Abstract
Background
Aluminium (Al) toxicity is the most important soil constraint for plant growth and development in acid soils. The mechanism of Al-induced inhibition of root elongation is still not well understood, and it is a matter of debate whether the primary lesions of Al toxicity are apoplastic or symplastic.
Scope
The present review focuses on the role of the apoplast in Al toxicity and resistance, summarizing evidence from our own experimental work and other evidence published since 1995.
Conclusions
The binding of Al in the cell wall particularly to the pectic matrix and to the apoplastic face of the plasma membrane in the most Al-sensitive root zone of the root apex thus impairing apoplastic and symplastic cell functions is a major factor leading to Al-induced inhibition of root elongation. Although symplastic lesions of Al toxicity cannot be excluded, the protection of the root apoplast appears to be a prerequisite for Al resistance in both Al-tolerant and Al-accumulating plant species. In many plant species the release of organic acid anions complexing Al, thus protecting the root apoplast from Al binding, is a most important Al resistance mechanism. However, there is increasing physiological, biochemical and, most recently also, molecular evidence showing that the modification of the binding properties of the root apoplast contributes to Al resistance. A further in-depth characterization of the Al-induced apoplastic reaction in the most Al-sensitive zone of the root apex is urgently required, particularly to understand the Al resistance of the most Al-resistant plant species.
Keywords: Aluminium, aluminum, resistance, apoplast, cell wall, pectin, root elongation
INTRODUCTION
It has been estimated that soils of 30 % of the ice-free land area of the world are acid, where crop productivity is limited by a range of growth-limiting factors related to soil acidity (von Uexküll and Mutert, 1995). Aluminium (Al) toxicity is the most important soil constraint for plant growth and development in acid soils. It is now well understood that the toxicity of Al in aquatic and terrestrial systems is not correlated with total Al concentrations (7 % of mineral soils), but is a function of the concentration of the biologically active fraction in solution (Lewis, 1989). Among the soluble Al species in the soil solution, the inorganic monomeric forms, particularly Al3+, are considered the most important (Kinraide et al., 1992). Organic and inorganic Al complexes are regarded as not phytotoxic, or considerably less so (Kerven et al., 1989).
As early as 1918, Al toxicity was implicated as the main cause of the inhibition of root growth of barley (Hordeum vulgare) and rye (Secale cereale) in an acid soil (Hartwell and Pember, 1918). Aluminium-induced inhibition of root elongation can be measured within hours or less after the roots have been exposed to excess Al supply (Llugany et al., 1995; Blamey et al., 2004, 2005). Ryan et al. (1993) were the first who unequivocally demonstrated the role of the root apex in the perception of Al toxicity in maize (Zea mays). They also clearly refuted the hypothesis put forward by Bennet et al. (1985) that the root cap plays a decisive role in the expression of Al-induced inhibition of root elongation. In a more refined methodological approach, Sivaguru and Horst (1998) presented evidence that in the Al-sensitive maize cultivar ‘Lixis’ the distal part of the transition zone (DTZ, 1–2 mm) is the most Al-sensitive apical root zone in maize. Application of Al only to the DTZ reduced cell elongation in the elongation zone (EZ) to the same extent as application to the entire 10 mm root apex. However, application of Al only to the EZ did not inhibit root elongation, needing signal transduction as proposed by Bennet et al. (1985) between the DTZ and the EZ (Kollmeier et al., 2000). These authors also provided evidence that basipetal auxin transport might be implicated in the signalling. The role of ethylene-mediated inhibition of polar auxin transport in Al-induced inhibition of root elongation has recently been substantiated in Arabidopsis (Sun et al., 2010). The importance of the TZ (1–2 mm) as a main target of Al was also confirmed in common bean (Phaseolus vulgaris) by Rangel et al. (2007). However, in contrast to maize, in common bean Al also reduced root elongation when applied only to the EZ, the zone initially impacted by Al in a digital microscopy study with mungbean (Vigna radiata) (Blamey et al., 2004).
Treatment with Al as well as with other metals causes transverse ruptures to develop in sub-apical regions of the root through the breaking and separation of the rhizodermis and outer cortical layers from the inner cortical cell layers (Blamey et al., 2004; Kopittke et al., 2008). It was proposed that these ruptures relate to the binding of Al to the cell wall thus increasing cell wall rigidity and decreasing elasticity. The differences between metals could be related to the strength with which they bind to the cell wall (Kopittke et al., 2009) in agreement with Kinraide (2009). However, the relationship between these ruptures and inhibition of root elongation is not well understood to date (Ryan et al., 1993; Kopittke et al., 2008).
Another sensitive indicator of Al injury in roots is the induction of callose synthesis (Wissemeier et al., 1987; Staß and Horst, 2009), particularly in the root apex (Wissemeier and Horst 1995; Sivaguru et al., 2006). Al-induced callose formation is an indicator of Al sensitivity and a reliable parameter for the classification of genotypes of different plant species in terms of Al resistance (Wissenmeier et al., 1992; Horst et al., 1997). Collet and Horst (2001) developed a rapid non-destructive screening procedure for maize cultivars for adaptation to acid soils with high Al supply, and Eticha et al. (2005c) successfully used Al-induced callose formation to study inheritance of Al resistance and adaptation to an acid, Al-toxic soil, using a 13 × 13 diallel of maize cultivars of largely different origin.
Much progress has been made during recent years in the physiological and molecular understanding of Al exclusion (for reviews, see Matsumoto 2000; Kochian et al., 2004; Zheng and Yang, 2005; Delhaize et al., 2007; Ma, 2007; Panda and Matsumoto, 2007). In agreement with Ryan and Delhaize (2010), in this review we use the expression ‘Al resistance’ consistently as a plant property which allows a plant to grow with little or no injury with elevated Al supplies. The plant mechanism conferring resistance may be exclusion from binding to and uptake by the roots (Al exclusion) or Al tolerance (Al binding and uptake without Al injury). In spite of the progress made, the Al exclusion mechanisms of some of the most Al-resistant plant species such as rice (Oryza sativa) (Yang et al., 2008; Huang et al., 2009), rye (Shi et al., 2009) and signalgrass (Brachiaria decumbens) (Wenzl et al., 2001; Watanabe et al., 2006) are still far from being understood. Furthermore, the primary target site of Al phytotoxicity leading to inhibition of root elongation is still not well defined. Indeed, the relative importance of symplastic vs. apoplastic lesions of Al toxicity remains a matter of debate, and the role of cell wall properties in Al resistance is not widely acknowledged. Horst (1995), Rengel (1996) and Blamey (2003) focused their attention on the role of the apoplast in Al toxicity and Al resistance. The additional experimental evidence since then justifies an updated consideration of the interactions of Al with apoplastic components. This review summarizes the current understanding of the role of the root apoplast in Al-induced inhibition of root elongation and in Al resistance of plants.
AL INTERACTIONS WITH APOPLASTIC BINDING SITES LEAD TO Al INJURY
Cell wall
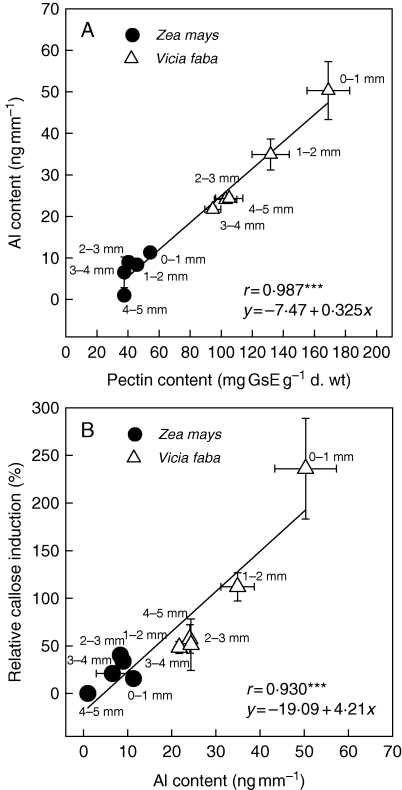
Aluminium is accumulated by roots, with a rapid initial phase and a lower rate thereafter (Zhang and Taylor, 1989, 1990). The rapid initial phase reflects the binding of Al in the apoplast, which has been demonstrated using fractionated extraction methods (Wang et al., 2004; Rangel et al., 2009a), surgical separation of the cell wall and symplast in the giant algae Chara corallina (Rengel and Reid, 1997; Taylor et al., 2000) and also in situ localization techniques such as X-ray microanalysis (Marienfeld and Stelzer, 1993) and secondary ion mass spectrometry (SIMS) (Horst et al., 2007). The primary binding site of Al3+ in the apoplast is probably the pectic matrix, with its negatively charged carboxylic groups having a particularly high affinity for Al3+ (Blamey et al., 1990; Chang et al., 1999). Short-term Al accumulation by roots is closely related to the pectin content and may explain the differences in Al contents between apical root sections of maize and faba bean (Vicia faba) (Fig. 1). It appears that the Al content and thus the binding of Al to the pectic matrix are closely positively correlated to Al-induced callose formation and thus Al sensitivity (Horst et al., 1999).
Fig. 1.
Relationships between (A) pectin and Al content, and (B) Al content and relative callose induction (digitonin = 100) of root sections of maize (Zea mays) and faba bean (Vicia faba). Roots were incubated for 3 h in nutrient solution ± 50 µm Al or 10 µm digitonin at pH 4·3. ***Significant at P < 0·001. From Horst et al. (2007).
The role of the pectin content in Al accumulation and Al sensitivity has been further substantiated using different approaches modifying the pectin content of intact maize plants (Horst et al., 1999) and maize cell suspension cultures (Schmohl and Horst, 2000). In fact, the factor responsible for Al binding to pectin is not the pectin content alone but its negative charge determined by its degree of methylation (DM) (Eticha et al., 2005a), which is controlled by pectin methylesterase (PME) (Bordenave, 1996; Gerendás, 2007). Schmohl et al. (2000) provided evidence that Al accumulation and Al sensitivity of maize cell suspension cells is modulated by the DM of their cell walls. Also, short-term treatment of intact maize roots with PME enhanced Al accumulation and Al-induced inhibition of root elongation (Horst et al., 2007).
