Abstract
Background
Low phosphorus (P) availability is a major constraint to soybean growth and production. Developing P-efficient soybean varieties that can efficiently utilize native P and added P in the soils would be a sustainable and economical approach to soybean production.
Scope
This review summarizes the possible mechanisms for P efficiency and genetic strategies to improve P efficiency in soybean with examples from several case studies. It also highlights potential obstacles and depicts future perspectives in ‘root breeding’.
Conclusions
This review provides new insights into the mechanisms of P efficiency and breeding strategies for this trait in soybean. Root biology is a new frontier of plant biology. Substantial efforts are now focusing on increasing soybean P efficiency through ‘root breeding’. To advance this area, additional collaborations between plant breeders and physiologists, as well as applied and theoretical research are needed to develop more soybean varieties with enhanced P efficiency through root modification, which might contribute to reduced use of P fertilizers, expanding agriculture on low-P soils, and achieving more sustainable agriculture.
Keywords: Soybean, genetic improvement, phosphorus efficiency, root breeding
INTRODUCTION
Soybean (Glycine max), as an essential source of protein, oil and micronutrients in human and animal diets, has become an increasingly important agricultural commodity, with a steady increase in worldwide annual production due to its excellent nutritional value and health benefits (Liu, 1997). Soybean is cultivated in tropical, subtropical and temperate areas, often on soils low in phosphorus (P) because of intensive erosion, weathering and strong P fixation by free Fe and Al oxides (Sample et al., 1980; Stevenson, 1986). Therefore, low P availability is often a major constraint to soybean growth and production. At the same time, the world is facing a future shortage of P fertilizer resources, as P fertilizers are increasing in cost and P ore deposits that can be economically mined and processed into fertilizer are limited and will eventually be depleted (Yan et al., 2009). Nutrient-efficient plants are defined as plants which could produce higher yields per unit of nutrient applied or absorbed compared with other plants grown under similar agroecological conditions (Fageria et al., 2008). Therefore, P-efficient varieties should play a major role in increasing soybean yield. Developing P-efficient soybean varieties that can efficiently utilize native P and added P in the soils, which are often in forms unavailable to plants, would be a sustainable and economical approach to soybean production. This review discusses the possible mechanisms for P efficiency and genetic strategies to improve P efficiency in soybean through root-based approaches. These studies might help scientists to gain new insights into the mechanisms of P efficiency and genetic strategies for better P efficiency in soybean, particularly through root modification.
POSSIBLE MECHANISMS FOR PHOSPHORUS EFFICIENCY
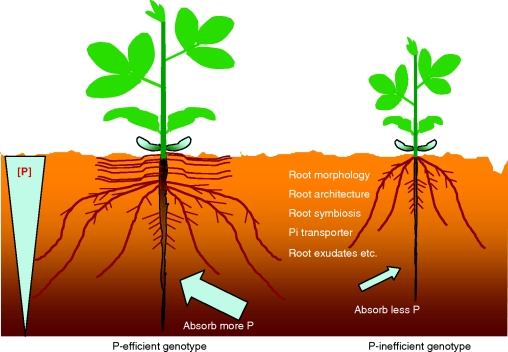
Many studies have demonstrated that there is substantial genetic variation for plant P efficiency. In soybean, our laboratory has identified P-efficient varieties that grow much better than standard varieties under P-deficient conditions in acid soils, where the concentration of available soil P is often lower than 20 mg kg−1, and show similar yields to standard varieties in P-fertilized soils (Fig. 1). There are a number of potential adaptive mechanisms that P-efficient plants can employ for better growth on low-P soils, including changes in root morphology and architecture, root symbiosis, activation of high-affinity phosphate (Pi) transporters, enhancement of internal phosphatase activity, and secretion of organic acids and phosphatases into the rhizosphere (Fig. 2; Raghothama, 1999; Vance et al., 2003; Gahoonia and Nielsen, 2004a). Therefore, it would be preferable to identify and select the specific traits that are directly related to P efficiency and can be modified. These specific traits could be used for more efficient screening in controlled environments, or tagged with the molecular markers and then improved through marker-assisted selection or gene transformation (Yan, 2005). Among the possibly beneficial traits for P efficiency, some studies have shown that root morphology and architecture, root symbiosis and root exudates were the most important traits for efficient P acquisition in soybean (Wang et al., 2004, 2009; Zhao et al., 2004; Cheng et al., 2008; Liu et al., 2008a).
Fig. 1.
Genetic variation for phosphorus efficiency in soybean. Two soybean varieties contrasting in P efficiency were grown on a P-deficient acid soil with or without P addition. Low P is no P added, high P is 160 kg P ha−1 added as triple sulfate phosphorus (TSP). The available P content of the tested soil was 12·60 mg kg−1 (photo by X. Yan).
Fig. 2.
Schematic representation of a number of adaptive mechanisms for better soybean growth on low-P soils.
