Abstract
Background and Aims
Most studies on seed position-dependent effects have focused on germination characteristics. Our aim was to determine the effects of seed position in the spikelet on differences in timing of germination and on the ecological life history of the grass Eremopyrum distans in its cold desert habitat.
Methods
For seeds in three spikelet positions, morphology, mass and dormancy/germination characteristics were determined in the laboratory, and seeds planted in field plots with and without watering were followed to reproduction to investigate seedling emergence and survival, plant size and seed production.
Key Results
After maturation, of the seeds within the spikelet, basal ones (group 1) are the largest and have the highest proportion with physiological dormancy, while distal ones (group 3) are the smallest and have the highest proportion of non-dormant seeds. A higher percentage of seeds after-ripened in groups 2 and 3 than in group 1. Seeds sown in the field in early summer and watered at short, regular intervals germinated primarily in autumn, while those under natural soil moisture conditions germinated only in spring. Both cohorts completed their life cycle in early summer. Seeds in group 1 had lower percentages of seedling emergence and higher percentages of seedling survival than those in groups 2 and 3. Also, plants from group 1 seeds were larger and produced more seeds per plant than those from groups 2 and 3.
Conclusions
Seed position-dependent mass was associated with quantitative differences in several life history traits of E. distans. The environmentally enforced (low soil moisture) delay of germination from autumn to spring results in a reduction in fitness via reduction in number of seeds produced per plant.
Keywords: Eremopyrum distans, physiological dormancy, plant size, seed mass, seed position-dependent effects, seed production, seedling survival
INTRODUCTION
The position of a seed or fruit on a plant can affect its morphology, mass and dormancy/germination characteristics (Gutterman, 2000; Baskin and Baskin, 2001; Moravcová et al., 2005), and these responses are described as ‘position-dependent effects’ (Cheplick and Sung, 1998; Moravcová et al., 2005). Variations in dormancy and germination may occur in seeds/fruits from subterranean versus aerial flowers of amphicarpic plants (Baskin and Baskin, 2001) or from flowers produced in/at different (a) parts of the same inflorescence (Datta et al., 1970), (b) inflorescences at different positions on a plant (Venable and Lawlor, 1980; Venable et al., 1987), (c) heights on the stem (Baskin and Baskin, 2001) or (d) positions in a bur (Baskin and Baskin, 2001) or fruit (Maun and Payne, 1989). These differences may be due to (a) resources not being allocated equally to all seeds (Datta et al., 1970) and/or (b) seeds produced at one position (e.g. at the base of an inflorescence) developing under different environmental conditions than those produced at another position (e.g. at the top of an inflorescence), including differences in physiological age of the mother plant at the time seeds are produced (Baskin and Baskin, 2001).
Recently, most studies of position-dependent effects have focused on germination characteristics of seeds at different positions. The best examples of how the position within an inflorescence influences germination of the progeny are found in a number of species of Asteraceae with both disc (central) and ray (peripheral) flowers in the head (Baskin and Baskin, 2001). Other examples of variation in germination responses by seeds produced in different parts of the same inflorescence include some species of Poaceae, e.g. Aegilops ovata (Datta et al., 1970), A. neglecta, A. geniculata and A. triuncialis (Marañon, 1987). In these grasses, the basal seed (caryopsis) in a spikelet is larger and less dormant than the upper one. The annual dunegrass Triplasis purpurea exhibits a position-dependent seed heteromorphism in seed number, mass and dormancy/germination characteristics (Cheplick and Sung, 1998). However, little is known about the effects of seed position in the spikelet on differences in timing of germination and the consequences on the ecological life history of annual grasses.
The effects of seed position in the spikelet on seed morphology and mass, seed dormancy/germination characteristics, seedling survival, plant size and seed production of the cold desert annual grass Eremopyrum distans were investigated. The primary objective of the research was to determine whether there are seed position-dependent effects in spikelets of E. distans and, if so, what the consequences are for the ecological life history of the species in its cold desert habitat with summer rainfall.
MATERIALS AND METHODS
Study species, seed collection site and germination test temperatures
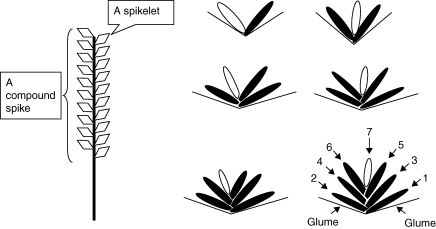
Eremopyrum distans (C. Koch) Nevski, a close relative of cultivated wheat (Liu and Ding, 1996), grows in the former Soviet Republics of central Asia, and in Caucasus, Asia Minor, Iran, Himalayas and the northern part of Xinjiang Province in China (Cui et al., 1996; Fedorov, 1999). In the Junggar Desert of Xinjiang Province, it is widely distributed and is an important component of the desert vegetation in early spring. The species has important ecological value since it increases the roughness of the ground and helps stabilize sandy soil. Also, it is one of the prime early-spring native forage species for livestock (Lv and Tan, 2005). There are several tillers on an individual plant, and each has one compound spike that contains several spikelets. The number of florets in a spikelet varies from 1 to 7, and florets in a spikelet were numbered from 1 (the most basal) to 7 (the most distal; Fig. 1). None of the most distal florets in spikelets produced seeds, and <1 % of florets in positions 4–6 produced seeds (A. B. Wang, pers. obs.). Thus, seeds in positions 1, 2 and 3 were used in this study and were placed in groups 1, 2 and 3, respectively.
Fig. 1.