Accumulation of Al in the cell wall can also be expected on the basis of the measured low rates of transport of Al through the plasma membrane into the symplast of the model plant C. corallina (Rengel and Reid, 1997; Taylor et al., 2000). However, both studies as well as those by Marienfeld et al. (2000) in maize also show that a rapid transfer of Al from the apoplast to the symplast does occur. Using different techniques Tice et al. (1992), Lazof et al. (1994) and Vázques et al. (1999) demonstrated the accumulation of Al in the symplast in wheat (Triticum aestivum), soybean (Glycine max) and maize, respectively, leading to a rather uniform cellular distribution of Al or even accumulation of Al in the symplast at the expense of the cell wall. However, we have challenged the results based on cellular Al localization using morin, because morin is unable to bind to cell wall-bound Al (Eticha et al., 2005b). The rapid uptake of Al into the symplast (Ma et al., 1998; B. Klug and W. J. Horst, Leibniz University Hannover, Germany, unpubl. res.), transfer to the central cylinder, xylem transport to the shoots and accumulation of Al in the vacuoles of the leaves are a typical feature of Al accumulator plant species such as hortensia (Hydrangea macrophylla) (Ma et al., 1997; Naumann and Horst, 2003), buckwheat (Fygopyrum esculentum) (Ma et al., 1998) and tea (Camellia sinensis) (Carr et al., 2003). The reasons for the difference in mobility of Al between Al excluders (most plant species) and includers are not yet understood (Jansen et al., 2002; Klug and Horst, 2010) and the role of Al tolerance in Al resistance is still unresolved (see below).
Cell elongation requires cell turgor pressure driving expansion, the release of cell wall components from the symplast to the apoplast for cell wall synthesis, and the formation and cleavage of Ca bonds with the pectic matrix controlling cell wall extensibility (Boyer, 2009). It has been shown that Al treatment reduces root cell wall extensibility (Tabuchi and Matsumoto, 2001; Ma et al., 2004). Strong binding of Al (and other metals) to the pectic matrix may prevent cell wall extension physically and/or physiologically by decreasing the effectiveness of cell wall-loosening enzymes (Wehr et al., 2004).
Plasma membrane
Aluminium rapidly affects the properties not only of the cell wall but also those of the plasma membrane (Ishikawa and Wagatsuma, 1998). Interaction of Al with membrane lipids and proteins (Akeson et al., 1989; Caldwell, 1989; Jones and Kochian, 1997) induces modifications of its structural properties such as fluidity and permeability (Vierstra and Haug, 1978; Wagatsuma et al., 2005a; Khan et al., 2009). Such a structural change in membrane properties is one of the prerequisites, in addition to an increase in the cytosolic Ca2+ activity, for the induction of callose synthesis (Kauss et al., 1989), the most sensitive response of root apices to Al (see above). Binding of Al to the plasma membrane alters its surface negativity (Kinraide et al., 1992; Kinraide, 2006), as shown by Ahn et al. (2001, 2004) in squash (Cucurbita pepo) and wheat. Also, in most studies, Al supply rapidly induced membrane depolarization (Lindberg et al., 1991; Olivetti et al., 1995) specifically in the most Al-sensitive root zone (the DTZ) (Sivaguru et al., 1999a). This may be related to inhibition of the H+-ATPase activity (Ahn et al. 2001) which in turn may lead to a disturbance of the H+ homeostasis in the cytosol (Lindberg and Strid, 1997; Plieth et al., 1999). These changes in plasma membrane properties by Al affect its ion transport properties. In soybean, Al treatment led to a rapid decrease of K+ efflux without changing K+ influx (Horst et al., 1992; Staß and Horst, 1995). In wheat the Al-enhanced release of malate was charge-balanced by a release of K+ (Ryan et al., 1995), which is in agreement with our results in maize where Al-induced citrate release through an anion channel was observed without affecting the K+ outward rectifier (Kollmeier et al., 2001).
Aluminium-induced impairment of membrane functions may be related to Al-enhanced oxidative stress through the formation of reactive oxygen species (ROS) leading to lipid peroxidation (Yamamoto et al., 1997, 2003; Jones et al., 2006) and protein oxidation (Boscolo et al., 2003). Oxidative stress genes are among the identified genes that are particularly expressed after Al treatment (Richards et al., 1998; Ezaki et al., 2005). Transformation of Arabidopsis thaliana with such genes conferred Al resistance (Ezaki et al., 2001). However, oxidative stress in roots appears not to be the primary cause for Al-induced inhibition of root elongation (Yamamoto et al., 2001), because, in most cases, it could be observed only after prolonged Al treatment (Cakmak and Horst, 1991; Maltais and Houde, 2002; Boscolo et al., 2003; Liu et al., 2008). However, sustained Al resistance may require protection mechanisms against oxidative stress.
In spite of these changes in plasma membrane structure and function it needs to be stressed that there is no indication that at physiological Al concentrations a severe disruption of plasma membrane functions is a prerequisite for inhibition of root elongation and callose formation (Horst et al., 1992). It appears that Al triggers signal transduction pathways leading to the observed symplastic physiological disorders. In this regard, the effect of Al on cytosolic Ca2+ seems to play a crucial role (Rengel and Zhang, 2003; Jones et al., 2006). An increase in cytosolic Ca2+ as an immediate response to Al treatment has been demonstrated in a range of plant species (Jones et al., 1998; Zhang and Rengel, 1999; Ma et al., 2002). The source of Ca2+ is probably the apoplastic Ca2+ pool, because Ca2+ bound in the apoplast is liberated by Al3+ and the change of the plasma membrane potential results in an activation of Ca2+ channels. However, the triggering by Al of a release of Ca2+ from symplastic Ca2+ pools cannot be excluded (Rengel and Zhang, 2003). Increasing cytosolic Ca2+ can explain two cellular distortions: callose formation and disorganization of the cytoskeleton (Rengel and Zhang, 2003). An increase in cytosolic Ca2+ is one of the prerequisites for the induction of callose synthesis by different elicitors (Kauss et al., 1989; Staß and Horst, 2009). Aluminium-induced alterations of the cytoskeleton have been reported by Blancaflor et al. (1998), Sivaguru et al. (1999b), Schwarzerova et al. (2002) and Frantzios et al. (2005). Amenós et al. (2009) also observed that Al resulted in disorganized arrangements of actin filaments in the stele cells of the TZ of maize root. Similarly, gene expression of actin and profilin was inhibited by Al (Zhang et al., 2007). Profilin, an actin-binding protein, regulates the polymerization of actin filaments and plays a significant role in cell elongation (Ramachandran et al., 2000). Although a direct effect of cytosolic Al on the cytoskeleton cannot be ruled out, an interaction of apoplastic Al with the cell wall–plasma membrane–cytoskeleton continuum appears more likely (Horst et al., 1999; Sivaguru et al., 2000b).
MODIFICATION OF APOPLASTIC AL BINDING MODULATES Al TOXICITY
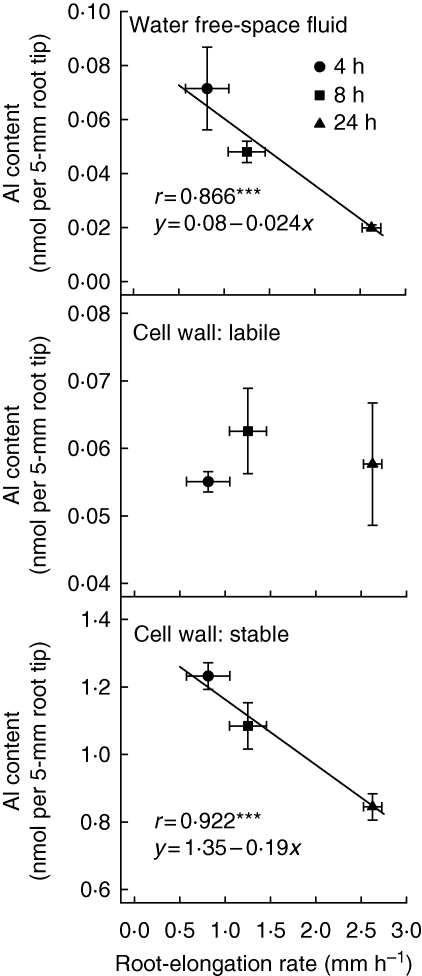
Aluminium accumulated in the root apoplast modifies cell wall composition and properties. Cellulose synthesis was inhibited in favour of callose synthesis in barley (Teraoka et al., 2002), and Al stress has been demonstrated to increase cell wall pectin content in a number of plant species such as squash (Van et al., 1994), maize (Eticha et al., 2005a), rice (Yang et al., 2008) and common bean (Rangel et al., 2009a). Increased pectin content caused by Al treatment has been interpreted by some researchers as a tolerance mechanism (Van et al., 1994), since the pectic substances bind or chelate Al3+ ions via their free carboxyl groups, resulting in cross-linking of pectin molecules (Klimashevskii and Dedov, 1975) and leading to detoxification of Al. However, there is increasing evidence that binding of Al to pectins appears to be more closely related to Al sensitivity since the Al-induced increase in pectin content of Al-sensitive cultivars was greater than that of Al-resistant cultivars (Eticha et al., 2005a; Yang et al., 2008). Also, in an attempt to explain the recovery from the initial (4 h treatment) Al-induced inhibition of root elongation in an Al-resistant common bean cultivar, Rangel et al. (2009a) related different cellular Al fractions to Al-induced cell elongation. They showed that recovery of root growth was closely negatively related to free apolastic and particularly strongly bound cell wall Al (Fig. 2). This suggests that the strong binding of Al to the pectic matrix of the cell wall is a main factor in Al toxicity and not a resistance mechanism in common bean. In contrast to the stably bound cell wall Al fraction, there was no indication that the labile bound (citrate-exchangeable) Al fraction was related to Al-induced inhibition of root elongation. This was unexpected, because in maize this fraction appeared to contribute to explaining silicon (Si)-mediated amelioration of Al toxicity (Wang et al., 2004). However, there seems to be a principal difference between monocots and dicots in Al binding to cell walls, which is not surprising given the difference in cell wall composition (Carpita and Gibeaut, 1993; Sarkar et al., 2009). This is well illustrated by the fact that treatment of cell walls with 50 mm BaCl2 removed about 20 % of the cell wall-bound Al in maize (Wang et al., 2004), and nearly all Al adsorbed on wheat cell walls could be exchanged with 2·5 mm CaCl2 (Zheng et al., 2004). In contrast, BaCl2 was unable to exchange any Al in common bean even after only short-term Al treatment (Staß et al., 2007). Overall, this clearly supports the view that the main role of Al-induced release of organic acid anions into the apoplast is the prevention of Al from binding to the pectic matrix. Wehr et al. (2003) showed that citrate and malate were able to remove Al from artificial Al–pectate gels, suggesting that exudation of organic acids would remove Al bound to pectin and this could alleviate toxicity. However, the decrease in the Al content of the stably bound cell wall Al fraction with increasing Al treatment duration, as shown in Fig. 2, by root-released citrate appears to be improbable because this fraction is defined as citrate non-exchangeable. It thus appears that once Al is firmly bound it is unlikely to be released by the citrate exuded from the cells, unless the citrate concentration in the apoplast is much higher than the concentration used for the exchange (33 mm). Therefore, it is more likely that citrate released into the apoplast reduces the binding of Al in the apoplast by complexing Al and decreasing the strength of Al binding, thus preventing the strong binding of Al to the cell wall (Zheng et al., 2004).