Root morphology and architecture
Root morphology refers to the surface features of a single root axis as an organ, including characteristics of the epidermis such as root hairs, root diameter, root cap, the pattern of appearance of daughter roots, undulations of the root axis and cortical senescence (Lynch, 1995). Important root developmental processes, such as root hair formation, primary root growth and lateral root formation, are particularly sensitive to changes in the internal and external concentration of nutrients (López-Bucio et al., 2002, 2003). Root morphological features, particularly root hairs, are important for plant P uptake from the soil by expanding the absorptive surface area of the root and increasing the soil volume explored by the roots. Studies with many species have shown a significant correlation between radius, number and length of root hairs and P influx in plants, indicating a close relationship between root hairs and P uptake from the soils (Foehse et al., 1991; Yan et al., 2004). In barley, genotypic variations in root hairs can significantly affect P uptake from the soil and grain yields in low-P fields (Gahoonia and Nielsen, 1997, 2004b). This also appears to be the case with soybean. In a study with two contrasting soybean genotypes and their F9-derived recombinant inbred lines (RILs), Wang et al. (2004) found that root hair traits, including root hair density, average root hair length and root hair length per unit root, varied significantly among different genetic materials and that these variations were highly associated with P status in soybean plants. However, the root hair traits are possibly controlled by quantitative trait loci (QTLs) and therefore can be subject to significant environmental influence.
Root system architecture, the spatial configuration of a root system in the soil, is used to describe the shape and structure of root systems (de Dorlodot et al., 2007). Plant root architecture, or the spatial configuration of roots in the soil, determines to a great extent the soil volume explored by the roots, and thus is very important to plant P acquisition in many crop species studied, particularly in legumes (Lynch, 1995; Liao et al., 2001a; Zhao et al., 2004). In common bean, comparative analysis of different genotypes contrasting in adaptation to low P availability suggested that root architectural parameters, including lateral root branching, and length and growth angle of basal roots, and root growth plasticity were related to P efficiency (Lynch and van Beem, 1993; Lynch and Beebe, 1995; Lynch 1998; Liao et al., 2001b). In soybean, a study employing an ‘applied core collection’ of soybean germplasm provided the first evidence for a close relationship between root architecture and P efficiency (Zhao et al., 2004). Their study found that plants with shallow root architecture had better spatial configuration in the higher-P cultivated soil surface layer and thus had higher P efficiency and yield. Interestingly, there seems to be a co-evolutionary pattern among shoot type, root architecture and P efficiency. Bush cultivated soybean plants have shallower root architecture and greater P efficiency, and climbing wild soybean plants have deeper root architecture and lower P efficiency, while the root architecture and P efficiency of semi-wild soybean lines are intermediate between cultivated and wild soybean (Fig. 3; Zhao et al., 2004). The co-evolutionary relationship between root architecture and P efficiency might be attributed to the higher nutrient availability in topsoil. A recent field study further indicated that root architecture and morphology were closely related to plant P efficiency (Liu et al., 2008b). Genotypes with shallower root architecture were found to have optimal three-dimensional root configurations and longer total root lengths, thus having higher P efficiency and seed yield under low P conditions. Root plasticity of genotypes with either deep or shallow root architecture was lower than those with intermediate root architecture, indicating that root architecture was more stable in the former two groups under low P conditions (Liu et al., 2008b).
Fig. 3.
Shoot and root architecture of a soybean cultivar, and a semi-wild and a wild genotype. Plants had been grown in the field for 3 months. The available P content of the tested soil was 17·30 mg kg−1 (photo by X. Yan).
Using suppression subtractive hybridization, Guo et al. (2008) constructed several low-P induced cDNA libraries from both P-efficient and P-inefficient soybean genotypes to identify Pi starvation-induced genes in soybean. The results showed that of 2000 clones, 210 genes were differentially expressed in roots and shoots of soybean seedlings under Pi starvation. Among them, a novel soybean β-expansin gene, GmEXPB2, was cloned and characterized from a Pi starvation-induced soybean cDNA library. The results from both transgenic soybean hairy roots carrying the promoter of GmEXPB2 and transgenic GmEXPB2-overexpressing Arabidopsis lines verified that this gene was strongly induced by Pi starvation, and that this induction was closely related to primary and lateral root elongation, which led to improved growth performance and P efficiency compared with control hairy roots or wild-type plants (Guo, 2008).
It is interesting to note that soybean root architecture might not only affect P efficiency of soybean per se but also affect N and P nutrition of maize in a soybean-maize intercropping system (Tang et al., 2005). The shallow-rooted and P-efficient soybean genotype had significantly greater advantages over the deep-rooted and P-inefficient genotype when intercropped with maize. The nutrient acquisition efficiency of the maize plant intercropped with the shallow-rooted soybean genotype was significantly greater than that intercropped with the deep-rooted soybean genotype, indicating that soybean root architecture may play an important role in the maize/soybean intercropping system. Maize has a fibrous root system, which is generally more deeply distributed than the basal roots of the tap root system, such as common bean and soybean. Those soybean genotypes with shallower roots might be able to take up P from topsoil, and also avoid competition for subsoil P acquisition with maize (Tang et al., 2005).
Root symbiosis
Significant positive nitrogen (N) and P interactions were found in field-grown soybean, possibly related to genetic attributes of root morphological and nodular traits (Kuang et al., 2005). Such a positive N and P interaction in the field might have resulted from better nodulation, longer root length and shallower root architecture, and these traits could be considered as selection criteria in further breeding programmes for better nutrient efficiency in legumes. P addition has considerable impact on rhizobium symbiosis and biological N2 fixation of some genotypes by increasing nodule formation and nitrogenase activity on the upper parts of the roots (Kuang et al., 2005). This at least in part explains the observations from the above described field experiment and may imply that selecting soybean genotypes with shallow root systems would not only increase P-uptake efficiency but also facilitate biological N2 fixation.
