Abstract
Background and Aims
Genotypic variation in tillering can be caused by differences in the carbon supply–demand balance within a plant. The aim of this study was to understand and quantify the effects of genotype on tillering as a consequence of the underlying internal competition for carbohydrates.
Methods
Five sorghum hybrids, derived from inbred lines with a common genetic background and with similar phenology and plant height but contrasting tillering, were grown in five experiments. The experiments covered a wide range in radiation and temperature conditions, so that number of tillers produced varied significantly. Data on leaf area, tiller number, and biomass accumulation and partitioning were collected at regular intervals. To quantify internal plant competition for carbohydrates, a carbohydrate supply–demand index (S/Dindex) was developed and related to variation in tillering.
Key Results
The appearance of main shoot leaves and tillers was highly co-ordinated across genotypes. High-tillering hybrids had a greater appearance frequency of early tiller ranks than low-tillering hybrids, and this was associated with narrower and hence smaller main shoot leaves. A generalized S/Dindex of internal plant competition accounted for most of the observed variation in maximum tiller number (Ntiller,max) across genotypes. However, genotypic differences in the relationship between the S/Dindex and Ntiller,max suggested that high-tillering hybrids also had a lower S/D threshold at which tillers appeared, possibly associated with hormonal effects.
Conclusions
The results support the hypothesis that genotypic differences in tillering were associated with differences in plant carbon S/D balance, associated with differences in leaf size and in the threshold at which tillers grow out. The results provide avenues for phenotyping of mapping populations to identify genomic regions regulating tillering. Incorporating the results in crop growth simulation models could provide insight into the complex genotype-by-management-by-environment interactions associated with drought adaptation.
Keywords: Carbohydrate supply–demand ratio, genotype-by-environment interaction, internal plant competition, leaf area development, leaf width, Sorghum bicolor, tiller number, tiller onset
INTRODUCTION
Tillering is generally recognized as one of the most plastic traits affecting accumulation of biomass and ultimately grain yield in many field crops. Depending on growing conditions and genotype, a wide range in tiller number has been observed in high-tillering cereals such as barley (Hordeum vulgare) (Aspinall, 1961; Canell, 1969), wheat (Triticum aestivum) (Friend, 1965; Kasperbauer and Karlen, 1986; Bos and Neuteboom, 1998), rice (Oryza sativa) (Honda and Okajima, 1970) and pearl millet (Pennistum glaucum) (Rai et al., 1999; van Oosterom et al., 2001) as well as in lower-tillering cereals such as sorghum (Sorghum bicolor) (Bruns and Horrocks, 1984). Genetic variation in tillering affects the dynamics of canopy development and hence the timing and nature of crop water limitation (Hammer, 2006). Simulation studies in sorghum (Hammer et al., 1996) indicated significant yield advantage of high-tillering types in high-yielding seasons when water was plentiful, whereas such types incurred a significant disadvantage in lower yielding water-limited circumstances. Hence, the selection of the best genotype is confounded by genotype-by-environment (GE) interactions for tillering (Hammer et al., 2005).
This GE interaction can be extended to the role of tillering across species in breeding of modern cereals (Doust, 2007). In high-yielding environments, one of the most critical characteristics of successful high-yielding varieties for rice or wheat was a semi-dwarf plant type with high tillering ability (Yoshida, 1972). Conversely, a uniculm plant, even for high-tillering species, could be more appropriate than freely tillering varieties under poorer growing conditions, as the smaller canopy size reduces pre-anthesis water use (Islam and Sedgley, 1981; Hammer, 2006). In addition, the presence of non-fertile tillers reduces grain yield in water-limiting environments (Jones and Kirby, 1977; Winward et al., 1983) via inefficient water use. As a consequence, wheat cultivars with a gene for tiller inhibition performed better than standard tillering cultivars under terminal drought (Duggan et al., 2005).
A sound understanding of the genetic and physiological basis underlying the GE interaction for tillering is not yet incorporated in current crop growth simulation models. Either tillers are not considered (Birch et al., 1990), or main culms and tillers are treated similarly (Rosenthal et al., 1989; Maas, 1993; Heiniger et al., 1997) or tillering is an input to the model (Hammer and Muchow, 1994). Although it is known that variation in tillering is highly heritable (Hart et al., 2001), the physiological basis of this heritability is not well understood. Dissecting variation in tillering into underlying component traits that are more robust across environments than tillering itself provides a means to develop such understanding. This could allow indirect selection for tillering in environments where the trait is poorly expressed, such as high-density experiments in glasshouses.
Environmental regulation of tillering in sorghum was characterized in a companion study (Kim et al., 2010). Tillering was found to be regulated by internal competition for resources during the early stages of development of the plant, and a generalized index of internal plant competition that accounted for assimilate supply and demand (S/Dindex) explained most of the variation in tiller number for a high-tillering hybrid grown in five contrasting experiments. The main environmental effect was on the appearance frequency of lower rank tillers. To explore the genetic regulation of tillering in sorghum within the context of plant internal competition for resources (Lauer and Simmons, 1985; Pieters et al., 2001; Dingkuhn et al., 2006; Luquet et al., 2006; Kim et al., 2010), the objective of this study was to understand and quantify the physiological basis of genotypic differences in tillering. A set of sorghum hybrids with similar genetic background, but known to differ in tillering, was grown in a wide range of environments. The S/Dindex for environmental regulation of tillering (Kim et al., 2010) was used as a basis to develop a more generic index that captured the effects of genotype, environment and their interaction on tillering as an emergent consequence of the underlying competition among axes for carbohydrates.
MATERIALS AND METHODS
Experimental details
Plant material.