Fig. 2.
Relationship between root elongation rate and the Al contents of three apoplastic fractions in 5 mm root tips of common bean genotype Quimbaya (Al-resistant) grown in a simplified nutrient solution containing 0·5 mm CaCl2, 0·5 mm KCl, 8 µm H3BO3 and 20 µm Al for up to 24 h, pH 4·5. ***Significant at P < 0·001. From Rangel et al. (2009a).
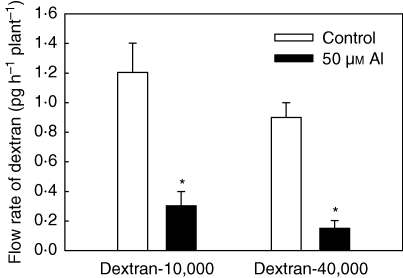
The Al-induced modifications of structural properties of the cell wall (Schildknecht and Vidal, 2002) affect the mobility of solutes in the apoplast of the root cortex. Schmohl and Horst (2002) demonstrated that the release of proteins in general, and acid phosphatase in particular, and pectins was inhibited by Al in maize root apices and maize cell suspension cultures. These effects were attributed to a reduction of cell wall porosity by Al binding in the cell wall. However, these results can also be explained by a lower permeability of the plasma membrane for macromolecules. More convincing evidence of an inhibition of the apoplastic solute bypass flow in maize root apices was provided by Sivaguru et al. (2006). They showed accumulation of fluorescent probes with mol. wts of 524 (8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt), 3000, 10 000 and 40 000 (neutral dextran–Texas red conjugates) in the outer cortical cells, especially in the DTZ, and inhibition of transfer of these solutes to the xylem and finally the shoot (Fig. 3). Water flow was not affected, in contrast to the expectations expressed by Blamey et al. (1993) showing a strong reduction by Al of water flow through an artificial pectate membrane. Since the inhibition of callose synthesis by pre-treatment of the roots with 2-deoxy-d-glucose (DDG) prior to Al treatment partially alleviated the Al-induced inhibition of solute bypass flow (Sivaguru et al., 2006), it is assumed that callose deposition contributes not only to the inhibition of cell to cell trafficking of solutes through plasmodesmata (Sivaguru et al., 2000a) but also to the apoplastic bypass flow in root cortical cell walls.
Fig. 3.
Effect of Al on the flow rate of dextran-10,000-TR and dextran-40,000-TR in root xylem exudates of maize cv. Lixis. Al and fluorescent tracers were applied to the 1 cm root apex for 2 h including the 1 h exudate collection period. Values are means of 8–10 independent replicates ±s.e. Means with * indicate significant differences at P < 0·05 (Tukey test). From Sivaguru et al. (2006).
COMPLEXATION OF AL IN THE APOPLAST CONFERS GENOTYPIC Al RESISTANCE BY REDUCING INTERACTIONS BETWEEN Al AND CELL WALL COMPONENTS
It is generally agreed that the release of Al-complexing solutes, particularly organic acid anions, in the Al-sensitive apical root zone is the most effective way to reduce the impact of Al on apoplastic functions (Ma et al., 2001; Ryan et al., 2001; Delhaize et al., 2007). Using the patch–clamp technique, it is well established that the Al-induced release of malate in wheat (Ryan et al., 1997; Zhang et al., 2001) and of citrate in maize (Kollmeier et al., 2001; Piñeros and Kochian, 2001) is mediated by plasma membrane anion channels. The frequency and magnitude of Al-induced anion currents are greater in Al-resistant than in Al-sensitive cultivars (Kollmeier et al., 2000; Zhang et al., 2001). The genes encoding these anion channel proteins have been identified and characterized in several plant species (see the review by Delhaize et al., 2007).
The role of the metabolism of organic acids in Al resistance is still a matter of discussion. Most studies have shown no clear relationship between the root content and release of organic acid anions (Ryan et al., 2001). Also, the activities of enzymes involved in the synthesis of organic acids did not differ significantly between Al-resistant and Al-sensitive genotypes. These findings and others led Ryan and Delhaize (2010) to suggest convergent evolution of Al resistance in Al-excluder plant species through mutation of transport proteins to organic acid anion permeases. However, based on a detailed study on release from and content of specific 1 mm apical root sections, Kollmeier and Horst (2001) showed that an Al-sensitive cultivar was not capable of maintaining the level of citrate in the apical root sections in spite of a lower citrate release rate. This was in agreement with a general trend of Al-enhanced activities of enzymes involved in citrate synthesis such as NAD-malate dehydrogenase (MDH) and phosphoenolpyruvate decarboxylase (PEPC), but not citrate synthase (CS), in the Al-resistant cultivar and of citrate-degrading aconitase in the Al-sensitive cultivar (Kollmeier and Horst, 2001). Also, sustained recovery from Al stress through citrate exudation in the Al-resistant common bean genotype Quimbaya after 24 h Al treatment relied on restoring the internal citrate pool and the constitutively high activity of CS fuelled by high PEPC activity (Rangel et al., 2009b). In the Al-sensitive genotype VAX-1 the citrate exudation and, thus, Al exclusion and root elongation could not be maintained, resulting in an exhaustion of the internal citrate pool and decreased CS activity.
There was no difference between the genotypes in the upregulation of MATE genes coding for citrate permeases (Eticha et al., 2010). Further evidence for an involvement of enhanced organic acid synthesis and reduced degradation in Al resistance comes from studies using transgenic plants with modified organic acid metabolism. Al-induced citrate exudation driven by Al-inducible expression of mitochondrial CS enhancing Al resistance in Paraserianthes falcaria, a leguminous tree, was reported: Al treatment increased the accumulation of mitochondrial CS transcripts, its activity and gene expression (Osawa and Kojima 2006). The over-expression of the mitochondrial CS gene from Citrus junos conferred Al resistance in Nicotiana benthaminana (Deng et al., 2006). Similarly, a successful transformation of several independent tobacco lines with rice mitochondrial CS resulted in increased citrate efflux and greatly enhanced Al resistance (Han et al., 2009). Not only the over-expression of CS, but also that of MDH (Tesfaye et al., 2001) and PEPC (Osaki et al., 2001), was reported to enhance Al resistance of plants. A role for PEPC in Al resistance of soybean genotypes through citrate exudation has also been convincingly demonstrated by Ermolayev et al. (2003). It thus appears that the maintenance of cytosolic organic acid anion concentrations and their release into the root tip apoplast through activation of anion permeases are both key factors for Al resistance in some plant species.
In addition to organic acid anions the release into the apoplast of polypeptides (Basu et al. 1999) and phenols (Heim et al., 1999; Kidd et al., 2001) may also be involved in genotypic Al resistance in wheat and maize, respectively.
MODIFICATION OF Al BINDING PROPERTIES OF THE APOPLAST CONFERS GENOTYPIC Al RESISTANCE
Negativity of the root apoplast
As shown above, Al binds readily to negative binding sites of the cell wall and the plasma membrane in the most Al-sensitive zones of the root apex. Since this may lead to enhanced transport of Al into the symplast and/or to impairment of root growth and functions (see above), it cannot only be expected, but has to be postulated, that reduced binding of Al in the apoplast is a prerequisite for Al resistance. Kinraide et al. (1992) were able to explain inhibition of root elongation by Al3+ in the presence of competing cations, including protons, on the basis of the computed cation distribution on a negatively charged root membrane surface. Blamey et al. (1992) and Grauer and Horst (1992) came to comparable conclusions based on similar but conceptually different approaches. A lower root cation exchange capacity as a measure of cell wall negativity has been reported in plant species adapted to acid soils with high Al supply (Blamey et al., 1990; Büscher et al., 1990). However, across a large range of plant species a clear relationship between root cation exchange capacity and Al resistance does not exist (Grauer, 1992).
The negativity of the cell wall depends mainly on the pectin content and its DM. Across all plant species studied so far, there is no consensus on differences in constitutive pectin contents of plants with regards to Al resistance. Without Al supply, Al-resistant and Al-sensitive maize cultivars did not differ in pectin content in the 5 mm root apex (Eticha et al., 2005a). However, in rice, the pectin content of the root apex in the Al-resistant cultivars was lower than in Al-sensitive cultivars (Yang et al., 2008). In common bean the initial (4 h Al supply) high Al sensitivity and Al accumulation by roots of the Al-resistant cultivar (Quimbaya) prior to the induction of citrate exudation (Rangel et al., 2009b) was related to a higher unmethylated pectin content in the 5 mm root tip (Rangel et al., 2009a).