Compound spike and spikelet of Eremopyrum distans. These diagrams represent gradations of spikelets from top to bottom of the compound spike. Numbers 1–7 refer to seeds in a spikelet. Seeds in positions 1, 2 and 3 were placed in study groups 1, 2 and 3, respectively. The shaded position in a spikelet indicates a filled seed (all seeds used in this study were filled and presumably viable), and non-shaded position indicates an empty seed.
The study site, on Yamalic Mountain, about 2 km from the laboratory in Urümqi, China, has typical desert vegetation, gravelly grey desert soil and a continental climate (Sun et al., 2009). The site is located on the southern edge of Junggar Desert of Xinjiang Province in north-west China (43°79′94·2″N, 87°56′70·4″E, 920 m a.s.l.). Whole spikelets containing fresh-matured caryopses (hereafter seeds) were collected from plants of E. distans growing at the study site on 8 June 2008, at which time spikelets were dispersing naturally. After collection, seeds were divided into three groups according to their position in the spikelets.
During the germination tests, seeds were incubated at daily (12/12 h) temperature regimes of 30/15, 25/15, 20/10, 15/2 and 5/2 °C in light (12-h daily photoperiod, about 100 µmol m−2 s−1, 400–700 nm, cool white fluorescent light) or in constant darkness (seeds in Petri dishes were put in black bags) for 28 d. A seed was considered to be germinated when the radicle had emerged. The four higher temperature regimes approximate mean daily maximum and minimum monthly air temperatures in the vicinity of Urümqi at different times during the growing season: March, April and November, 15/2 °C; May, September and October, 20/10 °C; June and August, 25/15 °C; and July, 30/15 °C (Sun et al., 2009). Since the winter temperature in the vicinity of Urümqi is below 0 °C and seeds would not germinate at this temperature, the 5/2 °C temperature regime was used to simulate late winter temperature and test the effects of low temperature on germination of the seeds (Sun et al., 2009).
Effect of seed position on seed morphology and mass
Average dimensions and mass of seeds produced were determined in each of the three spikelet positions. The lemma and palea were removed from 30 seeds of each group and their lengths and widths measured by a vernier caliper. Also, lengths of trichomes, awns and teeth were measured. Ten replicates of 100 seeds each were weighed using an electronic analytical balance.
Effect of seed position, light and temperature on germination of fresh seeds
To investigate germination responses of fresh seeds, four replicates of 25 fresh seeds each of the three groups were incubated on two layers of Whatman No. 1 filter paper moistened with 2·5 mL of distilled water in 9-cm-diameter plastic Petri dishes in light and in constant darkness at the five temperature regimes, as described above. Germination in light was examined daily for 28 d, and any seeds that had germinated were removed at each counting; distilled water was added as needed. Seeds incubated in constant darkness were checked only after 28 d, and thus they were not exposed to any light during the incubation period.
The rate of germination in light was estimated using the Timson index of germination velocity: germination index = ΣG/t, where G is seed germination percentage at 1-d intervals and t the germination period (Timson, 1965; Wang et al., 2008). In this equation, a high value indicates rapid germination, with the highest value obtainable being 100 (i.e. 2800/28).
Tetrazolium viability tests were conducted on ungerminated seeds in each group of E. distans seeds. Embryos were removed from seeds and cut into halves and then one part of each embryo was placed in 0·1 % aqueous 2,3,5-triphenyl tetrazolium chloride solution in the dark for 24 h at 30 °C. Embryos that stained red or pink were considered to be viable. The proportion of seeds in each group giving a positive test for viability was recorded.
Breaking seed dormancy
Germination of fresh seeds of each group in light and in darkness at all temperature regimes was <80 %. Seed viability tests using tetrazolium chloride gave a positive test in nearly 95 % of the ungerminated seeds in each group, indicating that most of the ungerminated seeds were still viable but dormant. Therefore, effects of dry storage and gibberellic acid (GA3) on dormancy-break were determined for seeds of the three groups.
Dry storage
After-ripening is one of the characteristics of seeds with non-deep physiological dormancy (PD) (Baskin and Baskin, 2001). Thus, this experiment aimed to determine if seeds come out of dormancy (after-ripened) during dry storage. Seeds of each group were stored for 0 (control) and 12 months in a closed cotton bag under room conditions (18–25 °C, 15–30 % relative humidity), beginning on 22 June 2008. After dry storage, four replicates of 25 seeds of each group were incubated in Petri dishes on two layers of filter paper moistened with distilled water at each temperature regime in light and in constant darkness for 28 d.
GA3 treatments
GA3 is a plant growth regulator that can break dormancy in seeds with non-deep PD (Nikolaeva, 1977). To test the effects of GA3 on dormancy break, four replicates of 25 fresh seeds of each group were incubated in 0 (distilled water control), 0·1, 1 and 10 mmol L−1 GA3 solutions in light at the optimal temperature regimes for each seed position of E. distans for 28 d. Germination was checked daily.
Effect of seed position on germination of seeds after burial in field
After 2 weeks of dry storage at room temperature, approx. 600 seeds of each group were placed in each of 12 fine-mesh nylon bags, i.e. the total number of bags for each group was 12. Each bag was buried to a depth of 7 cm in soil in a 16-cm-diameter, 19·5-cm-deep plastic pot with drainage holes, and then the pots were buried (the top of the pot was even with the soil surface) in gravel desert soil in the experimental garden of Xinjiang Agricultural University, in Urümqi, about 2 km from the site where seeds were collected. Seeds were subjected to natural temperature and soil moisture conditions; temperature and rainfall data were recorded at a weather station about 30 m from the pots.