In a recent study with isolated and purified rhizobium strains from the nodules of two soybean genotypes contrasting in P efficiency grown on different low-P acid soils with different soybean cultivation history, Cheng et al. (2008) found that inoculation with these rhizobial inoculants not only formed many nodules, but also increased soybean shoot biomass and yield, and resulted in improved N and P nutrient status. Improved N status with resulting enhanced root growth might be the mechanism by which soybean P uptake was increased in plants inoculated with the effective rhizobium strains on low-P acid soils. Inoculating with these effective rhizobial inoculants could significantly improve growth, as well as N and P content of soybean plants on low-P acid soils (Cheng et al., 2008).
Another beneficial root trait for P efficiency is enhanced root symbiosis with mycorrhizal fungi, which may both mobilize insoluble soil P through their exudates and extend root absorptive area through hyphae. Under low P conditions, plants often have higher mycorrhizal infection rate and mycorrhizae contribute more to P uptake (Smith, 2002). At the same time, it is now recognized that plant growth response to arbuscular mycorrhizae (AM) associations (i.e. the ‘mycorrhizal growth response’, MGR) varies widely among plant species and even varieties when soil P availability limits growth. Some plants ‘typically’ show large positive MGR, whereas others ‘typically’ show no MGR or even negative MGR (Tawaraya, 2003; Smith and Read, 2008; Smith et al., 2009). Some studies have clearly demonstrated the beneficial effects that both indigenous and inoculated AM had on legume growth and N uptake (Ganry et al., 1985; Ibijbijen et al., 1996; Chalk et al., 2006). Results from a pot experiment indicated that soybean genotypes differed in their mycorrhizal infection rate and that there was a significant interaction between soybean root architecture, soil P availability and AM colonization (Liu et al., 2008a). The soybean genotypes with intermediate and deep root architectures had higher infection rates with AM under low P conditions. The P-efficient soybean genotypes had either better root architecture or higher AM colonization, indicating that a complementary relationship between root architecture and AM colonization might contribute to soybean P efficiency (Liu et al., 2008a).
Root exudates
Low P availability may induce root exudation of a number of compounds, including organic acids, phosphatases and other compounds that can mobilize P from bound P pools. Exudation of organic acids into the rhizosphere has been proposed to increase P availability to the plant by mobilizing the sparingly soluble mineral P and, possibly, organic P sources (Dinkelaker et al., 1989; Jones and Darrah, 1994; Johnson et al., 1996). Cluster (proteoid) roots in the Proteacea (e.g. Lupinus albus) can release a large amount of citrate under P-deficiency conditions, and thus sufficiently increase P nutrition of plants (Dinkelaker et al., 1989; Neumann and Martinoia, 2002; Kochian et al., 2004). Dong et al. (2004) showed that soybean genotypes contrasting in P efficiency differed in the type and quantity of organic acids excreted from the roots under P stress, suggesting that organic acids might contribute to P uptake in P-efficient genotypes. Further studies have demonstrated that organic acid exudation was differentially induced by low P and aluminium (Al) toxicity in soybean plants, with specific induction of oxalate and malate by low P and citrate by Al toxicity, suggesting different functions in P efficiency and Al resistance for different organic acids (Liao et al., 2006). However, the roles of organic acids supported by in vitro experiments might not represent the roles of organic acids under real soil conditions. The reactions of organic acids in soils are extremely complex (Jones et al., 2003). Once exuded, organic acids can be almost instantly sorbed to anion exchange sites in acid soils and rapidly biograded in calcareous soils. Their bioavailability and effective nutrient mobilizing capacity can therefore be reduced quickly (Jones and Brassington, 1998; Ström et al., 2001). In addition, the concentration of organic acids in soil solution is generally low, ranging from 1 to 50 µm (Strobel, 2001). Even using 10 mm organic acid extractants at pH 7·5, there was only 1·9 and 0·8 µm P released by citrate and oxalate, respectively, far below the growth demands for plants (Ström et al., 2005). Therefore, fundamental research on organic acids and their interaction with soils still needs to be done to fully elucidate their roles in soil processes, and understand the factors controlling organic acid-mediated nutrient release in different soil types (Jones, 1998; Jones et al., 2003).