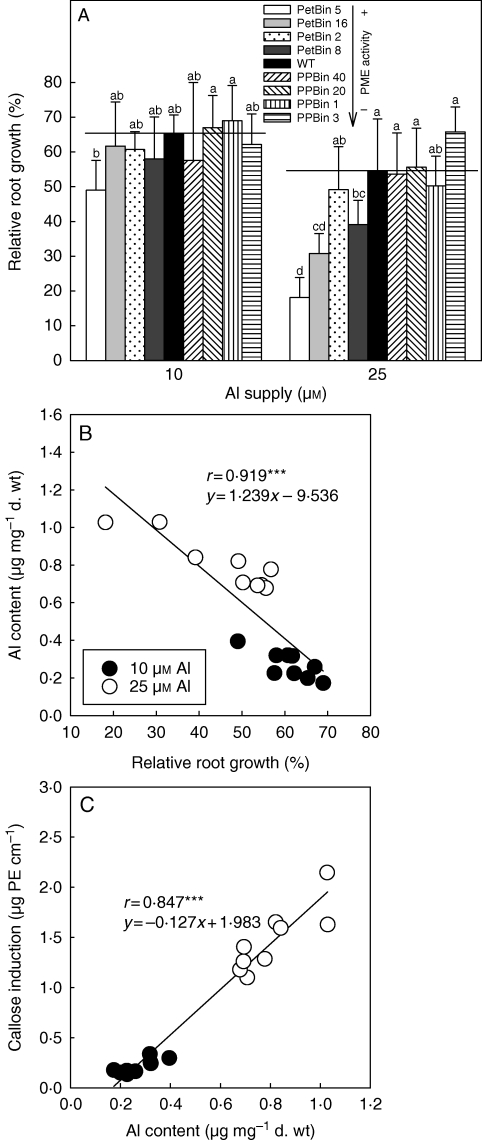
A modulating role for the DM of root cell walls in Al resistance is most convincingly supported by the comparison of potato transformants differing in the expression of PME from Petunia inflata (Schmohl et al., 2000): transformants with higher PME expression accumulated more Al, produced more callose and were more inhibited in root growth when exposed to Al than the wild type (Fig. 4). Applying a pectin immunolocalization method to root tips, Eticha et al. (2005a) demonstrated the importance of the DM of cell wall pectin for differential Al resistance of two maize cultivars. The cultivars did not differ in pectin content but in DM: the Al-sensitive cultivar had lower DM and consequently accumulated more Al and experienced more severe Al injury compared with the Al-resistant maize cultivar. Similarly, in rice (Yang et al., 2008), root tip cell wall PME activity was constitutively higher in the Al-sensitive cultivar than in the Al-resistant cultivar. Immunolocalization of pectins showed a higher proportion of demethylated pectins in the Al-sensitive cultivar, indicating a higher proportion of free pectic acid residues in the cell walls corresponding to the higher Al contents in root tips and the cell wall. In studying Al adsorption and desorption kinetics, Yang et al. (2008) also confirmed that root tip cell walls of the Al-sensitive rice cultivar bound more Al, and that the bound Al was retained more tightly, compared with the Al-resistant cultivar. Using similar experimental approaches S. J. Zheng (Zhejiang University, Hangzhou, China, pers. comm.) showed that the differential Al resistance of two buckwheat genotypes could also be related to differences in the Al binding capacity of root tip cell walls.
Fig. 4.
(A) Effect of Al supply on relative root growth (–Al = 100 %) of transgenic potato lines that differ in expression of PME. Different letters indicate significant differences between lines at P < 0·05 (Tukey test). (B) Relationship between relative root growth (–Al = 100 %) of potato genotypes (wild type and PME transformants) and Al contents, and (C) relationship between Al contents and Al-induced callose contents in root apices. Treatment of intact plants in nutrient solution for 24 h at pH 4·3. From Horst et al. (2007).
Besides the cell wall, the plasma membrane contributes to the negativity of the apoplast and may affect the toxicity of metals (Kinraide, 2006). Wagatsuma and Akiba (1989) and Wagatsuma et al. (1991, 2005b) related differences in Al resistance between plant species to the plasma membrane negativity of protoplasts, and Yermiyahu et al. (1997) ascribed the higher Al sensitivity of the wheat cultivar Scout to its higher plasma membrane negativity compared with the Al-resistant cultivar Atlas. Recently, Khan et al. (2009) provided evidence that genotypic Al tolerance in rice was related to a lower ratio of phospholipids to Δ5-sterols in the plasma membrane leading to a lower negativity and permeability compared with Al-sensitive cultivars. A role for the plasma membrane in Al resistance is also indicated by studies showing that the transformation of yeast and plants by a higher plant Δ8-sphingolipid desaturase modulated Al resistance (Da Silva et al., 2006; Ryan et al., 2007).
Silicon nutrition
There are several reports showing that Si nutrition enhances Al resistance of plants (Hodson and Evans, 1995). Both ex planta and in planta effects are involved, but the latter effects are only poorly understood (Cocker et al., 1998a). In maize, Kidd et al. (2001) provided evidence for an Al-induced enhanced release of phenolic compounds by Si-pre-treated plants, thus detoxifying Al in the apoplast. Wang et al. (2004), too, attributed Si-enhanced Al resistance of maize to a clear in planta effect. However, they could not relate Si-enhanced Al resistance to an increased release of phenols or organic acid anions, but rather to a decrease in Al binding capacity of the cell wall. Al treatment greatly enhanced Si accumulation in the cell wall fraction, reducing the mobility of apoplastic Al. In agreement with Cocker et al. (1998b), Wang et al. (2004) concluded from their data that Si treatment leads to the formation of hydroxyaluminum silicates (HAS) in the apoplast of the root apex, thus detoxifying Al.
Boron nutrition
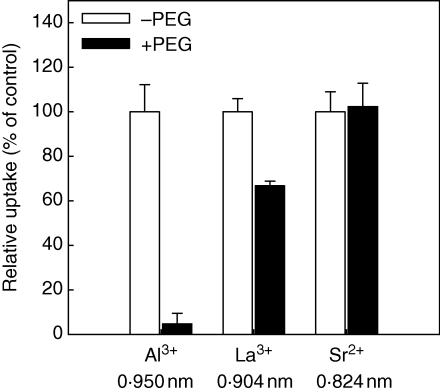
Boron (B) is a structural component in growing plant tissues (Brown and Hu, 1997). The formation of bis-diester bonds between the rhamnogalacturonan II (RG II) subunits of pectin chains and boric acid is a main function of B in plants (Brown et al., 2002; Goldbach and Wimmer, 2007). Boron-induced amelioration of Al toxicity has been reported in a number of plant species (LeNoble et al., 1996a, b; Lukaszewski and Blevins, 1996; Wojcik, 2003; Hossain et al., 2005; Staß et al., 2007; Corrales et al., 2008; Yu et al., 2009). However, the picture emerging on the mechanisms responsible for B amelioration of Al toxicity is not yet clear. Lukaszewski and Blevins (1996) reported that in squash both Al toxicity and B deficiency caused a reduction in ascorbate concentrations in root apices which was correlated with reduced root growth. They proposed that Al toxicity impaired the role of B in ascorbate metabolism which could be prevented by supplemental B (Lenoble et al. 1996b) and thus enhanced anti-oxidative defence (Corrales et al., 2008). However, Staß et al. (2007) and Yu et al. (2009) provided evidence that B-related changes in cell wall properties affect Al binding in root apices and thus Al toxicity. B deficiency increased the unmethylated pectin content of cell walls in common bean, which created additional binding sites for Al in the cell wall, thus enhancing Al accumulation and toxicity (Staß et al., 2007). Similarly, B supplementation reduced Al uptake by pea (Pisum sativum) root tips and Al binding to cell walls, which resulted in less Al toxicity (Yu et al., 2009). However, other studies have failed to find any ameliorative effect of B on Al toxicity (Taylor and Macfie, 1994; Corrales et al., 2008). In general, dicots display a stronger response to B supply than monocots, which may be due to the higher B requirement of dicots. Enhanced Al toxicity in B-deficient dicot plant species could also be related to the pore size of the cell wall which is affected by borate ester cross-linking of the pectic polysaccharide RG II (Fleischer et al., 1999). Reduced pore size of the cell wall could affect Al uptake. In a study on the interaction of Al toxicity and drought stress in common bean, Yang et al. (Z. Yang, Leibniz University Hannover, Germany, unpubl. res.) presented circumstantial evidence that osmotic stress induced by polyethylene glycol (PEG) treatment reduced the accumulation of cations (Al3+ > La3+ > Sr2+) in root tips by reducing cell wall porosity in agreement with their hydrated ionic diameter (Fig. 5).
Fig. 5.
Effect of polyethylene glycol (PEG) treatment on relative (no PEG = 100) Al, La and Sr accumulation of 10 mm root tips of Al-sensitive common bean genotype VAX-1. Plants were pre-treated with PEG (150 g L−1) for 8 h in a simplified solution (pH 4·5) containing 5 mm CaCl2, 1 mm KCl and 8 mm H3BO3. Then the plants were supplied with 25 mm AlCl3, 5 mm LaCl3 or 2·5 mm SrCl2 in the absence or presence of PEG (150 g L−1) in the same nutrient solution for 1 h. The hydrated ionic diameters are indicated under the ions. Bars represent means ± s.d. (n = 4). (Z. Yang et al., Leibniz University Hannover, Germany, unpubl. res.)
AL RESISTANCE IN Al ACCUMULATORS REQUIRES Al EXCLUSION MECHANISMS
A close relationship exists between Al exclusion from the root tip and Al resistance in Al-excluding plant species, e.g. in wheat (Delhaize et al., 1993), maize (Piñeros et al., 2005), triticale (Yang et al., 2005), rice (Yang et al., 2008) and common bean (Rangel et al., 2007). In all of these plant species Al accumulation in the root tip of Al-resistant genotypes was less than that of their Al-sensitive counterparts, indicating that Al resistance is achieved through Al exclusion particularly from the apoplast where the bulk of Al accumulates. Li et al. (2009b) reported that the amount of Al adsorbed to cell walls isolated from the Al-resistant wheat line ET8 and the Al-sensitive wheat line ES8 was similar, but the latter accumulated several times more Al in root tips. This was explained by the exudation of malate by ET8 under Al stress, thereby protecting the Al-sensitive sites in cell walls from binding Al.
The additional role of Al tolerance in Al resistance of Al excluders is not yet fully resolved. Higher symplastic Al contents may be indicative of enhanced or acquired Al resistance, as suggested by Vázquez et al. (1999) who observed internalization of Al into the symplast contributing to Al tolerance in an Al-resistant maize genotype. Also Illes et al. (2006) ascribed Al internalization into endosomal or vacuolar compartments contributing to the recovery from initial Al stress in Arabidopsis. However, the trend of increasing symplastic Al contents with the recovery from initial Al stress and the significantly higher symplastic Al contents in the Al-resistant common bean genotype Quimbaya compared with the Al-sensitive genotype VAX-1 seems to indicate that transport of Al into the symplast is not a prerequisite for Al toxicity (Rangel et al., 2009a). From their results it appears rather unlikely that transfer to and inactivation of Al in the symplast can explain enhanced Al resistance because of the quantitatively small Al fraction in the symplast.