Germination tests were conducted on seeds of each group that had been buried for 1–12 months. Beginning on 22 July 2008, seeds were exhumed on the same day of each month for 12 months (except in January and February 2009, when the soil was frozen and seeds could not be exhumed). All seeds were incubated in 9-cm-diameter plastic Petri dishes, and four replicates of 25 seeds each were placed in light at each temperature regime for 28 d. Germination was examined daily for 28 d. Any seeds that had germinated were removed at each counting, and distilled water was added when needed.
Effect of seed position on seedling emergence and survival, plant size and seed production
Six replicates of 100 seeds of each group were sown on 30 June 2008 in 1-m (length) × 0·5-m (width) plots in gravel desert soil in the experimental garden of Xinjiang Agricultural University. Seeds were protected from predation by formation of a 20-cm high ridge of soil around each plot, and two layers of fine-mesh netting were placed over the plots (resting on the ridge of soil). The netting was removed in late autumn, when seedlings were well established and ungerminated seeds had become covered by soil. Three replicates of each seed group were kept under natural soil moisture conditions, while the other three were watered to field capacity regularly. The watering intervals were set as follows: at 2-d intervals from June to August 2008 and from late March to May 2009 and at 3-d intervals in September and October 2008 and June 2009. During November to mid-March, seeds received natural soil moisture regimes; snow continuously covered the plots during this period of time. Plots were checked daily, and newly-emerged seeds were marked. The total number of germinated and living seedlings was recorded every week until 1 June 2009, by which time plants had flowered and set seeds. From these data, the number of seedlings that died each week could be determined. The viability (or not) of ungerminated seeds in the plot was not determined at the end of the study. For each surviving plant, the height, numbers of tillers per plant, spikelets per tiller and seeds per spikelet were recorded in June 2009. The number of seeds per plant and total number of seeds from all surviving plants were calculated. Temperature and rainfall data in the field where the plots were located were obtained from a weather station about 30 m from the plots.
Data analysis
Data were transformed as necessary to meet normality and homogeneity of variance assumptions. If the ANOVA assumptions were violated after data transformation, treatment differences were assessed by using the more conservative Kruskal–Wallis non-parametric test. While analysis was done on transformed data, non-transformed data are presented in the figures.
These data were analysed using SPSS for Windows, Version 13·0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA tested for effect of seed position on seed dimensions and mass, percentages of seedling emergence, percentages of seedling survival, plant size and seed production in the experimental garden. Two-way ANOVA was used to test for the significance of main effects (seed position and temperature) and their interaction on germination of buried seeds and on germination velocity of fresh seeds. Three-way ANOVA was used to test for significance of main effects (seed position, light and temperature) and their interaction on germination percentage of fresh seeds and main effects [seed age (fresh seeds or seeds after 12 months of dry storage), temperature and seed position] and their interaction on germination rate of seeds, and main effects (GA3 concentration, temperature and seed position) and their interaction on germination of fresh seeds. Four-way ANOVA was used to test for significance of main effects [seed age (fresh seeds or seeds after 12 months of dry storage), light condition, temperature and seed position] and their interaction on germination percentages. Tukey's HSD test was performed for multiple comparisons to test for significant (P < 0·05) differences between individual treatments. Statistical tests were conducted at P = 0·05. Values are means ± 1 standard error (Sokal and Rohlf, 1995).
RESULTS
Effect of seed position on seed morphology and mass
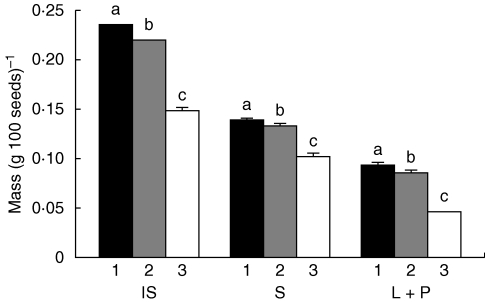
Seed position had significant effects on seed dimensions, except for length of palea and of palea teeth, with the dimension of each character decreasing as seed position increased (Table 1). Mass of the intact seed, of seed without lemma and palea and of lemma and palea decreased significantly as seed position increased (Fig. 2).
Table 1.
Dimensions (mm, mean ± standard deviation) of seeds and of lemma and palea and associated structures in the three seed groups of Eremopyrum distans (n = 30)
| Group | Length of lemma | Length of awn on lemma | Length of trichomes on lemma | Length of palea | Length of teeth on palea | Length of seed | Width of seed | Thickness of seed |
|---|---|---|---|---|---|---|---|---|
| Group 1 | 10·97 ± 0·28a | 2·63 ± 0·11a | 1·96 ± 0·08a | 7·04 ± 0·17a | 0·86 ± 0·05a | 3·31 ± 0·05a | 0·98 ± 0·02a | 0·92 ± 0·02a |
| Group 2 | 10·71 ± 0·27a | 2·51 ± 0·10a | 1·68 ± 0·06b | 6·88 ± 0·31a | 0·78 ± 0·04a | 3·22 ± 3·06a | 0·89 ± 0·03b | 0·92 ± 0·02a |
| Group 3 | 8·36 ± 0·23b | 1·47 ± 0·06b | 1·21 ± 0·10c | 6·48 ± 0·18a | 0·76 ± 0·04a | 3·07 ± 0·05b | 0·89 ± 0·02b | 0·69 ± 0·03b |
| P | <0·001 | <0·001 | <0·001 | 0·217 | 0·207 | 0·017 | 0·007 | <0·001 |
Different letters within a column indicate significant differences (Tukey's HSD, P = 0·05).