Tian et al. (2003) showed that there were large variations for leaf acid phosphatase (APase) activity among the tested 274 soybean genotypes with different origins, and APase activity was generally increased without P fertilizer addition. Also, the increase in leaf APase activity under low P stress was mainly due to an increase in the activity of existing isoforms and not by the induction of new isoforms. The results suggested that leaf APase activity could serve as an enzymatic indicator of low P status for soybean plants. Studies with other crop species demonstrated that APase and phytase secretion was a major contributor for plant assimilation of organic P from soils (Li et al., 1997; Hayes et al., 2000; Lung and Lim, 2006; George and Richardson, 2008). Over-expression of phytase genes from a soil fungus (Aspergillus sp.) or a soil bacteria (Bacillus subtilis) in transgenic model plants (such as Arabidopsis thaliana and Nicotiana tabacum) significantly increased exudation of phytase from plant roots (Richardson et al., 2001; Mudge et al., 2003; George et al., 2005; Lung et al., 2005), and several studies have reported that plant purple APase genes such as MtPHY1 and MtPAP1 were expressed in transgenic Arabidopsis and increased phytase exudation (Xiao et al., 2005, 2006). At the same time, results have shown that enhanced exudation of phytase might not help to improve plant P nutrition due to the low availability of phytate in the soil (George et al., 2005). Our studies found that over-expression of an Arabidopsis purple APase gene (AtPAP15) containing a carrot extracellular targeting peptide not only increased the secretion of APase from transgenic soybean plants, but also significantly enhanced intracellular APase activity in leaves, as well as P efficiency and yield on media of low organic P (Wang et al., 2009). This suggested that enhanced intracellular APase activity might be more important for crops to adapt to a wide range of low P soils.
In addition, root plasma membrane H+-ATPase was also found to be involved in the adaptation of soybean to Pi starvation. P uptake might be regulated in part via modulation of the activity of plasma membrane H+-ATPase under Pi starvation (Shen et al., 2006). They suggested that indole-3-acetic acid was involved in signal transduction of Pi starvation by activating the plasma membrane H+-ATPase in soybean roots (Shen et al., 2006).
ROOT BREEDING STRATEGIES TO IMPROVE PHOSPHORUS EFFICIENCY IN SOYBEAN
Plant breeding is the science of changing the genetics of plants to increase disease resistance, productivity and product quality in economically important species. Important techniques include conventional breeding, marker-assisted selection (MAS) and genetic engineering via transgenesis. Wide genotypic variation and high hereditability for root morphology and architecture, root symbiosis and root exudates in soybean provide opportunities for selection and breeding P-efficient soybean varieties through root trait selection.
Conventional breeding
During the 20th century, conventional breeding produced a vast number of varieties and hybrids that contributed immensely to higher grain yield, stability of harvests and farm income (Borlaug, 2000). Conventional breeding also plays an important role in the selection of new soybean varieties. Recently, conventional breeding methods (backcross breeding, recurrent selection, etc.) were employed to develop soybean varieties with superior root characteristics with a focus on root architecture and other important agronomic traits that enable better adaptation to acid soils (Yan et al., 2006). After being tested under various soil/climatic conditions through Chinese National Field Trials, seven new soybean varieties have been officially certified by the Chinese Ministry of Agriculture and now are being released to the farmers in nine provinces of southern China. These new varieties show substantial yield gains in low-P soils compared with conventional genotypes. They typically out-performed some local varieties two- to three-fold in both biomass and grain yield under low P conditions (Cheng et al., 2010).
Marker-assisted selection
The stock of DNA markers has been increasing year by year in various crop plants (Mackill et al., 1999; Brar, 2002). Developing strategies for using these markers in practical breeding has been one of the major research subjects in the past decade (Ishii and Yonezawa, 2007; Moose and Mumm, 2008). Due to the difficulty in directly measuring root traits and the significance of genotype–environment interactions, successful examples of using root-trait QTLs to facilitate genetic improvement in P efficiency remain scarce. In common bean, Liao et al. (2004), Beebe et al. (2006) and Ochoa et al. (2006) used QTL analysis to show the importance of basal roots and adventitious roots for P acquisition. Yan et al. (2004) identified multiple QTLs for H+ exudation, and root-hair density and length, which were associated with the QTLs for P efficiency in the field. In soybean, there are few reports on QTL analysis of P efficiency, and very few QTLs for root traits related to P efficiency have been identified (Li et al., 2005; Liang, 2007; Zhang et al., 2009). In one study, a population of 106 F9 RILs derived from a cross between BD2 and BX10, contrasting in both P efficiency and root architecture, was used for mapping and QTL analysis (Liang, 2007). Preliminary results indicated that most of the root traits showed a continuous, normal distribution of phenotypic traits, indicating that these were controlled by QTLs. Some of the root traits (such as root architecture, total root length and root surface area) within the populations were positively correlated with P-uptake efficiency of soybean plants. Some of these root traits had relatively high heritability, suggesting that these traits can be selected as breeding indices with large selective potential. Using simple sequence repeat markers as well as other types of molecular marker, Liang (2007) have successfully identified a number of QTLs associated with important root traits and/or P efficiency in soybean. Studies are underway to employ MAS for further improvement of P efficiency and other agronomic traits using the major QTLs identified.
Recently, QTLs controlling P deficiency tolerance using 152 RILs derived from a cross between P-stress-tolerant and -sensitive parents were mapped by Zhang et al. (2009). Five agronomic traits, namely shoot dry weight, root dry weight, total dry weight, P-use efficiency and P-acquisition efficiency, were used to map the QTLs associated with P efficiency. Thirty-four additive QTLs and eight pairs of epistatic QTLs were detected on 12 linkage groups and provided a basis for fine mapping and cloning of a number of P efficiency genes. The identified QTLs might be useful in improving the stress resistance of soybean against a complex nutritional disorder caused by P deficiency (Zhang et al., 2009). At the same time, breeders need to take into account the complexity of QTLs and to test the effects of individual loci in the targeted genetic background to obtain the expected phenotypes for the genes of interest (Yu et al., 2002). These markers might be able to play more direct roles in breeding programmes if they could be used explain a large portion of the genetic variability associated with P efficiency, and if the expression of the trait was not greatly affected by genotype–environment interactions.