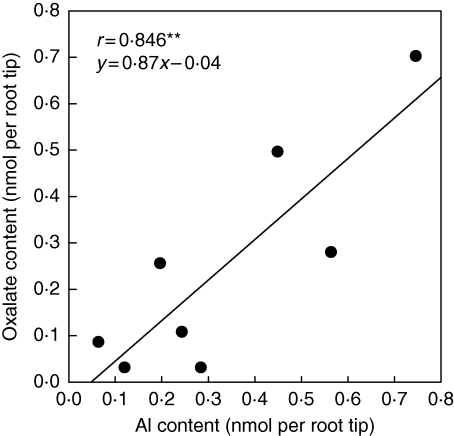
In contrast to Al excluder species, Al includer species which are among the most Al-resistant plant species can contain >1 mg (g d. wt)−1 Al in their leaves (Jansen et al., 2002). Thus, they can be classified as Al-tolerant. Al tolerance is attributed to the symplastic complexation of Al by organic ligands, particularly organic acids (Ma et al., 1997, 1998; Morita et al., 2008). Rapid transfer of Al into the symplast may contribute to keep the Al3+ activity lower in the apoplast. However, also in Al accumulators Al3+ will strongly interact with the negative binding sites of the apoplast. This assumption is supported by the results with the Al accumulator buckwheat in which a lower pectin content of the cell wall resulted in a lower Al content in the root tip of an Al-resistant compared with an Al-sensitive buckwheat genotype (S. J. Zheng, Zhejiang University, Hangzhou, China, pers. comm.). Thus Al accumulators not only symplastically complex Al but also release organic acid anions from the Al-sensitive root tips and complex Al in the root apoplast when exposed to Al. This has been shown for buckwheat (Zheng et al., 1998; Klug and Horst, 2010), tea (Morita et al., 2001) and hortensia (Naumann, 2001). In buckwheat, there was a close relationship between the Al and oxalate concentrations, not only in the symplast but also in the apoplast of root tips (Fig. 6). Klug and Horst conclude from their results that the formation of a 1 : 1 oxalate : Al complex in the root apoplast both protects root apoplastic binding sites from interaction with Al and is a prerequisite for rapid transport of Al into the symplast (Klug and Horst, 2010; B. Klug and W. J. Horst, Leibniz University Hannover, Germany, unpubl. res.).
Fig. 6.
Relationship between the contents of Al and oxalate in the water free-space fluid (WFSF) of adventitious buckwheat root tips. The WFSF was extracted by centrifugation of excised 10 mm root tips at 4000 g for 15 min. Plants were pre-treated for 0·5, 4 and 24 h at 75 µm Al in simplified nutrient solution (pH 4·3) containing 500 µm CaCl2, 8 µm H3BO3 and 100 µm K2SO4. Points represent single values. **Significant at P < 0·01. (B. Klug and W. J. Horst, Leibniz University Hannover, Germany, unpubl. res.)
MOLECULAR EVIDENCE FOR THE INVOLVEMENT OF CELL WALL PROPERTIES IN Al RESISTANCE
In recent years, more molecular information on the role of the root apoplast in Al toxicity and resistance has been accumulating. Using Al-hypersensitive mutants, Huang et al. (2009) discovered two genes contributing to Al resistance in rice. These genes, STAR1 and STAR2 (Sensitive To Al Rhizotoxicity), encode a nucleotide-binding domain and a transmembrane domain, respectively, of a bacterial-type ATP-binding cassette (ABC) transporter, and are expressed in the root particularly under Al stress. Disruption of either of these genes resulted in hypersensitivity to Al. The authors showed that the STAR1–STAR2 complex had efflux transport activity for UDP-glucose and hypothesized that UDP-glucose or glycosides derived from UDP-glucose modify the cell walls to mask potential Al-binding sites in the apoplast, resulting in Al resistance in rice.
In line with this, the transcriptional analysis of Al resistance in maize by Maron et al. (2008) indicated that several genes related to cell wall structure and composition exhibit differential expression upon Al treatment. Among these, PME, the enzyme responsible for the demethylation of pectin in the cell wall, was upregulated by Al treatment in both Al-resistant and Al-sensitive maize genotypes. The PME expression was constitutively higher and its upregulation was more enhanced in the Al-sensitive genotype. These results nicely back-up the immunolocalization study on unmethylated pectins in roots of two maize cultivars differing in Al resistance by Eticha et al. (2005a) and Li et al. (2009a). The results contribute to close the gap in the understanding of genotypic Al resistance in maize which cannot be fully explained by differential exudation of citrate (Piñeros et al., 2005).
In addition to PME, the expression and activity of apoplastic peroxidases were enhanced under Al stress (Kumari et al., 2008; Maron et al., 2008; Xue et al., 2008). Plant peroxidases constitute a large group of proteins encoded by a multigene family consisting of about 138 members in rice (Passardi et al., 2004) and 73 members in Arabidopsis (Tognolli et al., 2002). These enzymes catalyse diverse reactions and are involved in a wide range of physiological processes, such as auxin catabolism, lignin and suberin formation, cross-linking of cell wall components, defence against pathogens, and cell elongation (Hiraga et al., 2001). It thus may be assumed that apopalstic peroxidases are involved in both Al toxicity and Al resistance mechanisms.
In addition, Al caused changes in gene expression of a number of cell wall-modifying enzymes such as xyloglucanase, endotransglycosylases, polygalacturonases, glycosyl transferases, lipid transfer proteins and other wall-related genes in Arabidopsis (Kumari et al., 2008), indicating the important role of dynamic changes in the apoplast for the Al stress response. Moreover, several hundreds of other Al-responsive genes have been documented through transcriptional profiling studies in Arabidopsis (Kumari et al., 2008; Goodwin and Sutter, 2009), wheat (Guo et al., 2007; Houde and Diallo, 2008), maize (Maron et al., 2008) and Medicago truncatula (Chandran et al., 2008a, b). Their possible functions in relation to Al toxicity and resistance can only be speculated at this time. Therefore, future studies should focus on elucidating the significance of these genes for Al resistance of plants.
CONCLUSIONS AND FUTURE PERSPECTIVES
The binding of Al in the cell wall and to the apoplastic face of the plasma membrane in the most Al-sensitive root tip zone impairs both apoplastic and symplastic functions, disruption of which is a major factor leading to Al-induced inhibition of root elongation. Although symplastic lesions of Al toxicity cannot be ruled out, the protection of the root apoplast appears to be a prerequisite for Al resistance in both Al-excluder and Al-accumulator plant species. The most important Al resistance mechanism in most plant species is the sustained release of organic acid anions from the root apex. These organic acid anions complex Al, thus protecting the root apoplast from Al binding. However, there is increasing physiological, biochemical and, most recently, molecular evidence showing that the modification of the binding properties of the root apoplast contributes to Al resistance.
The physiological research during the last 15 years has contributed to consolidate the role of the apoplast in Al toxicity and resistance. Whereas the role of organic acid anions in the protection of the apoplast from Al binding, including its molecular basis, are well understood, the understanding of the mechanism of Al toxicity is still mainly circumstantial. Particularly our knowledge on the molecular mechanisms governing the Al–apoplast interactions leading to inhibition of root elongation, callose formation and disruption of tissue integrity is still scarce. The recent identification of a few genes which code for proteins involved in the modification of cell wall composition, structure and functions are mostly by-products of studies searching for genes involved in organic acid anion exudation and metabolism. A more focused search for genes related to the root tip apoplast is urgently needed. This would allow the function of these genes in Al toxicity and resistance to be proved using transgenic approaches.