Fig. 2.
Mass per 100 fresh seeds of the three groups (n = 10). IS = intact seeds, S = seeds without lemmas and paleae, L + P = lemmas and paleae. Columns within IS, S and L + P with different letters are significantly different (P < 0·05).
Effect of seed position, light and temperature on germination of fresh seeds
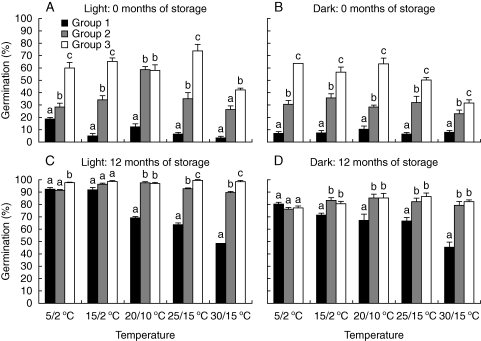
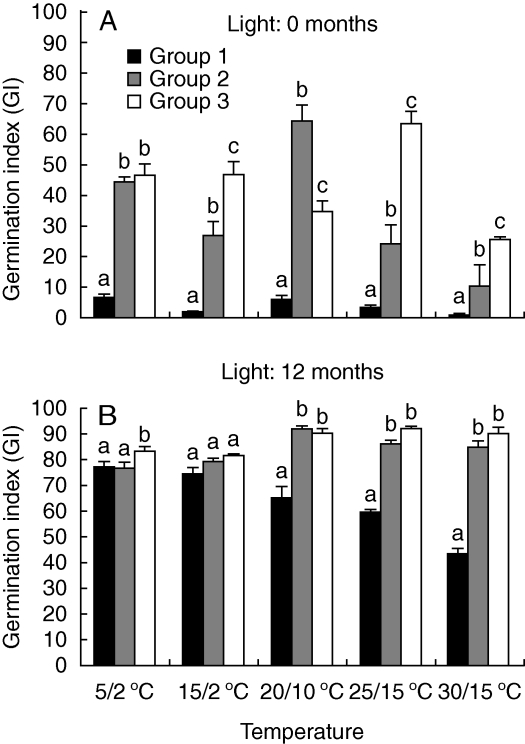
Germination percentage was significantly affected by seed position (P < 0·001), light (P < 0·001), temperature (P < 0·001), the interaction between seed position and temperature (P < 0·001), the interaction between seed position and light (P = 0·016) and the interaction between seed position, light and temperature (P < 0·001), but not by the interaction between light and temperature (P = 0·394). For fresh seeds, germination percentages increased as seed position increased at all temperature regimes both in light and in darkness (Fig. 3A and B). Germination velocity of fresh seeds was significantly affected by seed position (P < 0·001), temperature (P < 0·001) and their interaction (P < 0·001), and it increased as seed position increased at each temperature regime (Fig. 4A). In light, group 1, 2 and 3 seeds had the highest germination percentage and germination velocity at 5/2, 20/10 and 25/15 °C, respectively.
Fig. 3.
Germination percentages (mean + s.d.) of (A, B) fresh (0 months old), and (C, D) of 12 months dry-stored seeds of the three groups (n = 4). Columns within a temperature with different letters are significantly different (P < 0·05).
Fig. 4.
Germination index (mean + s.d.) of (A) fresh (0 months old) and (B) 12 months dry-stored seeds of the three groups in light (n = 4). Columns within a temperature with different letters are significantly different (P < 0·05).
Breaking seed dormancy
Dry storage
After 12 months of dry storage, germination percentages were higher than those of fresh seeds. Generally, germination percentages of 12-month-old seeds were higher for group 2 and 3 seeds than for group 1 seeds in both light and constant darkness (Fig. 3C and D). The main factors and their interactions significantly affected germination except for the interaction among seed age, light condition and seed position (Table 2). In light, germination of group 2 and group 3 seeds was >85 % at all temperature regimes, and maximum germination was 97·0 ± 0·5 % (at 20/10 °C) and 99·0 ± 0·3 % (at 25/15 °C) for the two groups, respectively. Maximum germination for group 1 seeds was 92·0 ± 0·5 % (at 5/2 °C). In constant dark, maximum germination was 80 ± 2·2 % (at 5/2 °C), 85 ± 3·8 % (at 20/10 °C) and 86 ± 2·9 % (at 25/15 °C) for groups 1, 2 and 3, respectively. After 12 months of dry storage, germination velocity of seeds was higher than that of fresh seeds and increased as seed position increased (Fig. 4B). Germination velocity was significantly affected by seed age (P < 0·001), temperature (P < 0·001), seed position (P < 0·001), the interaction between seed age and temperature (P = 0·003), the interaction between seed age and seed position (P < 0·001), the interaction between temperature and seed position (P < 0·001) and the interaction among the three main factors (P < 0·001).
Table 2.