Genetic engineering breeding
Conventional breeding programmes have sometimes failed for genetic reasons, such as sterility, incompatibility and lack of significant variability, and genetic engineering techniques have proven useful in overcoming some of these problems (Oldach et al., 2001; Doebley et al., 2006; Ulukan, 2009). In recent years, important successes in terms of soybean yield and quality, herbicide tolerance, pest and disease resistance, and other agronomic aspects have been achieved via genetic engineering (Nelson and Renner, 1999; Owen, 2000; De Ronde et al., 2004; Krishnan, 2005; MacRae et al., 2005; Pline-Srnic, 2005; Eckert et al., 2006; Tougou et al., 2006; Manavalan et al., 2009). However, few studies have reported on improvement of P efficiency in soybean by genetic engineering techniques (Chiera et al., 2004; Wang et al., 2009).
In one study, a transgenic approach was used to alter soybean seed phytate content by expressing a soybean phytase gene (GmPhy) during seed development to degrade accumulating phytic acid (IP6). IP6 levels in transgenic seeds decreased compared with control soybeans, and ectopic phytase expression during seed development reduced phytate content in soybean seeds, which therefore increased P availability in animal diets (Chiera et al., 2004).
In another study, the Arabidopsis purple APase gene PAP15 directed by an extracellular targeting sequence was successfully transformed into soybean (Wang et al., 2009). The transgenic lines exhibited significantly improved plant dry weight and plant P content when grown on sand culture containing phytate as the sole P source. Furthermore, the transgenic lines exhibited improved yields when grown on acid soils (Fig. 4). This study is the first report of the expression of a plant APase gene in soybean, which led to a significant improvement in P efficiency and plant yield (Wang et al., 2009).
Fig. 4.
Growth performance of transgenic soybean materials in low-P soils. Control: wild-type soybean; AtPAP15:OX: soybean lines over-expressing AtPAP15. The available P content of the tested soil was 15·39 mg kg−1 (photo by H. Liao).
CONCLUSIONS AND PERSPECTIVES
The above studies have helped us to gain new insight into the mechanisms of P efficiency in soybean that could be used in both the theory and the practice for genetic improvement of soybean varieties. Once clearly identified, these traits could be used for more efficient screening in controlled environments, or tagged with molecular markers and then incorporated into other varieties through MAS or transgenic approaches. Recent progress in plant molecular biology has provided both opportunities and challenges to plant biology research, resulting in the emergence of many new areas for study. Among these, root biology is a new frontier of plant biology for systematic studies of the many processes involved in plant nutrient mobilization, uptake, transport and translocation as well as their effects on plant growth, development and adaptability. Root biology will certainly facilitate the development of P-efficient soybean varieties. Further studies involving collaborations between plant breeders and physiologists, as well as additional applied and theoretical research are needed to develop crop species with enhanced P efficiency that will contribute to reducing the use of P fertilizers, expanding agriculture on low-P soils and achieving more sustainable agriculture.
ACKNOWLEDGEMENTS
We thank our colleagues Drs J. Tian, J. X. Wang and J. Zhao for helpful discussions and comments. This research was in part supported by the 7th International Symposium on Plant–Soil Interactions at Low pH, the National Natural Science Foundation of China and the McKnight Foundation Collaborative Crop Research Program.
LITERATURE CITED
- Beebe SE, Rojas-Pierce M, Yan XL, et al. Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Science. 2006;46:413–423. [Google Scholar]
- Borlaug N. Ending world hunger. The promise of biotechnology and the threat of antiscience zealotry. Plant Physiology. 2000;124:487–490. doi: 10.1104/pp.124.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar D. Molecular marker assisted breeding. In: Jain SM, Brar DS, Ahloowalia BS, editors. Molecular techniques in crop improvement. Dordrecht: Kluwer Academic Publishers; 2002. pp. 55–83. [Google Scholar]
- Cheng F, Cao G, Wang X, Zhao J, Yan X, Liao H. Identification and application of effective rhizobial strains for soybean on acid laterite soils in South China. Chinese Science Bulletin. 2008;53:2903–2910. [Google Scholar]
- Cheng F, Tu P, Yan X, Wang X, Liao H. Phosphorus nutrition characters for new soybean germplasms with phosphorus efficiency on acid red soils. Plant Nutrition and fertilizer Science. 2010;16:71–81. [Google Scholar]
- Chalk PM, Souza RF, Urquiaga S, Alves BJ, Boddey RM. The role of arbuscular mycorrhiza in legume symbiotic performance. Soil Biology & Biochemistry. 2006;38:2944–2951. [Google Scholar]
- Chiera J, Finer J, Grabau E. Ectopic expression of a soybean phytase in developing seeds of Glycine max to improve phosphorus availability. Plant Molecular Biology. 2004;56:895–904. doi: 10.1007/s11103-004-5293-6. [DOI] [PubMed] [Google Scholar]