Five hybrids of Sorghum bicolor L. Moench, obtained by crossing inbred lines from a mapping population with an elite male sterile parent (A23171), were grown in five experiments (expts 1–5; see table 1 in Kim et al., 2010). The inbred lines were derived from a cross between a low-tillering parent line (31945-2-2), developed by the breeding programme of Queensland Primary Industries & Fisheries, and Sorghum arundinaceum (S. bicolor ssp. arundinaceum), a wild sorghum from Africa with high tillering ability. The F1 of the cross was backcrossed to 31945-2-2, and then propagated for four generations through self-pollination, while selecting for contrasting tillering performance within agronomically desirable plant types. As a consequence, the five inbred lines from the mapping population used in this study were genetically very similar to 31945-2-2, apart from small segments of S. arundinaceum introgressed into the genome. The inbred lines were selected for similar plant height and phenology, but contrasting tillering behaviour. The lines were crossed with the elite male sterile parent (A23171), resulting in two low-tillering hybrids (Hybrids 1 and 2) and three high-tillering hybrids (Hybrids 4–6). The hybrids were included in all five experiments, except for Hybrid 6, which was not included in expts 4 and 5. The hybrid derived using the low-tillering parent (A23171/31945-2-2) was included as a low-tillering check (Hybrid 3) in all experiments except expt 1.
Table 1.
Maximum tiller number per plant (Ntiller,max) and total number of tillers for each tiller rank (T_T#), and total fertile tiller number per plant (FTN) and number of fertile tillers for each tiller rank (F_T#) for each genotype (G) across all environments (E) or each E across all G
| Ntiller,max | T_T1 | T_T2 | T_T3 | T_T4 | T_T5 | FTN | F_T1 | F_T2 | F_T3 | F_T4 | F_T5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hybrid 1 | 1·19 | 0·01 | 0·26 | 0·58 | 0·33 | 0·00 | 0·60 | 0·00 | 0·09 | 0·42 | 0·09 | 0·00 |
| Hybrid 2 | 1·49 | 0·03 | 0·33 | 0·69 | 0·43 | 0·00 | 1·04 | 0·00 | 0·11 | 0·56 | 0·38 | 0·00 |
| Hybrid 3 | 1·09 | 0·04 | 0·25 | 0·58 | 0·23 | 0·00 | 0·73 | 0·00 | 0·13 | 0·40 | 0·20 | 0·00 |
| Hybrid 4 | 2·24 | 0·14 | 0·69 | 0·89 | 0·49 | 0·03 | 1·78 | 0·04 | 0·40 | 0·84 | 0·47 | 0·02 |
| Hybrid 5 | 1·76 | 0·01 | 0·50 | 0·78 | 0·44 | 0·03 | 1·76 | 0·00 | 0·38 | 0·80 | 0·58 | 0·00 |
| Hybrid 6 | 1·90 | 0·00 | 0·47 | 0·83 | 0·60 | 0·00 | 1·33 | 0·00 | 0·37 | 0·70 | 0·27 | 0·00 |
| Expt 1 | 2·58 | 0·01 | 0·41 | 0·99 | 0·98 | 0·19 | 1·53 | 0·00 | 0·20 | 0·84 | 0·49 | 0·00 |
| Expt 2 | 2·66 | 0·09 | 0·60 | 0·92 | 0·87 | 0·18 | 1·86 | 0·03 | 0·40 | 0·83 | 0·56 | 0·03 |
| Expt 3 | 1·21 | 0·05 | 0·36 | 0·56 | 0·24 | 0·00 | 0·68 | 0·00 | 0·20 | 0·32 | 0·15 | 0·00 |
| Expt 4 | 1·52 | 0·10 | 0·65 | 0·65 | 0·12 | 0·00 | – | – | – | – | – | – |
| Expt 5 | 1·00 | 0·02 | 0·09 | 0·70 | 0·19 | 0·00 | – | – | – | – | – | – |
| G | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | *** | * |
| E | *** | * | *** | *** | *** | *** | *** | ns | *** | *** | *** | ns |
| G × E | + | ns | ns | ** | *** | ns | * | ns | * | ns | *** | ns |
Significance levels for G, E and G × E interaction effects are indicated: n.s., not significant (P > 0·1); + P < 0·10; *P < 0·05; **P < 0·01; ***P < 0·001.
Experimental set-up.
The experiments included three field (expts 1–3) and two controlled environment (expts 4 and 5) experiments that were described in detail by Kim et al. (2010).
The field experiments were conducted in south-east Queensland, Australia. Experiments 1 and 2 were sown at Warwick (25 October, 2004 and 2 March, 2005) and both experienced moderate temperature and high daily radiation during the period of tiller emergence. Experiment 3 was sown at Gatton on 16 January, 2006 and experienced high temperatures and high daily radiation during tillering. The contrasting temperature/radiation regimes ensured a range in tiller number across experiments (Kim et al., 2010). Field experiments were laid out as randomized complete block designs with three replicates. Plot size was four rows of 15 m, with a row spacing of 1 m. Plants were thinned to 5 m−2 around 2 weeks after emergence. All experiments were well watered and well fertilized. Experiments 1 and 2 were terminated at anthesis, and expt 3 at physiological maturity.
Controlled environment experiments were conducted in the summer of 2005 in a glasshouse (expt 4) and phytotron (expt 5) at CIRAD, Montpellier, France. Both experiments were laid out as a randomized block design with three replications. Seeds were germinated for 1 d at 30 °C in an illuminated culture chamber before sowing into drained 1-L pots containing fertilized soil. Pots were watered at least once daily to field capacity with a culture solution (pH 5·5) containing all essential micro-nutrients. Nevertheless, plants in expt 4 did show some symptoms of calcium deficiency. In expt 4, halogen lamps were used during cloudy days to maintain at least 300 µmol m−2 s−1 of photosynthetically active radiation (PAR). Night temperature was maintained at around 20 °C and a cooling system was used when temperature exceeded 35 °C during the day. In expt 5, air temperature was maintained at 28 °C/22 °C (day/night), and PAR was supplied with halogen lamps during a 13-h photoperiod. Experiments were terminated at the end of the tillering stage.
Plant measurements
Phenology and leaf size.