Four-way ANOVA of effects of seed age, light condition, temperature and seed position and their interactions on seed germination percentages of Eremopyrum distans (n = 4)
| Source | d.f. | SS | MS | F-value | P-value |
|---|---|---|---|---|---|
| Seed age (SA) | 1 | 147510·417 | 147510·417 | 6461·096 | <0·001 |
| Light (L) | 1 | 3920·417 | 3920·417 | 171·718 | <0·001 |
| Temperature (T) | 4 | 4945·933 | 1236·483 | 54·159 | <0·001 |
| Seed position (SP) | 2 | 47346·233 | 23673·117 | 1036·905 | <0·001 |
| SA × L | 1 | 570·417 | 570·417 | 24·985 | <0·001 |
| SA × T | 4 | 931·333 | 232·833 | 10·198 | <0·001 |
| L × T | 4 | 258·000 | 64·500 | 2·825 | 0·026 |
| SA × L × T | 4 | 367·000 | 91·750 | 4·019 | 0·004 |
| SA × SP | 2 | 8178·633 | 4089·317 | 179·116 | <0·001 |
| L × SP | 2 | 717·033 | 358·517 | 15·703 | <0·001 |
| SA × L × SP | 2 | 35·033 | 17·517 | .767 | 0·466 |
| T × SP | 8 | 4441·767 | 555·221 | 24·319 | <0·001 |
| SA × T × SP | 8 | 4208·367 | 526·046 | 23·041 | <0·001 |
| L × T × SP | 8 | 945·300 | 118·163 | 5·176 | <0·001 |
| SA × L × T × SP | 8 | 656·300 | 82·038 | 3·593 | 0·001 |
GA3 treatments
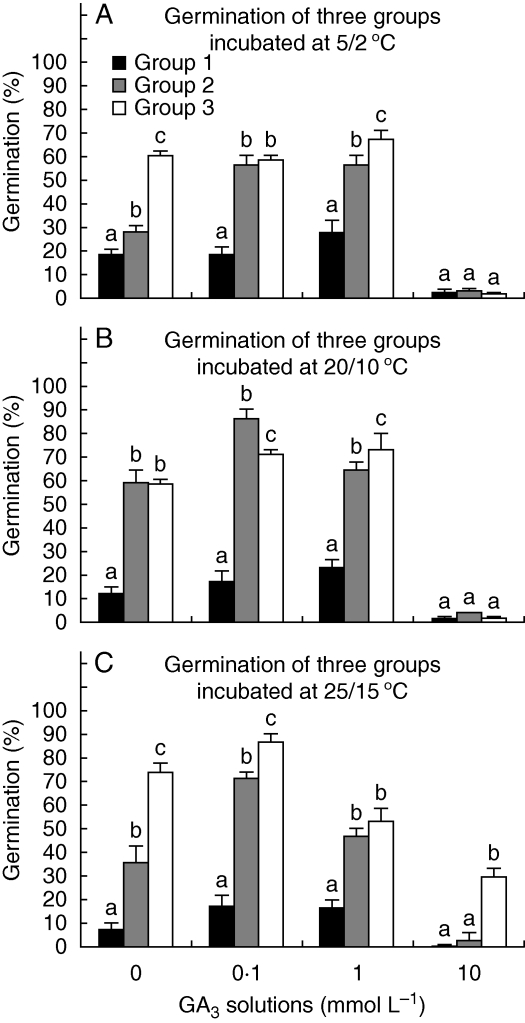
Germination percentages of fresh seeds were significantly different at different GA3 concentrations (P < 0·001) and seed positions (P < 0·001). However, temperature (P = 0·451) did not significantly affect germination. The highest germination for group 1, 2 and 3 seeds was 28 ± 4·3 % (at 1 mmol L−1 GA3, 5/2 °C), 86·0 ± 2·6 % (at 0·1 mmol L−1 GA3, 20/10 °C) and 87·0 ± 3·4 % (at 0·1 mmol L−1 GA3, 25/15 °C), respectively (Fig. 5).
Fig. 5.
Germination percentages (mean + s.d.) in light of the three groups of Eremopyrum distans seeds incubated in different concentrations of GA3 and at different temperatures (n = 4). Columns within a temperature and within a GA3 concentration with different letters are significantly different (P < 0·05).
Effect of seed position on germination of seeds after burial in field
After 1 month of burial in soil, group 1, 2 and 3 seeds germinated to a significantly higher percentage than did fresh ones at all temperature regimes in light (P < 0·001). At the five temperature regimes, germination after 1 month of burial ranged from 14·4 ± 2·0 to 36·7 ± 4·97 % for group 1, 45·0 ± 1·9 to 89·0 ± 1·0 % for group 2 and 68·6 ± 5·0 to 93·0 ± 1·9 % for group 3 seeds. After 2 months of burial, germination at the five temperature regimes had declined to 3·0 ± 1·0 to 32·4 ± 6·0 % for group 1, 24·0 ± 3·3 to 79·0 ± 1·9 % for group 2 and 7·0 ± 2·5 to 21·0 ± 1·9 % for group 3 seeds, and after 3 months of burial it was only 0·0 ± 0·0 to 4·0 ± 0·0 % for group 1, 11·0 ± 1·0 to 27·0 ± 0·4 % for group 2 and 0·0 ± 0·0 to 1·0 ± 0·1 % for group 3 seeds. Germination for all seed groups at all temperature regimes ranged from 0·0 ± 0·0 to 3·0 ± 1·9 % after 4 and 5 months of burial and from 0·0 ± 0·0 to 7·0 ± 4·7 % after 8, 9 and 10 months (data not shown). After 11 months of burial, none of the seeds germinated, and all of them collapsed when gently pinched with forceps, indicating that they were empty and dead.
Effect of seed position on seedling survival, plant size and seed production
Under natural temperature and soil moisture conditions, no seeds germinated in autumn, but they did germinate in spring (Figs 6C and 7). When watered regularly, seeds of each group germinated in summer–autumn and in spring; most seeds germinated in autumn and only a few in spring (Fig. 6A). Regular watering of each seed group resulted in percentages of seedling emergence (P < 0·001) and survival (P < 0·001) that were significantly higher for summer–autumn germination than for spring germination. Height of plants (P < 0·001), number of tillers per plant (P < 0·001), number of spikelets per tiller (P < 0·001) and number of seeds per spikelet (P < 0·001) of plants from seeds that germinated in summer–autumn were significantly greater than those of plants from seeds that germinated in spring (Table 3).