- De Ronde J, Cress W, Krügerd G, Strasser R, Van Staden J. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. Journal of Plant Physiology. 2004;161:1211–1224. doi: 10.1016/j.jplph.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.) Plant Cell Environment. 1989;12:285–292. [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Dong D, Peng X, Yan X. Organic acid exudation induced by phosphorus deficiency and/or aluminum toxicity in two contrasting soybean genotypes. Physiologia Plantarum. 2004;122:190–199. [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science. 2007;12:474–481. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Eckert H, La Vallee B, Schweiger B, Kinney A, Cahoon E, Clement T. Co-expression of the borage Delta Δ6 desaturase and the Arabidopsis Delta Δ15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta. 2006;224:1050–1057. doi: 10.1007/s00425-006-0291-3. [DOI] [PubMed] [Google Scholar]
- Fageria NK, Baligar VC, Li YC. The role of nutrient efficient plants in improving crop yields in the twenty first century. Journal of Plant Nutrition. 2008;31:1121–1157. [Google Scholar]
- Foehse D, Claassen N, Jungk A. Phosphorus efficiency of plants. II. Significance of root radius, root hairs and cation anion balance for phosphorus influx in seven plant species. Plant and Soil. 1991;132:261–272. [Google Scholar]
- Gahoonia TS, Nielsen NE. Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Plant and Soil. 1997;98:177–182. [Google Scholar]
- Gahoonia TS, Nielsen NE. Root traits as tools for creating phosphorus efficient crop varieties. Plant and Soil. 2004a;260:47–57. [Google Scholar]
- Gahoonia TS, Nielsen NE. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant and Soil. 2004b;262:55–62. [Google Scholar]
- Ganry F, Diem HG, Wey J, Dommergues YR. Inoculation with Glomus mosseae improves N2 fixation by field-grown soybeans. Biology and Fertility of Soils. 1985;1:15–23. [Google Scholar]
- George TS, Richardson AE. Potential and limitations to improving crops for enhanced phosphorus utilization. In: White PJ, Hammond JP, editors. Ecophysiology of plant–phosphorus interactions. Dordrecht: Springer; 2008. pp. 247–270. [Google Scholar]
- George TS, Simpson RJ, Hadobas PA, Richardson AE. Expression of a fungal phytase gene in Nicotiana tabacum improves phosphorus nutrition of plants grown in amended soils. Plant Biotechnology Journal. 2005;3:129–140. doi: 10.1111/j.1467-7652.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Guo W. Cloning and functional analysis of expansin gene GmEXPB2 in soybean. 2008 PhD thesis, South China Agricultural University, Guangzhou. [Google Scholar]
- Guo W, Zhang L, Zhao J, Liao H, Zhuang C, Yan X. Identification of temporally and spatially phosphate-starvation responsive genes in Glycine max. Plant Science. 2008;175:574–584. [Google Scholar]
- Hayes JE, Richardson AE, Simpson RJ. Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biology and Fertility of Soils. 2000;32:279–286. [Google Scholar]
- Ibijbijen J, Urquiaga S, Ismaili M, Alves BJR, Boddey RM. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition and nitrogen fixation of three varieties of common beans. New Phytologist. 1996;134:353–360. [Google Scholar]
- Ishii T, Yonezawa K. Optimization of the marker-based procedures for pyramiding genes from multiple donor lines: II. Strategies for selecting the objective homozygous plant. Crop Science. 2007;47:1878–1886. [Google Scholar]
- Johnson JF, Vance CP, Allan DL. Phosphorus deficiency in Lupinus albus. Altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiology. 1996;112:31–41. doi: 10.1104/pp.112.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL. Organic acids in the rhizosphere – a critical review. Plant and Soil. 1998;205:25–44. [Google Scholar]
- Jones DL, Brassington DS. Sorption of organic acids in acid soils and its implications in the rhizosphere. European Journal of Soil Science. 1998;49:447–455. [Google Scholar]
- Jones DL, Darrah PR. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant and Soil. 1994;166:247–257. [Google Scholar]
- Jones DL, Dennis PG, Owen AG, van Hees PAW. Organic acid behavior in soils – misconceptions and knowledge gaps. Plant and Soil. 2003;248:31–41. [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Krishnan H. Engineering soybean for enhanced sulfur amino acid content. Crop Science. 2005;45:454–461. [Google Scholar]
- Kuang R, Liao H, Yan X, Dong Y. Phosphorus and nitrogen interactions in field-grown soybean as related to genetic attributes of root morphological and nodular traits. Journal of Integrative Plant Biology. 2005;47:549–559. [Google Scholar]
- Li M, Osaki M, Rao IM, Tadano T. Secretion of phytase from the roots of several plant species under phosphorus-deficient conditions. Plant and Soil. 1997;195:161–169. [Google Scholar]
- Li YD, Wang YJ, Tong YP, Gao JG, Zhang JS, Chen SY. QTL mapping of phosphorus deficiency tolerance in soybean (Glycine max L. Merr.) Euphytica. 2005;142:137–142. [Google Scholar]