LITERATURE CITED
- Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant Physiology. 2001;126:1381–1390. doi: 10.1104/pp.126.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Rengel Z, Matsumoto H. Aluminum-induced plasma membrane surface potential and H+-ATPase activity in near-isogenic wheat lines differing in tolerance to aluminum. New Phytologist. 2004;162:71–79. [Google Scholar]
- Akeson MA, Munns DN, Burau RG. Adsorption of Al3+ to phosphatidylcholine vesicles. Biochimica et Biophysica Acta. 1989;986:33–40. doi: 10.1016/0005-2736(89)90269-1. [DOI] [PubMed] [Google Scholar]
- Amenós M, Corrales I, Poschenrieder C, Illés P, Baluska F, Barceló J. Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant and Cell Physiology. 2009;50:528–540. doi: 10.1093/pcp/pcp013. [DOI] [PubMed] [Google Scholar]
- Basu U, Good AG, Aung T, et al. A 23-kDa, root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physionogia Plantarum. 1999;106:53–61. [Google Scholar]
- Bennet RJ, Breen CM, Fey MV. Aluminium uptake sites in the primary root of Zea mays L. South African Journal of Plant and Soil. 1985;2:1–7. [Google Scholar]
- Blamey FPC. A role for pectin in the control of cell expansion. Soil Science and Plant Nutrition. 2003;49:775–783. [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. Role of root cation-exchange capacity in differential aluminum tolerance of Lotus species. Journal of Plant Nutrition. 1990;13:729–744. [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. Empirical models to approximate calcium and magnesium ameliorative effects and genetic differences in aluminium tolerance in wheat. Plant and Soil. 1992;144:281–287. [Google Scholar]
- Blamey FPC, Asher CJ, Edwards DG, Kerven GL. In vitro evidence of aluminium effects on solution movement through root cell walls. Journal of Plant Nutrition. 1993;16:555–562. [Google Scholar]
- Blamey FPC, Nishizawa NK, Yoshimura E. Timing, magnitude, and location of initial soluble aluminum injuries to mungbean roots. Soil Science and Plant Nutrition. 2004;50:67–76. [Google Scholar]
- Blamey FPC, Nishizawa NK, Yoshimura E. Digital microscopy: a useful technique for measuring root elongation in solution. Soil Science and Plant Nutrition. 2005;51:705–708. [Google Scholar]
- Blancaflor EB, Jones DL, Gilroy S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiology. 1998;118:159–172. doi: 10.1104/pp.118.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenave M. Analysis of pectin methyl esterases. In: Linskens H, Jackson J, editors. Plant cell wall analysis. Berlin: Springer; 1996. pp. 165–180. [Google Scholar]
- Boscolo PRS, Menossi M, Jorge RA. Aluminum-induced oxidative stress in maize. Phytochemistry. 2003;62:181–189. doi: 10.1016/s0031-9422(02)00491-0. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Cell wall biosynthesis and the molecular mechanism of plant enlargement. Functional Plant Biology. 2009;36:383–394. doi: 10.1071/FP09048. [DOI] [PubMed] [Google Scholar]
- Brown PH, Hu H. Does boron play only a structural role in the growing tissues of higher plants? Plant and Soil. 1997;196:211–215. [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, et al. Boron in plant biology. Plant Biology. 2002;4:205–223. [Google Scholar]
- Büscher P, Koedam N, Van Speybroek D. Cation exchange properties and adaptation to soil acidity in bryophytes. New Phytologist. 1990;115:177–186. [Google Scholar]
- Caldwell CR. Analysis of aluminum and divalent cation binding to wheat root plasma membrane proteins using Terbium phosphorescence. Plant Physiology. 1989;91:233–241. doi: 10.1104/pp.91.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiologia Plantarum. 1991;83:463–468. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carr HP, Lombi E, Küpper H, McGrath SP, Wong MH. Accumulation and distribution of aluminium and other elements in tea (Camellia sinensis) leaves. Agronomie. 2003;23:705–710. [Google Scholar]
- Chandran D, Sharopova N, Ivashuta S, Gantt J, VandenBosch K, Samac D. Transcriptome profiling identified novel genes associated with aluminum toxicity, resistance and tolerance in Medicago truncatula. Planta. 2008a;228:151–166. doi: 10.1007/s00425-008-0726-0. [DOI] [PubMed] [Google Scholar]
- Chandran D, Sharopova N, VandenBosch K, Garvin D, Samac D. Physiological and molecular characterization of aluminum resistance in Medicago truncatula. BMC Plant Biology. 2008b;8:89. doi: 10.1186/1471-2229-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell and Environment. 1999;22:1009–1017. [Google Scholar]
- Cocker KM, Evans DE, Hodson MJ. The amelioration of aluminium toxicity by silicon in higher plants: solution chemistry or an in planta mechanism? Physiologia Plantarum. 1998a;104:608–614. [Google Scholar]
- Cocker KM, Evans DE, Hodson MJ. The amelioration of aluminium toxicity by silicon in wheat (Triticum aestivum L.): malate exudation as evidence for an in planta mechanism. Planta. 1998b;204:318–323. [Google Scholar]
- Collet L, Horst W. Characterisation of maize cultivars in their adaptation to acid soils on the single plant level. In: Horst WJ, Schenk MK, Bürckert A, et al., editors. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Kluwer Academic Publishers; 2001. pp. 86–87. [Google Scholar]
- Corrales I, Poschenrieder C, Barceló J. Boron-induced amelioration of aluminium toxicity in a monocot and a dicot species. Journal of Plant Physiology. 2008;165:504–513. doi: 10.1016/j.jplph.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Da Silva ALS, Sperling P, Horst W, et al. A possible role of sphingolipids in the aluminium resistance of yeast and maize. Journal of Plant Physiology. 2006;163:26–38. doi: 10.1016/j.jplph.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.) (I. Uptake and distribution of aluminum in root apices) Plant Physiology. 1993;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Letters. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Deng W, Luo K, Li D, et al. Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. Journal of Experimental Botany. 2006;57:4235–4243. doi: 10.1093/jxb/erl201. [DOI] [PubMed] [Google Scholar]
- Ermolayev V, Weschke W, Manteuffel R. Comparison of Al-induced gene expression in sensitive and tolerant soybean cultivars. Journal of Experimental Botany. 2003;54:2745–2756. doi: 10.1093/jxb/erg302. [DOI] [PubMed] [Google Scholar]
- Eticha D, Staß A, Horst WJ. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell and Environment. 2005a;28:1410–1420. [Google Scholar]
- Eticha D, Staß A, Horst WJ. Localization of aluminium in the maize root apex: can morin detect cell wall-bound aluminium? Journal of Experimental Botany. 2005b;56:1351–1357. doi: 10.1093/jxb/eri136. [DOI] [PubMed] [Google Scholar]
- Eticha D, The C, Welcker C, Narro L, Staß A, Horst WJ. Aluminium-induced callose formation in root apices: inheritance and selection trait for adaptation of tropical maize to acid soils. Field Crops Research. 2005c;93:252–263. [Google Scholar]
- Eticha D, Zahn M, Bremer M, Yang Z, Rangel AF, Rao IM, Horst WJ. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Annals of Botany. 2010;105:1119–1128. doi: 10.1093/aob/mcq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Katsuhara M, Kawamura M, Matsumoto H. Different mechanisms of four aluminum (Al)-resistant transgenes for Al toxicity in Arabidopsis. Plant Physiology. 2001;127:918–927. [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Sasaki K, Matsumoto H, Nakashima S. Functions of two genes in aluminium (Al) stress resistance: repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1gene. Journal of Experimental Botany. 2005;56:2661–2671. doi: 10.1093/jxb/eri259. [DOI] [PubMed] [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiology. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantzios G, Galatis B, Apostolakos P. Aluminium causes variable responses in actin filament cytoskeleton of the root tip cells of Triticum turgidum. Protoplasma. 2005;225:129–140. doi: 10.1007/s00709-005-0100-z. [DOI] [PubMed] [Google Scholar]
- Gerendás J. Significance of polyamines for pectin methylesterase activity and the ion dynamics in the apoplast. In: Sattelmacher B, Horst W, editors. The apoplast of higher plants: compartment of storage, transport, and reactions. Dordrecht: Kluwer Academic Publishers; 2007. pp. 67–83. [Google Scholar]
- Goldbach HE, Wimmer MA. Boron in plants and animals: is there a role beyond cell-wall structure? Journal of Plant Nutrition and Soil Science. 2007;170:39–48. [Google Scholar]
- Goodwin SB, Sutter TR. Microarray analysis of Arabidopsis genome response to aluminum stress. Biologia Plantarum. 2009;53:85–99. [Google Scholar]
- Grauer UE. Faktoren der Aluminium-Toleranz bei verschiedenen Pflanzen. 1992. PhD Thesis, Universität Hohenheim, Germany. [Google Scholar]
- Grauer UE, Horst WJ. Modeling cation amelioration of aluminum phytotoxicity. Soil Science Society of America Journal. 1992;56:166–171. [Google Scholar]
- Guo P, Bai G, Carver B, Li R, Bernardo A, Baum M. Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Molecular Genetics and Genomics. 2007;277:1–12. doi: 10.1007/s00438-006-0169-x. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang W, Zhang B, Zhang S, Wang W, Ming F. One novel mitochondrial citrate synthase from Oryza sativa L. can enhance aluminum tolerance in transgenic tobacco. Molecular Biotechnology. 2009;42:299–305. doi: 10.1007/s12033-009-9162-z. [DOI] [PubMed] [Google Scholar]
- Hartwell BL, Pember FR. The presence of aluminium as a reason for the difference in the effect of so-called acid soil on barley and rye. Soil Science. 1918;6:259–281. [Google Scholar]
- Heim A, Luster J, Brunner I, Frey B, Frossard E. Effects of aluminium treatment on Norway spruce roots: aluminium binding forms, element distribution, and release of organic substances. Plant and Soil. 1999;216:103–116. [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant and Cell Physiology. 2001;42:462–468. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- Hodson MJ, Evans DE. Aluminium/silicon interactions in higher plants. Journal of Experimental Botany. 1995;46:161–171. doi: 10.1093/jxb/eraa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Zeitschrift für Pflanzenernährung und Bodenkunde. 1995;158:419–428. [Google Scholar]
- Horst WJ, Asher CJ, Cakmak I, Szulkiewicz P, Wissemeier AH. Short-term responses of soybean roots to aluminium. Journal of Plant Physiology. 1992;140::174–178. [Google Scholar]
- Horst WJ, Püschel A-K, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant and Soil. 1997;192:23–30. [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluska F, Sivaguru M. Does aluminium affect root growth of maize through interaction with the cell wall–plasma membrane–cytoskeleton continuum? Plant and Soil. 1999;215:163–174. [Google Scholar]
- Horst WJ, Kollmeier M, Schmohl N, et al. Significance of the root apoplast for aluminium toxicity and resistance of maize. In: Sattelmacher B, Horst W, editors. The apoplast of higher plants: compartment of storage, transport, and reactions. Dordrecht: Kluwer Academic Publishers; 2007. pp. 49–66. [Google Scholar]
- Hossain AKMZ, Asgar M, Hossain MA, Tosaki T, Koyama H, Hara T. Boron–calcium synergically alleviates aluminum toxicity in wheat plants (Triticum aestivum L.) Soil Science and Plant Nutrition. 2005;51:43–49. [Google Scholar]
- Houde M, Diallo AO. Identification of genes and pathways associated with aluminum stress and tolerance using transcriptome profiling of wheat near-isogenic lines. BioMed Central Genomics. 2008;9:400. doi: 10.1186/1471-2164-9-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. The Plant Cell. 2009;21:655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovecka M. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behavior, and nitric oxide production. Journal of Experimental Botany. 2006;57:4201–4213. doi: 10.1093/jxb/erl197. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Wagatsuma T. Plasma membrane permeability of root-tip cells following temporary exposure to Al ions is a rapid measure of Al tolerance among plant species. Plant and Cell Physiology. 1998;39:516–525. [Google Scholar]