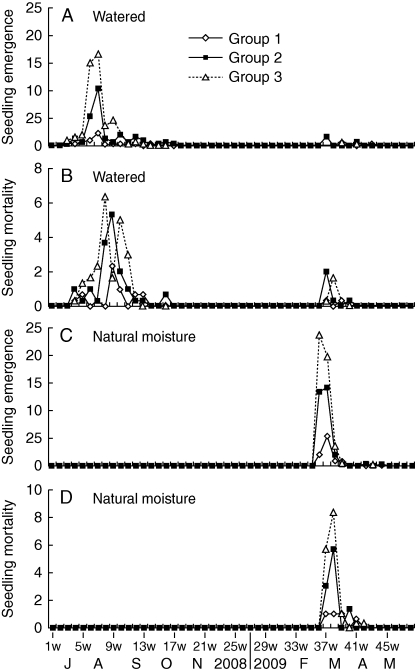
Fig. 6.
Number of seedlings emerging (A, C) and dying (B, D) in field plots of the three groups of Eremopyrum distans seeds watered regularly (A, B) and exposed to natural soil moisture conditions (C, D) (n = 3).
Table 3.
Plant size and seed production of Eremopyrum distans in the experimental garden (n = 3)
| Seed groups | G1 | G2 | G3 | P |
|---|---|---|---|---|
| Su-A (W) | ||||
| No. of surviving plants per plot | 4·7 ± 1·7 | 15·0 ± 1·3 | 22·0 ± 2·4 | |
| Height of plants (cm) | 36·1 ± 0·5 | 31·2 ± 0·4 | 24·2 ± 0·5 | <0·001 |
| No. of tillers per plant | 10·3 ± 0·8 | 6·8 ± 0·2 | 4·5 ± 0·2 | <0·001 |
| No. of spikelets per tiller | 10·9 ± 0·5 | 12·0 ± 0·6 | 15·3 ± 0·4 | <0·001 |
| No. of seeds per spikelet | 3·2 ± 0·1 | 2·7 ± 0·1 | 2·2 ± 0·1 | <0·001 |
| No. of seeds per plant | 375 | 229 | 147 | |
| Total no. of seeds from all plants | 1762 | 3428 | 3240 | |
| S (W) | ||||
| No. of surviving plants per plot | 1·7 ± 0·7 | 2·0 ± 0·6 | 2·3 ± 0·9 | |
| Height of plants (cm) | 30·6 ± 1·2 | 27·0 ± 1·1 | 21·9 ± 0·5 | 0·001 |
| No. of tillers per plant | 6·2 ± 1·1 | 3·6 ± 0·8 | 3·3 ± 0·3 | 0·003 |
| No. of spikelets per tiller | 10·8 ± 0·7 | 11·9 ± 0·3 | 11·9 ± 0·6 | 0·012 |
| No. of seeds per spikelet | 2·4 ± 0·1 | 2·4 ± 0·1 | 2·1 ± 0·1 | 0·005 |
| No. of seeds per plant | 159 | 102 | 86 | |
| Total no. of seeds from all plants | 271 | 3082 | 4209 | |
| S (N) | ||||
| No. of surviving plants per plot | 3·7 ± 0·3 | 12·0 ± 1·5 | 13·7 ± 2·1 | |
| Height of plants (cm) | 34·3 ± 0·6 | 29·8 ± 0·6 | 23·0 ± 0·5 | 0·044 |
| No. of tillers per plant | 6·6 ± 0·5 | 4·8 ± 0·2 | 3·4 ± 0·2 | <0·001 |
| No. of spikelets per tiller | 10·9 ± 0·7 | 12·6 ± 0·5 | 13·3 ± 0·4 | 0·001 |
| No. of seeds per spikelet | 2·6 ± 0·1 | 2·5 ± 0·1 | 2·2 ± 0·1 | 0·055 |
| No. of seeds per plant | 184 | 142 | 94 | |
| Total no. of seeds from all plants | 682 | 1706 | 1282 | |
Values are means ± s.d. G, Group; Su-A (W), summer–autumn germination of seeds that were watered at regular intervals; Sp (W), spring germination of seeds that were watered at regular intervals; Sp (N), spring germination of seeds under natural soil moisture conditions. All of the data are from plants that survived.
Seedling emergence (%) in summer–autumn (watering; P = 0·001) and spring (natural moisture; P < 0·001) increased significantly as seed position increased (Table 4). Percentages of seedling emergence in spring (watering) were not significantly affected by seed position (P = 0·159), but they increased as seed position increased. Seedling survival in summer–autumn (watering; P = 0·340), spring (watering; P = 0·858) and spring (natural moisture; P = 0·472) was not significantly affected by seed position, but decreased as seed position increased (Table 4). Height of plants, number of tillers per plant and number of seeds per spikelet from seedlings derived from summer–autumn (watering), spring (natural moisture) and spring (watering) germination all decreased significantly as seed position increased (P < 0·05). However, the number of seeds per spikelet produced by plants from spring (natural moisture) germination was not significantly affected by seed position (P = 0·055). On the other hand, the number of spikelets per tiller increased significantly as seed position increased (P < 0·05). The heights of plants decreased significantly as seed position increased (P < 0·001), regardless of whether or not seeds were watered or when they germinated (summer–autumn versus spring). The surviving plants from group 1 seeds produced a larger number of seeds per plant than those from either group 2 or group 3 seeds. However, the total number of surviving plants from group 2 or group 3 seeds was larger than that from group 1 seeds, and all surviving plants from group 1 seeds produced a smaller total number of seeds than surviving plants from either group 2 or group 3 seeds (Table 3).
