, Duron F, Guerci B, Jeandidier N, Mosnier-Pudar H, Assayag M, Dubois-Laforgue D, Velho G, Timsit J: The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2008;57:503–508 [DOI] [PubMed] [Google Scholar]
- 22.Harries LW, Ellard S, Stride A, Morgan NG, Hattersley AT: Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1α show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum Mol Genet 2006;15:2216–2224 [DOI] [PubMed] [Google Scholar]
- 23.Rebouissou S, Vasiliu V, Thomas C, Bellanné-Chantelot C, Bui H, Chrétien Y, Timsit J, Rosty C, Laurent-Puig P, Chauveau D, Zucman-Rossi J: Germline hepatocyte nuclear factor 1α and 1β mutations in renal cell carcinomas. Hum Mol Genet 2005;14:603–614 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Ellard S, Colclough K: Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1α (HNF1A) and 4α (HNF4A) in maturity-onset diabetes of the young. Hum Mutat 2006;27:854–869 [DOI] [PubMed] [Google Scholar]
- 26.Hainaut P, Pfeifer GP: Patterns of p53 G→T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis 2001;22:367–374 [DOI] [PubMed] [Google Scholar]
- 27.Denissenko MF, Pao A, Tang M, Pfeifer GP: Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 1996;274:430–432 [DOI] [PubMed] [Google Scholar]
- 28.Michaels ML, Miller JH: The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J Bacteriol 1992;174:6321–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusyn I, Asakura S, Pachkowski B, Bradford BU, Denissenko MF, Peters JM, Holland SM, Reddy JK, Cunningham ML, Swenberg JA: Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res 2004;64:1050–1057 [DOI] [PubMed] [Google Scholar]
- 30.Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP: Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat Genet 2002;30:227–232 [DOI] [PubMed] [Google Scholar]
- 31.Audebert M, Chevillard S, Levalois C, Gyapay G, Vieillefond A, Klijanienko J, Vielh P, El Naggar AK, Oudard S, Boiteux S, Radicella JP: Alterations of the DNA repair gene OGG1 in human clear cell carcinomas of the kidney. Cancer Res 2000;60:4740–4744 [PubMed] [Google Scholar]
- 32.Slupska MM, Baikalov C, Luther WM, Chiang JH, Wei YF, Miller JH: Cloning and sequencing a human homolog (hMYH) of the Escherichia coli mutY gene whose function is required for the repair of oxidative DNA damage. J Bacteriol 1996;178:3885–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byers PH: Killing the messenger: new insights into nonsense-mediated mRNA decay. J Clin Invest 2002;109:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frischmeyer PA, Dietz HC: Nonsense-mediated mRNA decay in health and disease. Hum Mol Genet 1999;8:1893–1900 [DOI] [PubMed] [Google Scholar]
- 35.Harries LW, Hattersley AT, Ellard S: Messenger RNA transcripts of the hepatocyte nuclear factor-1α gene containing premature termination codons are subject to nonsense-mediated decay. Diabetes 2004;53:500–504 [DOI] [PubMed] [Google Scholar]
- 36.Bressac B, Kew M, Wands J, Ozturk M: Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 1991;350:429–431 [DOI] [PubMed] [Google Scholar]
- 37.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC: Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 1991;350:427–428 [DOI] [PubMed] [Google Scholar]
- 38.Greenblatt MS, Bennett WP, Hollstein M, Harris CC: Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994;54:4855–4878 [PubMed] [Google Scholar]
- 39.Hollstein M, Sidransky D, Vogelstein B, Harris CC: p53 mutations in human cancers. Science 1991;253:49–53 [DOI] [PubMed] [Google Scholar]
- 40.Denissenko MF, Pao A, Pfeifer GP, Tang M: Slow repair of bulky DNA adducts along the nontranscribed strand of the human p53 gene may explain the strand bias of transversion mutations in cancers. Oncogene 1998;16:1241–1247 [DOI] [PubMed] [Google Scholar]
- 41.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG: Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A 1997;94:10937–10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yager JD, Liehr JG: Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol 1996;36:203–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.