- Jansen S, Broadley MR, Robbrecht W, Smets E. Aluminum hyperaccumulation in angiosperms: a review of its phylogenetic significance. Botanical Review. 2002;68:235–269. [Google Scholar]
- Jones DL, Kochian LV. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Letters. 1997;400:51–57. doi: 10.1016/s0014-5793(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Jones DL, Gilroy S, Larsen PB, Howell SH, Kochian LV. Effect of aluminum on cytoplasmic Ca2+ homeostasis in root hairs of Arabidopsis thaliana (L.) Planta. 1998;206:378–387. doi: 10.1007/s004250050413. [DOI] [PubMed] [Google Scholar]
- Jones DL, Blancaflor EB, Kochian LV, Gilroy S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant, Cell and Environment. 2006;29:1309–1318. doi: 10.1111/j.1365-3040.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- Kauss H, Waldmann T, Jeblick W, Euler G, Ranjeva R, Domard A. Ca2+ is an important but not the only signal in callose synthesis induced by chitosan, saponins and polyene antibiotics. In: Lugtenberg BJJ, editor. Signal molecules in plant and plant–microbe interactions. Berlin: Springer-Verlag; 1989. pp. 107–116. [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kobot S. Aluminium determination in soil solution. I. Evaluation of existing colorimetric and separation methods for the determination of inorganic monomeric aluminium in the presence of organic acid ligands. Australian Journal of Soil Research. 1989;27:79–90. [Google Scholar]
- Khan MSH, Tawaraya K, Sekimoto H, et al. Relative abundance of delta5-sterols in plasma membrane lipids of root-tip cells correlates with aluminum tolerance of rice. Physiologia Plantarum. 2009;135:73–83. doi: 10.1111/j.1399-3054.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunsé B, Barceló J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) Journal of Experimental Botany. 2001;52:1339–1352. [PubMed] [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiology. 1992;99:1461–1468. doi: 10.1104/pp.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Plasma membrane surface potential (‘psi’ PM) as a determinant of ion bioavailability: a critical analysis of new and published toxicological studies and a simplified method for the computation of plant ‘psi’ PM. Environmental Toxicology and Chemistry. 2006;25:3188–3198. doi: 10.1897/06-103r.1. [DOI] [PubMed] [Google Scholar]
- Kinraide TB. Improved scales for metal ion softness and toxicity. Environmental Toxicology and Chemistry. 2009;28:525–533. doi: 10.1897/08-208.1. [DOI] [PubMed] [Google Scholar]
- Klimashevskii EL, Dedov VM. Localization of growth inhibiting action of aluminum ions in elongating cell walls. Fiziologiia Rastenii. 1975;22:1183–1190. [Google Scholar]
- Klug B, Horst WJ. Spatial characteristics of aluminium uptake and translocation in roots of buckwheat (Fagopyrum esculentum Moench) Physiologia Plantarum. 2010 doi: 10.1111/j.1399-3054.2010.01355.x. (in press) [DOI] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Horst WJ. Aluminium-induced exudation of citrate from the root tip of Zea mays (L.): are differential impacts of Al on citrate metabolism involved in genotypical differences? In: Horst WJ, Schenk MK, Bürckert A, et al., editors. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Kluwer Academic Publishers; 2001. pp. 492–493. [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiology. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R. Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiology. 2001;126:397–410. doi: 10.1104/pp.126.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Blamey FPC, Menzies NW. Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant and Soil. 2008;303:217–227. [Google Scholar]
- Kopittke PM, McKenna BA, Blamey FPC, Wehr JB, Menzies NW. Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant and Soil. 2009;322:303–315. [Google Scholar]
- Kumari M, Taylor GJ, Deyholos MK. Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Molecular Genetics and Genomics. 2008;279:339–357. doi: 10.1007/s00438-007-0316-z. [DOI] [PubMed] [Google Scholar]
- Lazof DB, Goldsmith JG, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips. A microanalytical study using secondary ion mass spectrometry. Plant Physiology. 1994;106:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNoble ME, Blevins DG, Sharp RE, Cumbie BG. Prevention of aluminium toxicity with supplemental boron. I. Maintenance of root elongation and cellular structure. Plant, Cell and Environment. 1996a;19:1132–1142. [Google Scholar]
- LeNoble ME, Blevins DG, Miles RJ. Prevention of aluminium toxicity with supplemental boron. II. Stimulation of root growth in an acidic, high-aluminium subsoil. Plant, Cell and Environment. 1996b;19:1143–1148. [Google Scholar]
- Lewis TE. Environmental chemistry and toxicology of aluminium. Michigan: Lewis Publishers; 1989. [Google Scholar]
- Li YY, Yang JL, Zhang YJ, Zheng SJ. Disorganized distribution of homogalacturonan epitopes in cell walls as one possible mechanism for aluminium-induced root growth inhibition in maize. Annals of Botany. 2009a;104:235–241. doi: 10.1093/aob/mcp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Zhang YJ, Zhou Y, Yang JL, Zheng SJ. Protecting cell walls from binding aluminum by organic acids contributes to aluminum resistance. Journal of Integrative Plant Biology. 2009b;51:574–580. doi: 10.1111/j.1744-7909.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Strid H. Aluminium induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum) Physiologia Plantarum. 1997;99:405–414. [Google Scholar]
- Lindberg S, Szynkier K, Greger M. Aluminium effects on transmembrane potential in cells of fibrous roots of sugar beet. Physiologia Plantarum. 1991;83:54–62. [Google Scholar]
- Liu Q, Yang JL, He LS, Li YY, Zheng SJ. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biologia Plantarum. 2008;52:87–92. [Google Scholar]
- Llugany M, Poschenrieder C, Barceló J. Monitoring of aluminium-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminium and proton toxicity. Physiologia Plantarum. 1995;93:265–271. [Google Scholar]
- Lukaszewski KM, Blevins DG. Root growth inhibition in boron-deficient or aluminum-stressed squash may be a result of impaired ascorbate metabolism. Plant Physiology. 1996;112:1135–1140. doi: 10.1104/pp.112.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. International Review of Cytology. 2007;264:225–252. doi: 10.1016/S0074-7696(07)64005-4. [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in hydrangea (identification of Al form in the leaves) Plant Physiology. 1997;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Matsumoto H. High aluminum resistance in buckwheat. II. Oxalic acid detoxifies aluminum internally. Plant Physiology. 1998;117:753–759. doi: 10.1104/pp.117.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science. 2001;6:273–278. doi: 10.1016/s1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Zhao Z, et al. Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant and Cell Physiology. 2002;43:652–659. doi: 10.1093/pcp/pcf081. [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen RF, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant and Cell Physiology. 2004;45:583–589. doi: 10.1093/pcp/pch060. [DOI] [PubMed] [Google Scholar]
- Maltais K, Houde M. A new biochemical marker for aluminium tolerance in plants. Physiologia Plantarum. 2002;115:81–86. doi: 10.1034/j.1399-3054.2002.1150109.x. [DOI] [PubMed] [Google Scholar]
- Marienfeld S, Stelzer R. X-ray microanalyses in roots of Al-treated Avena sativa plants. Journal of Plant Physiology. 1993;141:569–573. [Google Scholar]
- Marienfeld S, Schmohl N, Klein M, Schröder WH, Kuhn AJ, Horst WJ. Localization of aluminium in root tips of Zea mays and Vicia faba. Journal of Plant Physiology. 2000;156:666–671. [Google Scholar]
- Maron LG, Kirst M, Mao C, Milner MJ, Menossi M, Kochian LV. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytologist. 2008;179:116–128. doi: 10.1111/j.1469-8137.2008.02440.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminium toxicity and tolerance in higher plants. International Review of Cytology. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- Morita A, Fujii Y, Yokota H. Effect of aluminium on exudation of organic acid anions in tea plants. In: Horst WJ, Schenk MK, Bürckert A, et al., editors. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Kluwer Academic Publishers; 2001. pp. 508–509. [Google Scholar]
- Morita A, Yanagisawa O, Takatsu S, Maeda S, Hiradate S. Mechanism for the detoxification of aluminum in roots of tea plant (Camellia sinensis (L.) Kuntze) Phytochemistry. 2008;69:147–153. doi: 10.1016/j.phytochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Naumann A. Aufnahme und Verlagerung von Aluminium bei Hortensie (Hydrangea macrophylla) in Beziehung zur Aluminiumtoleranz und zur Blaufärbung der Sepalen. 2001. PhD Thesis, Leibniz Universität Hannover, Germany. [Google Scholar]
- Naumann A, Horst WJ. Effect of aluminium supply on aluminium uptake, translocation and blueing of Hydrangea macrophylla (Thunb.) ser. cultivars in a peat–clay substrate. Journal of Horticultural Science and Biotechnology. 2003;78:463–469. [Google Scholar]
- Olivetti GP, Cumming JR, Etherton B. Membrane potential depolarization of root cap cells precedes aluminum tolerance in snapbean. Plant Physiology. 1995;109:123–129. [Google Scholar]
- Osaki M, Nursyamsi D, Begum HH, Watanabe T. Study on aluminium resistance in relation to organic-acid anion exudation from roots of PEPC transgenic rice plants. In: Horst WJ, Schenk MK, Bürckert A, et al., editors. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Kluwer Academic Publishers; 2001. pp. 514–515. [Google Scholar]
- Osawa H, Kojima K. Citrate-release-mediated aluminum resistance is coupled to the inducible expression of mitochondrial citrate synthase gene in Paraserianthes falcataria. Tree Physiology. 2006;26:565–574. doi: 10.1093/treephys/26.5.565. [DOI] [PubMed] [Google Scholar]
- Panda S, Matsumoto H. Molecular physiology of aluminum toxicity and tolerance in plants. Botanical Review. 2007;73:326–347. [Google Scholar]
- Passardi F, Longet D, Penel C, Dunand C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry. 2004;65:1879–1893. doi: 10.1016/j.phytochem.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Piñeros MA, Kochian LV. A patch–clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiology. 2001;125:292–305. doi: 10.1104/pp.125.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Carvalho Alves VM, Kochian LV. Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiology. 2005;137:231–241. doi: 10.1104/pp.104.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Sattelmacher B, Hansen UP, Knight MR. Low-pH-mediated elevations in cytosolic calcium are inhibited by aluminium: a potential mechanism for aluminium toxicity. The Plant Journal. 1999;18:643–650. doi: 10.1046/j.1365-313x.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Christensen HEM, Ishimaru Y, et al. Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis. Plant Physiology. 2000;124:1637–1647. doi: 10.1104/pp.124.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. Journal of Experimental Botany. 2007;58:3895–3904. doi: 10.1093/jxb/erm241. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Intracellular distribution and binding state of aluminum in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiologia Plantarum. 2009a;135:162–173. doi: 10.1111/j.1399-3054.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Braun H-P, Horst WJ. Aluminium resistance in common bean (Phaseolus vulgaris L.) involves induction and maintenance of citrate exudation from root apices. Physiologia Plantarum. 2009b;138:176–190. doi: 10.1111/j.1399-3054.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Rengel Z. Uptake of aluminium by plant cells. New Phytologist. 1996;134:389–406. [Google Scholar]
- Rengel Z, Reid RJ. Uptake of Al across the plasma membrane of plant cells. Plant and Soil. 1997;192:31–35. [Google Scholar]