The number of emerged plants was scored daily on 2 m per row in each plot until complete emergence in field experiments and on all individual plants in controlled environment experiments. In the field experiments, five consecutive plants in one of the two middle rows of each plot were tagged after thinning for weekly counts of the number of visible and fully expanded leaves for all axes. A leaf was considered visible if its leaf tip was visible inside the whorl, and fully expanded if its ligule was located above the ligule of the previous leaf. Tillers were labelled according to leaf axil of origin, e.g. T3 appeared from the axil of leaf 3. Anthesis was scored on these five plants as the date when 50 % of plants had exserted anthers midway down the panicle.
Individual leaf size in expt 1 was measured with a planimeter (Delta-T) on fully expanded leaves from three plants per plot that were destructively sampled at three growth stages (six and 12 fully expanded leaves and flag leaf on the main shoot). In all other experiments, the area of all fully expanded leaves was estimated by non-destructive measurements of leaf blade length and maximal width on the five tagged plants per plot in expts 2 and 3 and on one plant per genotype per replication in expts 4 and 5. Leaf area (LA) was obtained by multiplying length and width by a shape coefficient (Kim et al., 2010). Coefficients were independent of culm origin and environment and genotypic differences were assumed to be negligible.
Destructive biomass samples.
Biomass accumulation in the field was determined by destructively harvesting an area of 2 m2 (ten plants) at regular intervals, starting before tiller appearance for the first sample and finishing around anthesis (expts 1 and 2) or maturity (expt 3) for the last sample. Five or six samples were taken per plot. Plants were cut at ground level and transported to a laboratory, where the number of plants and tillers (by rank) were recorded. Identification of tiller rank was facilitated by marking in the field at the time of tiller appearance. Samples were separated into main shoots and individual tiller ranks (T1–T5), and each sub-sample was separated into green leaves, dead leaves, stems (including leaf sheaths) and panicles. Green leaf area was measured using a planimeter. All samples were dried at 80 °C for at least 5 d in a fan-forced oven before recording dry mass. Data were used to calculate leaf area index (LAI), leaf area ratio (LAR, leaf area per unit total shoot biomass, m2 g−1) and specific leaf area (SLA, green leaf area per unit green leaf mass, cm2 g−1).
In the controlled environment experiments, two (expt 4) or three (expt 5) samples were taken for shoot and root dry mass and plant leaf area prior to and during tiller emergence. Shoot dry mass samples were divided into organs and axes, similar to the field experiments. Plants were sampled early in the morning to minimize variation in dry mass caused by accumulation of carbohydrate reserves. After each destructive sampling, pots were rearranged to maintain a canopy of five plants per m2 (similar to the field experiments) with border plants.
Data analysis
Thermal time was calculated from hourly data, using a broken linear relationship with cardinal temperatures of 11, 30 and 42 °C for the base, optimum and maximum temperature (Hammer et al., 1993).
Data were analysed using standard analysis of variance procedures in R (R Development Core Team, 2007). Combined analyses of variance across experiments were performed using the AOV procedure, after verifying homogeneity of variance errors. Locations and replications were considered random factors and the remaining effects as fixed. Comparisons between lines within an experiment were performed using Tukey's least-significant difference (LSD) method.
Kim et al. (2010) developed a plant carbohydrate supply/demand (S/D) index to quantify environmental effects on tillering:
| 1 |
where RADLED5 is the average incident global radiation per unit thermal time (MJ m−2 °Cd−1) during the period of expansion of main shoot leaf 5 (LED5, °Cd−1), LA5 the fully expanded area of L5, which was expanding at the start of tillering, and LLIR(4–9) the linear rate of increase in leaf length between L4 and L9. The product in the numerator is an index of carbohydrate supply to the plant during tillering. The size of L5 is an indicator of plant leaf area and reflects the assumption that early in the season all leaf area was intercepting light (Lafarge and Hammer, 2002). The term in the denominator [LLIR(4–9)] represents the rate of increase in leaf area at the onset of tillering and hence demand for carbohydrate. Equation (1) was used as a basis to develop an S/Dindex that incorporated genotypic effects on tillering.
RESULTS
Genotypic differences in frequency of early tiller ranks
The maximum total tiller number (Ntiller,max) and final fertile tiller number (FTN) showed significant (P < 0·001) genotype (G) and environment (E) effects (Table 1). However, the ranking of hybrids was generally consistent across experiments except for hybrids 2 and 5 in expt 4 (Fig. 1). Based on tiller number, hybrids could be classified into a low-tillering (LT, hybrids 1–3) and high-tillering (HT, hybrids 4–6) group, which differed significantly for Ntiller,max and FTN according to Tukey's LSD method. The GE interaction for Ntiller,max was small compared with the G and E main effects, although significant GE interactions were observed for some individual tiller ranks (Table 1).
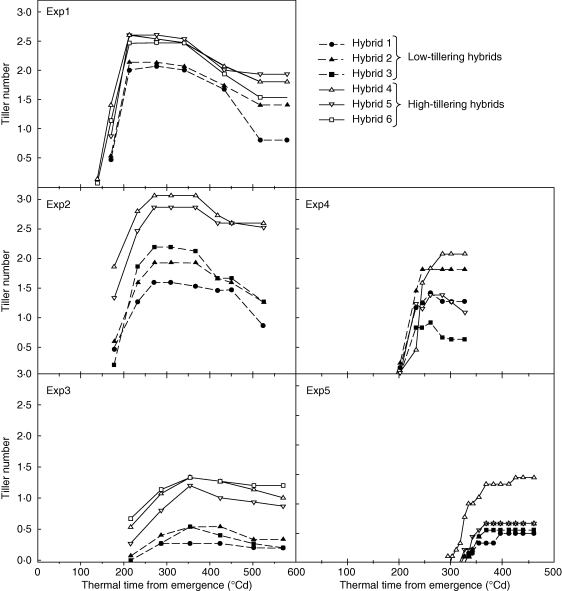
Fig. 1.
Tillering dynamics of each hybrid in field (expts 1–3) and controlled environment experiments (expts 4 and 5). Low-tillering hybrids (hybrids 1–3) are depicted by filled symbols and dotted lines and high-tillering hybrids (hybrids 4–6) by empty symbols and solid lines. Experiments 1 and 2 are high-tillering environments, expts 3 and 5 are low-tillering, whereas expt 4 is intermediate. Standard errors were below 0·2 and are not shown.