Table 4.
Germination percentages (mean ± standard deviation) per plot in three seed groups of Eremopyrum distans that emerged in the field in autumn and in spring and survival (mean ± s.d.) of seedlings to reproductive maturity per plot under regular watering and natural soil moisture conditions (n = 3)
| Watered at regular intervals |
Natural moisture condition |
|||||
|---|---|---|---|---|---|---|
| Summer–autumn germination |
Spring germination |
Spring germination |
||||
| Germination (%) | Survival | Germination (%) | Survival | Germination (%) | Survival | |
| Group 1 | 8·3 ± 2·2 | 68·3 ± 4·3 | 4·5 ± 1·1 | 50·0 ± 1·7 | 12·1 ± 2·3 | 42·7 ± 0·8 |
| Group 2 | 33·3 ± 4·7 | 59·6 ± 5·9 | 7·4 ± 2·3 | 54·6 ± 1·4 | 37·9 ± 1·8 | 39·3 ± 5·3 |
| Group 3 | 55·4 ± 3·2 | 49·8 ± 5·0 | 8·2 ± 3·1 | 50·0 ± 2·9 | 61·3 ± 3·3 | 28·3 ± 4·0 |
| P | 0·001 | 0·340 | 0·159 | 0·858 | <0·001 | 0·472 |
DISCUSSION
Caryopses (seeds) from three positions in spikelets of E. distans differed significantly in size, with the basal seed being largest and the distal one smallest, which is the same as that in some other Poaceae such as Aegilops spp. (Datta et al., 1970; Marañon, 1989). When resources are limited, fruits or seeds at the position nearest to the source of the photosynthetic product and nutrition have priority in space to compete for resources, and fruits or seeds that develop earlier have the priority in time to compete for resources (Wyatt, 1980, 1982; Webb and Bawa, 1985; Vaughton, 1993). Thus, seeds of E. distans at the basal position in spikelets developed first, and there was a greater time period over which seeds could increase in size. Seed filling in grass spikelets occurs with the growth of plants, and the length of the seed maturation period decreases with increasing seed position. Thus, the variation in seed mass associated with seed position may be a function of variation in the timing and duration of reproductive development (Cheplick and Clay, 1989).
In many species, including grasses, the position on the mother plant where the seed matures has an effect on seed dormancy and germination. In various grasses, including speices of Aegilops (Marañon, 1987), Agrostis (Gonzalez-Rabanal et al., 1994) and Avena (Salimi and Ghorbanli, 2001), the basal seed in the spikelet is larger and less dormant than the upper one. In Avena fatua, the basal seed is non-dormant, but the second one has PD (Raju and Ramaswamy, 1983). Thus, seeds in the spikelet of E. distans are similar to those of Aegilops, Agrostis and Avena in that the basal seed (group 1) had greater mass than group 2 or group 3 seeds. However, in contrast to the species mentioned above, the basal (group 1) seeds in spikelets of E. distans were more dormant than group 2 or group 3 seeds, as reflected by lower germination percentages of fresh group 1 than fresh group 2 or 3 seeds.
The only kind of dormancy known to occur in the Poaceae is non-deep PD (Baskin and Baskin, 1998, 2001). Depending on the species, freshly matured seeds of grasses may be non-dormant (Grime et al., 1981; Washitani and Masuda, 1990), or they may have PD (Gutterman et al., 1996; Baskin and Baskin, 1985a). In the present study, evidence for presence of non-deep PD in the three groups of E. distans seeds is 2-fold. (1) A portion of seeds in each group after-ripened during dry storage under room conditions and also while they were buried in the field from June to July. However, more seeds of groups 2 and 3 after-ripened than did those in group 1. (2) GA3 broke dormancy in a substantial portion of group 2 and group 3 seeds but only in a small portion of group 1 seeds. In E. distans, there is a mixture of non-dormant and PD seeds in the collection, like the summer annual grasses Leptochloa panicoides (Baskin et al., 1993) and Setaria glauca (Baskin et al., 1996). In E. distans, however, seeds differed in dormancy among the groups. Group 3 had the highest proportion of non-dormant seeds, while group 1 had the highest proportion of seeds with PD.
Dormancy break via after-ripening of seeds during summer means they are capable of germinating in autumn if soil moisture and light/dark conditions are favourable; this timing of dormancy break and germination is a characteristic of seeds of winter annuals (Baskin and Baskin, 2001). A portion of seeds in each group of E. distans after-ripened in summer and thus exhibited the winter annual pattern of timing of dormancy break. However, unlike seeds of winter annuals that exhibit changes in the range of temperatures during dormancy break at which seeds will germinate (Baskin and Baskin, 1985b), the three groups of E. distans seeds exhibited no increase or decrease in the temperature range for germination, i.e. fresh, as well as after-ripened group 1, 2 and 3 seeds germinated at all five temperature regimes. During the after-ripening period, germination percentages increased at all temperatures, but temperature range for germination did not change.