- Rengel Z, Zhang WH. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytologist. 2003;159:295–314. doi: 10.1046/j.1469-8137.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiology. 1998;116:409–418. doi: 10.1104/pp.116.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany. 1993;44:437–446. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterisation of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyerman SD. Aluminum activates an anion channel in the apical cells of wheat roots. Proceedings of the National Academy of Sciences, USA. 1997;94:6547–6552. doi: 10.1073/pnas.94.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Biology. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Liu Q, Sperling P, Dong B, Franke S, Delhaize E. A higher plant Delta8 sphingolipid desaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiology. 2007;144:1968–1977. doi: 10.1104/pp.107.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E. The convergent evolution of aluminium resistance in plants exploits a convenient currency. Functional Plant Biology. 2010 (in press) [Google Scholar]
- Sarkar P, Bosneaga E, Auer M. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. Journal of Experimental Botany. 2009;60:3615–3635. doi: 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- Schildknecht PHPA, Vidal BC. A role for the cell wall in Al3+ resistance and toxicity: crystallinity and availability of negative charges. International Archives of Biosciences. 2002;2000:1087–1095. [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant, Cell and Environment. 2000;23:735–742. [Google Scholar]
- Schmohl N, Horst WJ. Effect of aluminium on the activity of apoplastic acid phosphatase and the exudation of macromolecules by roots and suspension-culture cells of Zea mays L. Journal of Plant Physiology. 2002;159:1213–1218. [Google Scholar]
- Schmohl N, Pilling J, Fisahn J, Horst WJ. Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiologia Plantarum. 2000;109:419–427. [Google Scholar]
- Schwarzerova K, Zelenkova S, Nick P, Opatrny Z. Aluminum-induced rapid changes in the microtubular cytoskeleton of tobacco cell lines. Plant and Cell Physiology. 2002;43:207–216. doi: 10.1093/pcp/pcf028. [DOI] [PubMed] [Google Scholar]
- Shi BJ, Gustafson JP, Button J, et al. Physical analysis of the complex rye (Secale cereale L.) Alt4 aluminium (aluminum) tolerance locus using a whole-genome BAC library of rye cv. Blanco. Theoretical and Applied Genetics. 2009;119:695–704. doi: 10.1007/s00122-009-1080-8. [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiology. 1998;116:155–163. [Google Scholar]
- Sivaguru M, Baluska F, Volkmann D, Felle HH, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex. Short-term effects on the distal part of the transition zone. Plant Physiology. 1999a;119:1073–1082. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Yamamoto Y, Matsumoto H. Differential impacts of aluminium on microtubule organisation depends on growth phase in suspension-cultured tobacco cells. Physiologia Plantarum. 1999b;107:110–119. [Google Scholar]
- Sivaguru M, Fujiwara T, Samaj J, et al. Aluminum-induced 1–3-β-d-glucan inhibits cell-to-cell traffiking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiology. 2000a;124:991–1005. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Matsumoto H, Horst WJ. Control of the response to aluminium stress. In: Nick P, editor. Plant microtubules: potential for biotechnology. Berlin: Springer Verlag; 2000b. pp. 103–120. [Google Scholar]
- Sivaguru M, Horst WJ, Eticha D, Matsumoto H. Aluminum inhibits apoplastic flow of high-molecular weight solutes in root apices of Zea mays L. Journal of Plant Nutrition and Soil Science. 2006;169:679–690. [Google Scholar]
- Staß A, Horst WJ. Effect of aluminium on membrane properties of soybean (Glycine max) cells in suspension culture. Plant and Soil. 1995;171:113–118. [Google Scholar]
- Staß A, Horst WJ. Callose in abiotic stress. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, biochemistry, and biology of (1→3)-β-glucans and related polysaccharides. Burlington, MA: Academic Press; 2009. pp. 499–524. [Google Scholar]
- Staß A, Kotur Z, Horst WJ. Effect of boron on the expression of aluminium toxicity in Phaseolus vulgaris. Physiologia Plantarum. 2007;131:283–290. doi: 10.1111/j.1399-3054.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- Sun P, Tian Q-Y, Chen J, Zhang W-H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. Journal of Experimental Botany. 2010;61:347–356. doi: 10.1093/jxb/erp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A, Matsumoto H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiologia Plantarum. 2001;112:353–358. doi: 10.1034/j.1399-3054.2001.1120308.x. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Macfie SM. Modeling the potential for boron amelioration of aluminum toxicity using the Weibull function. Canadian Journal of Botany. 1994;72:1187–1196. [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, et al. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiology. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka T, Kaneko M, Mori S, Yoshimura E. Aluminum rapidly inhibits cellulose synthesis in roots of barley and wheat seedlings. Journal of Plant Physiology. 2002;159:17–23. [Google Scholar]
- Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiology. 2001;127:1836–1844. [PMC free article] [PubMed] [Google Scholar]
- Tice KR, Parker DR, DeMason DA. Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiology. 1992;100:309–318. doi: 10.1104/pp.100.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognolli M, Penel C, Greppin H, Simon P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene. 2002;288:129–138. doi: 10.1016/s0378-1119(02)00465-1. [DOI] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant and Soil. 1995;171:1–15. [Google Scholar]
- Van HL, Kuraishi S, Sakurai N. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiology. 1994;106:971–976. doi: 10.1104/pp.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez MD, Poschenrieder C, Corrales I, Barceló J. Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiology. 1999;119:435–444. doi: 10.1104/pp.119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R, Haug A. The effect of Al3+ on the physical properties of membrane lipids in Thermoplasma acidophilum. Biochemical and Biophysical Research Communications. 1978;84:138–143. doi: 10.1016/0006-291x(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Wagatsuma T, Akiba R. Low surface negativity of root protoplasts from aluminum-tolerant plant species. Soil Science and Plant Nutrition. 1989;35:443–452. [Google Scholar]
- Wagatsuma T, Nakashima T, Twaraya K. Identification of aluminium-tolerant protoplasts in the original root protoplast population from several plant species differing in aluminium tolerance. In: Wright RJ, Baligar VC, Murrmann RP, editors. Plant–soil interactions at low pH. Dordrecht: Kluwer Academic Publishers; 1991. pp. 789–793. [Google Scholar]
- Wagatsuma T, Ishikawa S, Uemura M, et al. Plasma membrane lipids are the powerful components for early stage aluminum tolerance in triticale. Soil Science and Plant Nutrition. 2005a;51:701–704. [Google Scholar]
- Wagatsuma T, Khan M, Rao IM, et al. Methylene blue stainability of root-tip protoplasts as an indicator of aluminum tolerance in a wide range of plant species, cultivars and lines. Soil Science and Plant Nutrition. 2005b;51:991–998. [Google Scholar]
- Wang Y, Staß A, Horst WJ. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiology. 2004;136:3762–3770. doi: 10.1104/pp.104.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Osaki M, Yano H, Rao I. Internal mechanisms of plant adaptation to aluminum toxicity and phosphorus starvation in three tropical forages. Journal of Plant Nutrition. 2006;29:1243–1255. [Google Scholar]
- Wehr JB, Menzies NW, Blamey FPC. Model studies on the role of citrate, malate and pectin esterification on the enzymatic degradation of Al- and Ca-pectate gels: possible implications for Al-tolerance. Plant Physiology and Biochemistry. 2003;41:1007–1010. [Google Scholar]
- Wehr JB, Menzies NW, Blamey FPC. Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiology and Biochemistry. 2004;42:485–492. doi: 10.1016/j.plaphy.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Wenzl P, Patino GM, Chaves AL, Mayer JE, Rao IM. The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiology. 2001;125:1473–1484. doi: 10.1104/pp.125.3.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissemeier AH, Klotz F, Horst WJ. Aluminium induced callose synthesis in roots of soybean (Glycine max L.) Journal of Plant Physiology. 1987;129:487–492. [Google Scholar]
- Wissemeier AH, Diening A, Hergenröder A, Horst WJ, Mix-Wagner G. Callose formation as parameter for assessing genotypical plant tolerance of aluminium and manganese. Plant and Soil. 1992;146:67–75. [Google Scholar]
- Wissemeier AH, Horst WJ. Effect of calcium supply on aluminium-induced callose formation, its distribution and persistence in roots of soybean (Glycine max (L.) Merr.) Journal of Plant Physiology. 1995;145:470–476. [Google Scholar]
- Wojcik P. Impact of boron on biomass production and nutrition of aluminium-stressed apple rootstocks. Journal of Plant Nutrition. 2003;26:2439–51. [Google Scholar]
- Xue YJ, Ling T, Yang ZM. Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. Journal of Agricultural and Food Chemistry. 2008;56:9676–9684. doi: 10.1021/jf802001v. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hachiya A, Matsumoto H. Oxidative damage to membranes by a combination of aluminum and iron in suspension-cultured tobacco cells. Plant and Cell Physiology. 1997;38:1333–1339. [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiology. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Oxidative stress triggered by aluminum in plant roots. Plant and Soil. 2003;255:239–243. [Google Scholar]
- Yang JL, Zheng SJ, He YF, Tang CX, Zhou GD. Genotypic differences among plant species in response to aluminum stress. Journal of Plant Nutrition. 2005;28:949–961. [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermiyahu U, Brauer DK, Kinraide TB. Sorption of aluminum to plasma membrane vesicles isolated from roots of Scout 66 and Atlas 66 cultivars of wheat. Plant Physiology. 1997;115:1119–1125. doi: 10.1104/pp.115.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Shen R, Xiao H, et al. Boron alleviates aluminum toxicity in pea (Pisum sativum) Plant and Soil. 2009;314:87–98. [Google Scholar]
- Zhang G, Taylor GJ. Kinetics of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars of Triticum aestivum L. Plant Physiology. 1989;91:1094–1099. doi: 10.1104/pp.91.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Taylor GJ. Kinetics of aluminum uptake in Triticum aestivum L. Identity of the linear phase of Al uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars. Plant Physiology. 1990;94:577–584. doi: 10.1104/pp.94.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, He Z, Tian H, Zhu G, Peng X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. Journal of Experimental Botany. 2007;58:2269–2278. doi: 10.1093/jxb/erm110. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Rengel Z. Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Australian Journal of Plant Physiology. 1999;26:401–409. [Google Scholar]
- Zhang WH, Ryan PR, Tyerman SD. Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiology. 2001;125:1459–1472. doi: 10.1104/pp.125.3.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL. Target sites of aluminum phytotoxicity. Biologia Plantarum. 2005;49:321–331. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat. I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiology. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Lin X, Yang J, Liu Q, Tang C. The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistant wheat (Triticum aestivum L.) cultivar. Plant and Soil. 2004;261:85–90. [Google Scholar]