In general, onset of tillering was slightly later in LT than in HT hybrids (Fig. 1). This was associated with a reduced frequency of occurrence of lower order tillers (T1 and T2) in LT hybrids. In the two high-tillering experiments (expts 1 and 2), the difference between HT and LT hybrids in appearance frequency of T2 accounted for 76 % (expt 1) and 51 % (expt 2) of the difference in Ntiller,max. In the low-tillering experiment (expt 3), the difference in appearance frequency of T2 plus T3 accounted for over 80 % of the difference in Ntiller,max between LT and HT hybrids.
Phenology and coordination of main shoot and fertile tillers
Main shoot phenology was similar across hybrids. There were no significant G effects or GE interactions for both the tip and ligule phyllochron and final main shoot leaf number (data not shown). Consequently, thermal time from emergence to full flag leaf expansion was also similar across hybrids. As main shoot leaf number and time to flag leaf appearance differed significantly among the field experiments (Kim et al., 2010), the absence of significant G and GE effects for main shoot leaf number and time to full flag leaf expansion indicated that hybrids responded similarly to environmental cues.
Within an experiment, leaf appearance rates (tip and ligule) on fertile tillers were highly synchronized with main shoot leaf appearance (Fig. 2A, B). The HT and LT hybrids did not differ in the timing of appearance of T3 if expressed in terms of physiological age (Fig. 2A, B) and T3 appeared around tip appearance of main shoot L5–L6 in field experiments and L6–L7 in controlled environments. The delayed onset of tillering in LT compared with HT hybrids (Fig. 1) was therefore not associated with a delayed appearance of tillers of a specific rank. Total leaf number of tillers was lower than for main shoots. This compensated for their later appearance, causing a synchronization of phenology between tillers and main shoot.
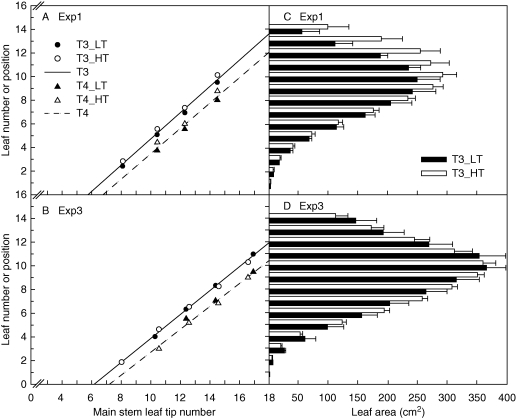
Fig. 2.
(A, B) Fully expanded leaf number on fertile T3 and T4 versus main shoot (MS) leaf tip number in a high- (expt 1) and low- (expt 3) tillering environment for low-tillering (LT) (hybrids 1–3) and high-tillering (HT) (hybrids 4–6) groups. (C, D) Individual leaf size profiles for fertile T3 of LT and HT groups in expts 1 and 3.
Genotypic differences in leaf area dynamics
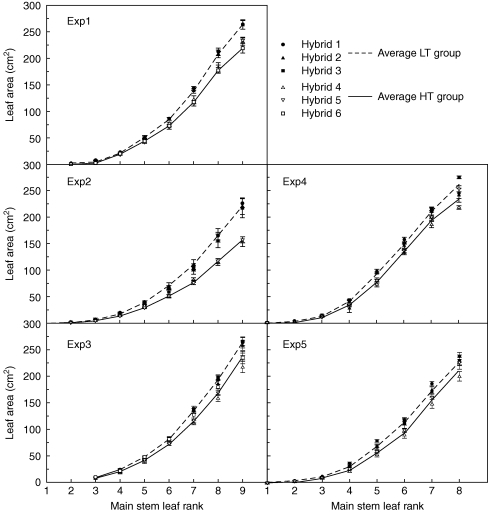
Individual leaf size on the main shoot differed significantly among hybrids from L5 onwards in the field and from L4 onwards in controlled environments (Fig. 3). In particular, LT hybrids had significantly larger leaf size than HT hybrids between L5 and L9 and this was predominantly due to wider rather than longer leaves (Table 2). Both the length and the width of successive leaves increased linearly with leaf rank between L4 and L9, but only the leaf width increase rate (LWIR) differed significantly among hybrids (Table 2). The significant environmental effect on LWIR was predominantly due to expt 4, where the high LWIR was associated with symptoms of calcium deficiency. Within experiments, Ntiller,max was negatively related to LWIR (Fig. 4). For fertile tillers, however, LT and HT hybrids did not differ significantly in leaf size for each tiller rank until the largest leaf was reached (Fig. 2C, D).
Fig. 3.
Individual leaf size profiles (from L1 to L9) for main shoot (MS) of low-tillering (LT) and high-tillering (HT) hybrids in field (expts 1–3) and controlled environments (expts 4 and 5) experiments. The dotted line represents the average of LT hybrids, the solid line the average of HT hybrids. Vertical bars indicate s.e.m.
Table 2.
Mean leaf width increase rate (LWIR; cm leaf−1) between main shoot L5 and L9 for each genotype (G) and environment (E) (except expt 1, which was similar to expt 3)
| Genotype | Expt 2 | Expt 3 | Expt 4 | Expt 5 |
|---|---|---|---|---|
| Hybrid 1 | 1·11 | 1·14 | 1·40 | 0·98 |
| Hybrid 2 | 1·13 | 1·10 | 1·37 | 0·94 |
| Hybrid 3 | 0·84 | 1·19 | 1·28 | 0·95 |
| Hybrid 4 | 0·72 | 0·97 | 1·22 | 0·86 |
| Hybrid 5 | 0·75 | 1·03 | 1·30 | 0·91 |
| Hybrid 6 | – | 0·97 | – | – |
| G | *** | |||
| E | *** | |||
| G × E | * |
Significance levels for G, E and G × E interaction effects are indicated: *P < 0·05; ***P < 0·001.