- Liang Q. Genetic studies and SSR-based QTL analysis of root traits related to P efficiency in soybean. 2007 doi: 10.1093/aob/mcq097. PhD thesis, South China Agricultural University, Guangzhou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Ge Z, Yan X. Ideal root architecture for phosphorus acquisition of plants under water and phosphorus coupled stresses: from simulation to application. Chinese Science Bulletin. 2001a;46:1346–1351. [Google Scholar]
- Liao H, Rubio G, Yan X, Cao A, Brown K, Lynch JP. Effect of phosphorus availability on basal root shallowness in common bean. Plant and Soil. 2001b;232:69–79. [PubMed] [Google Scholar]
- Liao H, Yan XL, Rubio G, Beebe SE, Blair MW, Lynch JP. Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Functional Plant Biology. 2004;31:959–970. doi: 10.1071/FP03255. [DOI] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian L. Phosphorus and aluminum interactions in soybean in relation to Al tolerance: exudation of specific organic acids from different regions of the intact root system. Plant Physiology. 2006;141:674–684. doi: 10.1104/pp.105.076497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. Soybeans: chemistry, technology, and utilization. Gaithersburg, MD: Aspen Publishers Inc. Press; 1997. pp. 1–22. [Google Scholar]
- Liu L, Liao H, Wang X, Yan X. Regulation effect of soil P availability on mycorrhizal infection in relation to root architecture and P efficiency of Glycine max. Chinese Journal of Applied Ecology. 2008a;19:564–568. [PubMed] [Google Scholar]
- Liu L, Liao H, Wang X, Yan X. Adaptive changes of soybean genotypes with different root architectures to low phosphorus availability as related to phosphorus efficiency. Scientia Agricultura Sinica. 2008b;41:1089–1099. [Google Scholar]
- López-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. Phosphorus availability alters root architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramĺrez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Lung SC, Lim BL. Assimilation of phytate-phosphorus by the extracellular phytase activity of tobacco (Nicotiana tabacum) is affected by the availability of soluble phytate. Plant and Soil. 2006;279:187–199. [Google Scholar]
- Lung SC, Chan WL, Yip W, Wang L, Yeung EC, Lim BL. Secretion of beta-propeller phytase from tobacco and Arabidopsis roots enhances phosphorus utilisation. Plant Science. 2005;169:341–349. [Google Scholar]
- Lynch JP. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Root architecture and phosphorus acquisition efficiency in common bean. In: Lynch JP, Deikman J, editors. Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. Rockville, MD: American Society of Plant Physiologists; 1998. pp. 81–91. [Google Scholar]
- Lynch JP, Beebe SE. Adaptation of beans (Phaseolus vulgaris L.) to low phosphorus availability. HortScience. 1995;30:1165–1171. [Google Scholar]
- Lynch JP, van Beem J. Growth and architecture of seedling roots of common bean genotypes. Crop Science. 1993;33:1253–1257. [Google Scholar]
- Mackill DJ, Nguyen HT, Zhang JX. Use of molecular markers in plant improvement programs for rainfed lowland rice. Field Crops Research. 1999;64:177–185. [Google Scholar]
- MacRae TC, Baur ME, Boethel DJ, et al. Laboratory and field evaluations of transgenic soybean exhibiting high-dose expression of a synthetic Bacillus thuringiensis cry1A gene for control of Lepidoptera. Journal of Economic Entomology. 2005;98:577–587. doi: 10.1093/jee/98.2.577. [DOI] [PubMed] [Google Scholar]
- Manavalan LP, Guttikonda SK, Tran LSP, Nguyen HT. Physiological and molecular approaches to improve drought resistance in soybean. Plant and Cell Physiology. 2009;50:1260–1276. doi: 10.1093/pcp/pcp082. [DOI] [PubMed] [Google Scholar]
- Moose SP, Mumm RH. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiology. 2008;147:969–977. doi: 10.1104/pp.108.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Smith FW, Richardson AE. Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter promoter enables Arabidopsis to grow on phytate as a sole P source. Plant Science. 2003;165:871–878. [Google Scholar]
- Nelson KA, Renner KA. Weed management in wide- and narrow-row glyphosate-resistant soybean. Journal of Production Agriculture. 1999;12:460–465. [Google Scholar]
- Neumann G, Martinoia E. Cluster roots – an underground adaptation for survival in extreme environments. Trends in Plant Science. 2002;7:162–167. doi: 10.1016/s1360-1385(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Ochoa IE, Blair MW, Lynch JP. QTL analysis of adventitious root formation in common bean under contrasting phosphorus availability. Crop Science. 2006;46:1609–1621. [Google Scholar]
- Oldach KH, Becker D, Lörz H. Heterologous expression of genes mediating enhanced fungal resistance in transgenic wheat. Molecular Plant–Microbe Interactions. 2001;14:832–838. doi: 10.1094/MPMI.2001.14.7.832. [DOI] [PubMed] [Google Scholar]
- Owen MDK. Current use of transgenic herbicide-resistant soybean and corn in the USA. Crop Protection. 2000;19:765–771. [Google Scholar]
- Pline-Srnic W. Technical performance of some commercial glyphosphate-resistant crops. Pest Management Science. 2005;61:225–234. doi: 10.1002/ps.1009. [DOI] [PubMed] [Google Scholar]