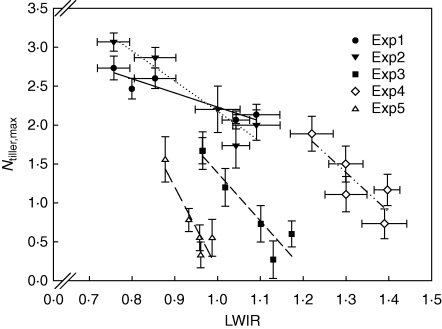
Fig. 4.
Maximum tiller number (Ntiller,max) versus leaf width increase rate (LWIR) for hybrids in each experiment. LWIR is calculated from main shoot leaf 4 to leaf 9. Lines represent linear regressions across hybrids within experiments. All regressions were significant at P < 0·05.
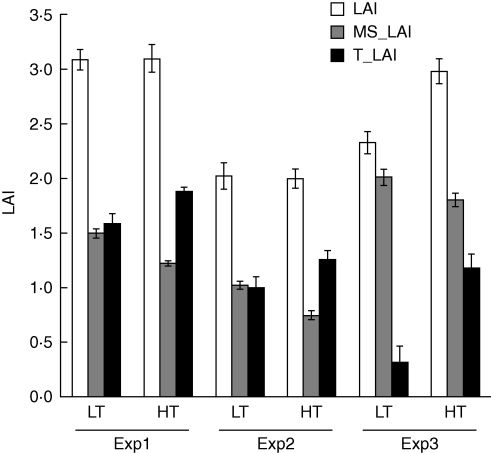
The larger size of individual leaves on the main shoot of LT hybrids, combined with the absence of genotypic differences in phyllochron and final leaf number, resulted in a consistently greater main shoot LAI for LT hybrids than HT hybrids throughout the pre-anthesis period (Fig. 5). In expts 1 and 2, this difference was compensated for by a difference in tiller leaf area, resulting in similar LAI at crop level for LT and HT hybrids (Fig. 5). In expt 3, however, the difference in tiller leaf area between the two groups was much larger, resulting in a significantly greater LAI at the flag leaf stage for HT than for LT hybrids.
Fig. 5.
Total leaf area index (LAI) and its allocation between main shoot (MS_LAI) and tillers (T_LAI) at flag leaf stage in field experiments for low-tillering (LT: hybrids 1–3) and high-tillering (HT: hybrids 4–5) hybrids. Vertical bars indicate s.e.m.
Biomass accumulation
Results for total above-ground biomass accumulation were consistent with those for LAI as there were no genotypic differences in total biomass accumulation within an experiment, either during the tillering phase or at the flag leaf stage. The only exception was expt 3, where HT hybrids had significantly greater biomass, associated with greater LAI. Consequently, the LAR did not differ significantly among hybrids. Consistent with these results, there were no genotypic differences in biomass partitioning among organs. Prior to stem elongation in expt 1, 68 % of above-ground dry mass was allocated to leaf blades, independent of hybrid and axis. Hybrids did not differ in SLA within experiments, but SLA decreased from approximately 300 cm2 g−1 (L5 stage) to 160 cm2 g−1 (flag leaf stage) in the field and from 500 cm2 g−1 (L4 stage) to 250 cm2 g−1 (L8 stage) in controlled environments. Hybrids also did not differ significantly in root mass and root/shoot ratio during the pre-tillering and tiller emergence phases in either expt 4 or expt 5 (data not shown). However, they differed consistently in biomass partitioning among axes, as LT hybrids had significantly lower tiller biomass per unit of main shoot biomass than HT hybrids (data not shown).
Developing a S/Dindex incorporating genotypic effects on tillering
The relationship between tillering and leaf width (Fig. 4) indicated that genotypes with larger main shoot leaves, and thus greater leaf area growth and demand for assimilate, produced fewer tillers. This supports the hypothesis that genotypic differences in tillering are associated with differences in internal plant competition for assimilates. Therefore, the S/Dindex developed by Kim et al. (2010) was enhanced by incorporating the main genotypic factor controlling tillering (LWIR) into the term representing plant leaf area growth and assimilate demand in the denominator of the index:
| 2 |
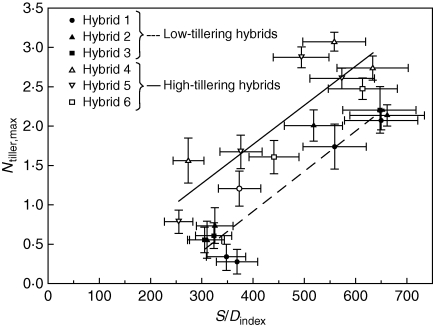
The relationship between this revised S/Dindex and Ntiller,max explained a high proportion of the variation associated with genotypic and environmental effects on Ntiller,max, but the relationship differed for HT and LT hybrids (Fig. 6). There was a significant reduction in the variation explained (approx. 20 %) if the LWIR term was not included in the S/Dindex, indicating its relevance and value in capturing some of the genotypic effects. The slopes of the relationships for HT (0·0050 ± 0·0001) and LT hybrids (0·0051 ± 0·0001) did not differ significantly, but the intercept with the x-axis was less for HT hybrids, suggesting a higher propensity to tiller, in addition to the effects associated with internal plant competition.
Fig. 6.
Ntiller,max versus S/Dindex for all hybrids and experiments. The S/Dindex incorporates genotypic difference in main shoot leaf size (LWIR) in the plant demand component (see text). The solid line represents linear regression for HT hybrids [y = 0·0050(±0·0001)x – 0·22, R2 = 0·75**] and dashed line represents regression for LT hybrids [y = 0·0051(±0·0001)x – 1·12, R2 = 0·89**]. Results for expt 4 were excluded due to the effect of calcium deficiency on leaf width.
DISCUSSION
This study quantified genotypic differences in tillering of sorghum through a carbohydrate S/Dindex of internal plant competition that was based on data from five hybrids with a similar genetic background, but contrasting tillering behaviour, grown in a range of high- and low-tillering environments. Low-tillering hybrids had wider leaves than high-tillering hybrids, which resulted in greater main shoot leaf area during tillering. The associated high demand for carbon restricted carbon availability for tillering, resulting in genotypic differences in the frequency of appearance of tillers at lower leaf ranks. A generalized S/Dindex that incorporated both genotypic and environmental effects on tillering explained a large proportion of the observed variation in tillering. However, some residual genotypic effects suggested that hybrids may also differ in their propensity to produce tillers, which was independent of the carbon supply/demand status.
High tillering was associated with small leaf size
Genotypic differences in tillering were associated with differences in main shoot leaf area around the onset of tillering, which were in turn a consequence of differences in leaf size (Fig. 3), associated with differences in leaf width (Table 2). The negative association between main shoot leaf area or leaf width and tillering (Fig. 4) has also been reported for pearl millet (van Oosterom et al., 2001), wheat (Rebetzke et al., 2004) and rice (Tivet et al., 2001). Genotypic differences in leaf width could be due to differences in meristem size, and a causal relationship between the ontogenetic increase in meristem size and increased width of successive leaves has been suggested (Wardlaw, 1952). Environmental effects on the relationship between LWIR and Ntiller,max (Fig. 4) were probably associated with environmental effects on leaf length of sorghum (Kaitaniemi et al., 1999; Lafarge and Tardieu, 2002; Kim et al., 2010). For example, the relatively low Ntiller,max in expt 3 (Fig. 4) was associated with a 20 % greater LLIR than in expt 2 (Kim et al., 2010), which had the same effect on main shoot leaf area as an increase in LWIR. In contrast, the low Ntiller,max in expt 5 (Fig. 4) was associated with an extremely slow phyllochron, probably a response to low radiation levels (Kim et al., 2010). This may have caused supply limitations for tillering (eqn 2). These disparate effects are, however, accommodated by the generalized S/Dindex, as shown by the consistency of its relationship with Ntiller,max across experiments (Fig. 6).
Genotypic differences in tillering were evident in the frequency of appearance of lower-rank tillers (Table 1). Processes determining differences in tiller appearance thus already operated at the onset of tiller outgrowth. This supports the hypothesis that genotypic differences in main shoot growth rate around this stage are critical determinants of genotypic differences in tillering (Bos and Neuteboom, 1998; Dingkuhn et al., 2001). In the absence of consistent genotypic differences in biomass partitioning, it is likely that a greater main shoot leaf area around tiller emergence was associated with greater main shoot biomass. A high growth rate of the main shoot could generate high carbon demand, which reduced the availability of carbon for tillering.
High tillering was also associated with propensity to tiller
The HT hybrids also had a higher propensity to tiller than LT hybrids and this was independent of the S/Dindex (Fig. 6). This finding suggests genotypic differences in the threshold S/Dindex at which tiller buds start to grow. This could be due to differences in either hormonal signalling or responsiveness to sugar levels in the plant. Recent studies have identified novel hormonal triggers for branching (Gomez-Roldan et al., 2008) and genes regulating polar auxin transport (Multani et al., 2003) that are known to affect tiller outgrowth in sorghum (George-Jaeggli, 2009). Other studies have identified many genomic regions associated with tillering in sorghum (Hart et al., 2001; Feltus et al., 2006) so that numerous regulatory mechanisms are likely.
Genotypic and environmental effects on tillering could be integrated in a single S/Dindex
The observation that environments differed in LLIR (Kim et al., 2010), whereas hybrids differed in LWIR, suggests that the developmental processes that control leaf length and width are under independent genetic control. This is consistent with findings in Arabidopsis, where specific genes regulating meristematic activities in cell division and proliferation have been identified (Tsuge et al., 1996; Tsukaya, 2005). This justified the use of both LLIR and LWIR in the S/Dindex (eqn 2).
The present results support the hypothesis that genotypic differences in tillering were associated with differences in the carbon supply–demand balance (leaf width) and in the propensity to tiller (possibly associated with hormonal signalling). However, the S/Dindex can also account for differences in tillering due to other physiological processes that affect the carbon S/D balance of a crop. For example, low tillering in sorghum has also been linked to high main shoot leaf area in response to a rapid leaf appearance rate (van Oosterom et al., 2008). A rapid leaf appearance rate would reduce LED5, causing a reduction in the S/Dindex and hence in tillering. The S/Dindex thus provides a robust means to integrate environmental and genotypic effects that regulate tillering.
This robustness provides an avenue for phenotyping of mapping populations to pursue identification of genomic regions and genes associated with traits that control tillering. The association of genetic variation in tillering with leaf width and propensity to tiller could be used to identify quantitative trait loci that are stable across environments in a similar manner to that shown for leaf extensive growth in maize (Reymond et al., 2004). This could potentially allow screening for tillering in environments where genotypic differences in tillering are poorly expressed, such as high-density experiments conducted in glasshouses.
Implications for adaptation to drought
The genotypic differences in leaf size, and consequently in tillering, could result in differences in leaf area dynamics over time that would affect leaf area at anthesis (Fig. 5) and hence adaptation to drought (Borrell et al., 2000; Hammer, 2006; van Oosterom et al., 2008). The larger main shoot leaf size (Fig. 3) of LT hybrids resulted in greater main shoot LAI early in the season. In environments without genotypic differences in LAI at anthesis, such early vigour of the main shoot would result in greater LAI for most of the pre-anthesis period. This could increase pre-anthesis water use of LT hybrids compared with HT hybrids. However, in environments where differences in tillering are substantial (expt 3, Fig. 5), the greater tiller LAI of HT hybrids would more than compensate for their lower main shoot LAI, resulting in greater plant size and hence potentially greater water use for HT hybrids. The effect of tillering on drought adaptation is therefore not straightforward and depends on specific environmental conditions and management practices.