- Raghothama KG. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. The Plant Journal. 2001;25:641–649. doi: 10.1046/j.1365-313x.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- Sample EC, Soper RJ, Racz GJ. Reaction of phosphate fertilizers in soils. In: Khasawneh FE, Sample EC, Kamprath EJ, editors. The role of phosphorus in agriculture. Madison, WI: American Society of Agronomy; 1980. pp. 263–310. [Google Scholar]
- Shen H, Chen J, Wang Z, et al. Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. Journal of Experimental Botany. 2006;57:1353–1362. doi: 10.1093/jxb/erj111. [DOI] [PubMed] [Google Scholar]
- Smith FA, Grace EJ, Smith SE. More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist. 2009;182:347–358. doi: 10.1111/j.1469-8137.2008.02753.x. [DOI] [PubMed] [Google Scholar]
- Smith SE. Soil microbes and plants – raising interest, mutual gains. New Phytologist. 2002;156:142–144. doi: 10.1046/j.1469-8137.2002.00514.x. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. London: Academic Press; 2008. [Google Scholar]
- Stevenson FJ. Cycles of soil: carbon, nitrogen, phosphorus, sulfur, micronutrients. New York: John Wiley & Sons; 1986. pp. 350–400. [Google Scholar]
- Strobel BW. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution – a review. Geoderma. 2001;99:169–198. [Google Scholar]
- Ström L, Owen AG, Godbold DL, Jones DL. Organic acid behaviour in a calcareous soil: sorption reactions and biodegradation rates. Soil Biology & Biochemistry. 2001;33:2125–2133. [Google Scholar]
- Ström L, Owen AG, Godbold DL, Jones DL. Organic acid behaviour in a calcareous soil implications for rhizosphere nutrient cycling. Soil Biology & Biochemistry. 2005;37:2046–2054. [Google Scholar]
- Tang J, Mborehal I, She L, et al. Nutritional effects of soybean root architecture in a soybean/maize intercropping system. Scientia Agricultura Sinica. 2005;38:1196–1203. [Google Scholar]
- Tawaraya K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Science and Plant Nutrition. 2003;49:655–668. [Google Scholar]
- Tian J, Liao H, Wang X, Yan X. Phosphorus starvation-induced expression of leaf acid phosphatase isoforms in soybean. Acta Botanica Sinica. 2003;45:1037–1042. [Google Scholar]
- Tougou M, Furutani N, Yamagishi N, Shizukawa Y, Takahata Y, Hidaka S. Development of resistant transgenic soybeans with inverted repeat-coat protein genes of soybean dwarf virus. Plant Cell Reports. 2006;25:1213–1218. doi: 10.1007/s00299-006-0186-6. [DOI] [PubMed] [Google Scholar]
- Ulukan H. The evolution of cultivated plant species: classical plant breeding versus genetic engineering. Plant Systematics and Evolution. 2009;280:133–142. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Liao H, Yan X, Zhuang B, Dong Y. Genetic variability for root hair traits as related to phosphorus status in soybean. Plant and Soil. 2004;261:77–84. [Google Scholar]
- Wang X, Wang Y, Tian J, Lim B, Yan X, Liao H. Overexpressing AtPAP15 enhances phosphorus efficiency in soybean. Plant Physiology. 2009;151:233–240. doi: 10.1104/pp.109.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Harrison M, Wang ZY. Transgenic expression of a novel M. truncatula phytase gene results in improved acquisition of organic phosphorus by Arabidopsis. Planta. 2005;222:27–36. doi: 10.1007/s00425-005-1511-y. [DOI] [PubMed] [Google Scholar]
- Xiao K, Katagi H, Harrison M, Wang ZY. Improved phosphorus acquisition and biomass production in Arabidopsis by transgenic expression of a purple acid phosphatase gene from M. truncatula. Plant Science. 2006;170:191–202. [Google Scholar]
- Yan X. The roots of phosphorus-efficient soybean: theories and practices. In: Li CJ, Zhang FS, Dobermann A, et al., editors. Plant nutrition for food security, human health and environmental protection. Beijing: Tsinghua University Press; 2005. pp. 36–37. [Google Scholar]
- Yan X, Liao H, Beebe SE, Blair MW, Lynch JP. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant and Soil. 2004;265:17–29. [Google Scholar]
- Yan X, Wu P, Ling H, Xu G, Xu F, Zhang Q. Plant nutriomics in China: an overview. Annals of Botany. 2006;98:473–482. doi: 10.1093/aob/mcl116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liao H, Nian H. Root breeding for better plant nutrient efficiency on acid soils. In: Liao H, Yan X, Kochian L, editors. Plant–soil interactions at low pH: a nutriomic approach. Guangzhou: South China University of Technology Press; 2009. pp. 5–6. [Google Scholar]
- Yu SB, Li JX, Xu CG, Tan YF, Li XH, Zhang Q. Identification of quantitative trait loci and epistatic interactions for plant height and heading date in rice. Theoretical and Applied Genetics. 2002;104:619–625. doi: 10.1007/s00122-001-0772-5. [DOI] [PubMed] [Google Scholar]
- Zhang D, Cheng H, Geng L, et al. Detection of quantitative trait loci for phosphorus deficiency tolerance at soybean seedling stage. Euphytica. 2009;167:313–322. [Google Scholar]
- Zhao J, Fu J, Liao H, et al. Characterization of root architecture in an applied core collection for phosphorus efficiency of soybean germplasm. Chinese Science Bulletin. 2004;49:1611–1620. [Google Scholar]