To provide a better insight into complex genotype-by-management-by-environment (GME) interactions associated with tillering and adaptation to drought, the understanding of G and E effects on the dynamics of tillering generated in this study could be incorporated into suitably structured crop growth simulation models (Keating et al., 2003; Luquet et al., 2006). GME combinations likely to improve grain yield in specific water-limited environments could be identified by exploring the GME adaptation landscape via simulation (Hammer et al., 2005, 2006), similar to the approach reported for leaf elongation rate in maize (Chenu et al., 2008, 2009).
ACKNOWLEDGEMENTS
We thank Ian Broad, Zongjian Yang, Vijaya Singh, Kurt Deifel and Janette Wood for assistance in collecting field data. We also acknowledge David Jordan from Queensland Primary Industries & Fisheries, Department of Employment, Economic Development and Innovation, for providing the genetic material issued from its sorghum breeding programme.
LITERATURE CITED
- Aspinall D. The control of tillering in the barley plant. 1. The pattern of tillering and its relation to nutrient supply. Australian Journal of Biological Sciences. 1961;14:493–505. [Google Scholar]
- Birch CJ, Carberry PS, Muchow RC, McCown RL, Hargreaves JNG. Development and evaluation of a sorghum model based on CERES-Maize in a semi-arid tropical environment. Field Crops Research. 1990;24:87–104. [Google Scholar]
- Borrell AK, Hammer GL, Douglas ACL. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Science. 2000;40:1026–1037. [Google Scholar]
- Bos HJ, Neuteboom JH. Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): responses to temperature and light intensity. Annals of Botany. 1998;81:131–139. [Google Scholar]
- Bruns HA, Horrocks RD. Relationship of yield components of main culms and tillers of grain sorghum. Field Crops Research. 1984;8:125–133. [Google Scholar]
- Canell RQ. The tillering pattern in barley varieties. II. The effect of temperature, light intensity and day-length on the frequency of occurrence of the coleoptile node and second tillers in barley. Journal of Agricultural Science. 1969;72:423–435. [Google Scholar]
- Chenu K, Chapman SC, Hammer GL, McLean G, Tardieu F. Short term responses of leaf growth rate to water deficit scale up to whole plant and crop levels. An integrated modelling approach in maize. Plant, Cell and Environment. 2008;31:378–391. doi: 10.1111/j.1365-3040.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Chenu K, Chapman SC, Tardieu F, McLean G, Welcker C, Hammer GL. Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize: a ‘gene-to-phenotype’ modeling approach. Genetics. 2009;183:1507–1523. doi: 10.1534/genetics.109.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingkuhn M, Tivet F, Siband P, Asch F, Audebert A, Sow A. Varietal differences in specific leaf area: a common physiological determinant of tillering ability and early growth vigor? In: Peng S, Hardy B, editors. Rice research for food security and poverty alleviation. Los Banos, Philippines: International Rice Research Institute; 2001. pp. 95–108. [Google Scholar]
- Dingkuhn M, Luquet D, Kim HK, Tambour L, Clement-Vidal A. Ecomeristem, a model of morphogenesis and competition among sinks in rice: 2. Simulating genotype responses to phosphorus deficiency. Functional Plant Biology. 2006;33:325–337. doi: 10.1071/FP05267. [DOI] [PubMed] [Google Scholar]
- Doust A. Architectural evolution and its implications for domestication in grasses. Annals of Botany. 2007;100:941–950. doi: 10.1093/aob/mcm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, van Herwaarden AF, Fettell NA. Agronomic evaluation of a tiller inhibition gene (tin) in wheat. I. Effect on yield, yield components, and grain protein. Australian Journal of Agricultural Research. 2005;56:169–178. [Google Scholar]
- Feltus FA, Hart GE, Schertz KF, et al. Alignment of genetic maps and QTLs between inter- and intra- specific sorghum populations. Theoretical and Applied Genetics. 2006;112:1295–1305. doi: 10.1007/s00122-006-0232-3. [DOI] [PubMed] [Google Scholar]
- Friend DJC. Tillering and leaf production in wheat as affected by temperature and light intensity. Canadian Journal of Botany. 1965;43:1063–1076. [Google Scholar]
- George-Jaeggli B. The physiology and genetics of height-yield associations in sorghum. 2009 PhD thesis, The University of Queensland. [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Hammer GL. Pathways to prosperity: breaking the yield barrier in sorghum. The Journal of the Australian Institute of Agricultural Science and Technology. 2006;19:16–22. [Google Scholar]
- Hammer GL, Muchow RC. Assessing climatic risk to sorghum production in water-limited subtropical environments. I. Development and testing of a simulation model. Field Crops Research. 1994;36:221–234. [Google Scholar]
- Hammer GL, Carberry PS, Muchow RC. Modelling genotypic and environmental control of leaf area dynamics in grain sorghum. I. Whole plant level. Field Crops Research. 1993;33:293–310. [Google Scholar]
- Hammer GL, Butler D, Muchow RC, Meinke H. Integrating physiological understanding and plant breeding via crop modelling and optimisation. In: Cooper M, Hammer GL, editors. Plant adaptation and crop improvement. Wallingford, UK: CAB International, ICRISAT & IRRI; 1996. pp. 419–441. [Google Scholar]
- Hammer GL, Chapman SC, van Oosterom EJ, Podlich DW. Trait physiology and crop modelling as a framework to link phenotypic complexity to underlying genetic systems. Australian Journal of Agricultural Research. 2005;56:947–960. [Google Scholar]
- Hammer G, Cooper M, Tardieu F, et al. Models for navigating biological complexity in breeding improved crop plants. Trends in Plant Science. 2006;11:587–593. doi: 10.1016/j.tplants.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hart GE, Schertz KF, Peng Y, Syed NH. Genetic mapping of Sorghum bicolor (L.) Moench QTLs that control variation in tillering and other morphological characters. Theoretical and Applied Genetics. 2001;103:1232–1242. [Google Scholar]
- Heiniger RW, Vanderlip RL, Welch SM. Developing guidelines for replanting grain sorghum: I. Validation and sensitivity analysis of the SORKAM sorghum growth model. Agronomy Journal. 1997;89:75–92. [Google Scholar]
- Honda T, Okajima H. Environmental light conditions and tiller development in the rice plant. 3. Effects of partial shading and temperature on the development of tiller buds and dry matter increments. Bulletin of the Institute for Agricultural Research. 1970;22:1–15. [Google Scholar]
- Islam TMT, Sedgley RH. Evidence of a ‘uniculm effect’ in spring wheat (Triticum aestivum L.) in a Mediterranean environment. Euphytica. 1981;30:277–282. [Google Scholar]
- Jones HG, Kirby EJM. Effects of manipulation of number of tillers and water supply on grain yield in barley. Journal of Agricultural Science. 1977;88:391–397. [Google Scholar]
- Kaitaniemi P, Room PM, Hanan JS. Architecture and morphogenesis of grain sorghum, Sorghum bicolor (L.) Moench. Field Crops Research. 1999;61:51–60. [Google Scholar]
- Kasperbauer MJ, Karlen DL. Light-mediated bioregulation of tillering and photosynthate partitioning in wheat. Physiologia Plantarum. 1986;66:159–163. [Google Scholar]
- Keating BA, Carberry PS, Hammer GL, et al. An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy. 2003;18:267–288. [Google Scholar]
- Kim HK, van Oosterom EJ, Dingkuhn M, Luquet D, Hammer GL. Regulation of tillering in sorghum: environmental effects. Annals of Botany. 2010;106:57–67. doi: 10.1093/aob/mcq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarge TA, Hammer GL. Predicting plant leaf area production: shoot assimilate accumulation and partitioning, and leaf area ratio, are stable for a wide range of sorghum population densities. Field Crops Research. 2002;77:137–151. [Google Scholar]
- Lafarge T, Tardieu F. A model co-ordinating the elongation of all leaves of a sorghum cultivar was applied to both Mediterranean and Sahelian conditions. Journal of Experimental Botany. 2002;53:715–725. doi: 10.1093/jexbot/53.369.715. [DOI] [PubMed] [Google Scholar]
- Lauer JG, Simmons SR. Photoassimilate partitioning of main shoot leaves in field-grown spring barley. Crop Science. 1985;25:851–855. [Google Scholar]
- Luquet D, Dingkuhn M, Kim HK, Tambour L, Clement-Vidal A. EcoMeristem, a model of morphogenesis and competition among sinks in rice. 1. Concept, validation and sensitivity analysis. Functional Plant Biology. 2006;33:309–323. doi: 10.1071/FP05266. [DOI] [PubMed] [Google Scholar]
- Maas SJ. Parameterised model of gramineous crop growth: I. Leaf area and dry mass simulation. Agronomy Journal. 1993;85:348–353. [Google Scholar]
- Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS. Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science. 2003;302:81–84. doi: 10.1126/science.1086072. [DOI] [PubMed] [Google Scholar]
- van Oosterom EJ, Carberry PS, Hargreaves JNG, O'Leary GJ. Simulating growth, development, and yield of tillering pearl millet II. Simulation of canopy development. Field Crops Research. 2001;72:67–91. [Google Scholar]
- van Oosterom E, Hammer G, Kim HK, McLean G, Deifel K. Unkovich M, editor. Plant design features that improve grain yield of cereals under drought. Global issues, paddock action. Proceedings of the 14th Australian Society of Agronomy Conference. 2008:21–25. September 2008, Adelaide, South Australia. CD ROM proceedings (ISBN 1 920842 34 9). Gosford, Australia: The Regional Institute. www.agronomy.org.au . [Google Scholar]
- Pieters AJ, Paul MJ, Lawlor DW. Low sink demand limits photosynthesis under P deficiency. Journal of Experimental Botany. 2001;52:1083–1091. doi: 10.1093/jexbot/52.358.1083. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. http://www.R-project.org://www.R-project.org . [Google Scholar]
- Rai KN, Murty DS, Andrews DJ, Bramel-Cox PJ. Genetic enhancement of pearl millet and sorghum for the semi-arid tropics of Asia and Africa. Genome. 1999;42:617–628. [Google Scholar]
- Rebetzke GJ, Botwright TL, Moore CS, Richards RA, Condon AG. Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crops Research. 2004;88:179–189. [Google Scholar]
- Reymond M, Muller B, Tardieu F. Dealing with the genotype×environment interaction via a modeling approach: a comparison of QTLs of maize leaf length or width with QTLs of model parameters. Journal of Experimental Botany. 2004;55:2461–2472. doi: 10.1093/jxb/erh200. [DOI] [PubMed] [Google Scholar]
- Rosenthal WD, Vanderlip RL, Jackson BS, Arkin GF. SORKAM: a grain sorghum crop growth model. Texas Agricultural Experimental Station: Miscellaneous Publications; 1989. MP-1669. [Google Scholar]
- Tivet F, da Silveira Pinheiro B, de Raissac M, Dingkuhn M. Leaf blade dimensions of rice (Oryza sativa and Oryza glaberrima Steud.). Relationships between tillers and main stem. Annals of Botany. 2001;88:507–511. [Google Scholar]
- Tsuge T, Tsukaya H, Uchimiya H. Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thatliana (L.) Heynh. Development. 1996;122:1589–1600. doi: 10.1242/dev.122.5.1589. [DOI] [PubMed] [Google Scholar]
- Tsukaya H. Leaf shape: genetic controls and environmental factors. International Journal of Developmental Biology. 2005;49:547–555. doi: 10.1387/ijdb.041921ht. [DOI] [PubMed] [Google Scholar]
- Wardlaw CW. Experimental and analytical studies of pteridophytes. XVIII. The nutritional status of the apex and morphogenesis. Annals of Botany. 1952;16:207–218. [Google Scholar]
- Winward D, Hanks RJ, Dewey WG, Albrechtsen RS. Influence of detillering and irrigation on wheat and barley yields. Utah Agricultural Experiment Station Research Report. 1983:1–27. [Google Scholar]
- Yoshida S. Physiological aspects of grain yield. Annual Review of Plant Physiology. 1972;23:437–464. [Google Scholar]