Seed mass can have an influence on germination percentages and rates. In general, large seeds of a species germinate to higher percentages than small ones, e.g. the grass Leymus arenarius (Greipsson and Davy, 1995), but sometimes small seeds of a species germinate to high percentages than large ones, e.g. Lolium spp. (Akpan and Bean, 1977). Germination rates are often higher for the small than the large seeds of a species, e.g. Leymus arenarius (Greipsson and Davy, 1995) with exceptions in some species such as Psammochloa villosa (Zhu et al., 2005). In E. distans, germination percentages and rates were highest for group 3 seeds, which had the smallest mass, and lowest for group 1 seeds, which had the greatest mass. It is not known why the large group 1 seeds germinated more slowly and to a lower percentage than the smaller group 2 and 3 seeds. However, the germination percentages and rates of different groups/ sizes of E. distans seeds do not seem to be exceptional in view of the response of seeds of various sizes in other grass species.
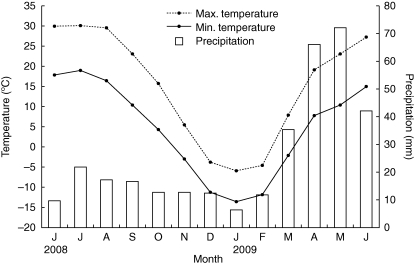
Regardless of whether seeds after-ripened in dry storage or in the soil in the field, they gained the ability to germinate at autumn temperatures. By autumn, the temperatures both in the habitat and those required for germination overlap, but seeds usually cannot germinate in autumn because sufficient soil moisture is not present. In 2008, precipitation in the garden in Urümqi, where the experimental plots were located, was 9·6 mm, 21·4 mm, 17·1 mm and 16·8 mm for June, July, August and September, respectively (Fig. 7). Thus, without regular watering the soil under natural moisture conditions was dry most of the time in August and September. With regular watering, some seeds in groups 1, 2 and 3 germinated in August and September, and the number of seeds that germinated increased as seed position increased. Thus, some seeds of each group are capable of germinating in autumn, as would be expected for a winter annual, if water is supplied.
Fig. 7.
Monthly precipitation and mean daily minimum and maximum temperatures during the study period (2008–2009).
Under natural soil moisture conditions, none of the seeds germinated in autumn 2008, thereby preventing the species from behaving as a winter annual. Many of the seedlings (67 %, 56 % and 46 % for seed groups 1, 2 and 3, respectively) that germinated under the regular watering regime in autumn survived. Thus, it can be concluded that if seeds germinate in autumn, the resulting plants behave as winter annuals. In spring, precipitation (including snow) in Urümqi was 35 mm, 66 mm and 75 mm in March, April and May 2009, respectively (Fig. 7), suggesting the soil probably was moist most of the time. Under natural soil moisture conditions in spring, some seeds in each group of E. distans germinated; the number of seeds that germinated increased as seed position increased. The plants from seeds that germinated in spring were short-lived summer annuals or spring ephemerals. Thus, E. distans has the potential to behave as a winter annual, but since natural soil moisture conditions prevent germination in autumn its seeds normally germinate in spring. It should be noted that some seedlings of E. distans have been observed in the field during relatively moist autumns and that the seedlings survive during the winter (A. B. Wang and D. Y. Tan, pers. obs.). In autumn 2008, no seedlings of E. distans were observed in the field, except in watered plots.
The delay of germination until spring also occurs for some seeds of temperate-zone facultative winter annuals, but the delay in germination of E. distans and facultative winter annuals may be for different reasons. In facultative winter annuals, seeds exposed to natural low-temperature regimes in late autumn and winter lose the ability to germinate at high, but not at low, temperatures, i.e. they enter conditional dormancy. Thus, seeds can germinate at low habitat temperatures in spring (Baskin and Baskin, 1981). The buried seeds of E. distans died, so it is not known if there were any changes in temperature responses of the seeds during winter. However, germination of seeds in the natural soil-moisture plots show that they not only remained viable on the soil surface but were capable of germinating at early spring temperatures.
Although more buried seeds of group 2 than of groups 1 and 3 were alive after 2, 3 and 4 months of burial, essentially all seeds of the three groups were dead after 5 months of burial. Seed survival from June 2008 to March and April 2009, when germination occurred in the field, obviously was higher for seeds on the soil surface than for those buried in the soil. The reason why all the buried seeds died is not known, but these results suggest that E. distans would not form a persistent soil seed bank. Seeds of many species of grasses do not form a soil seed bank (Baskin and Baskin, 1998); thus, the short life span of E. distans seeds buried in the soil is not too surprising. Since E. distans apparently does not form a soil seed bank, it is important that the species sets seeds each year. Having the flexibility to germinate in either autumn (if soil moisture is sufficient) or spring (when soil moisture is almost always sufficient for germination and plant growth) helps ensure the survival of the species.
Seed position also had an effect on seedling emergence and survival in the field experiment, and on subsequent plant size and seed production; however, this effect was always associated with seed mass. Regardless of soil moisture regime in the field or season of germination, fewer seeds from group 1 germinated, but a higher proportion of seedlings from group 1 seeds survived than from group 2 or 3 seeds. Likewise, seedlings from large seeds of the grass Amphicarpum purshii survived better than those from small seeds (Cheplick and Quinn, 1982, 1983). In each soil moisture condition and at each germination time, plants from group 1 seeds were larger and produced more seeds than those from either group 2 or 3 seeds. The reason for this phenomenon may be that, compared with small seeds, large seeds always had more food reserves (Stanton, 1985), and seedlings from large seeds were larger (Venable and Levin, 1985; Imbert et al., 1997) and had a greater ability to grow (Ellison, 1987; Beneke et al., 1993). Thus, plants from large seeds had more reproductive output than those from small seeds (Venable and Levin, 1985). Given the vulnerability of the seedling phase in many species (Fenner, 1987) and the potential difficulties of seedling establishment in the desert environment (Sun et al., 2009), in some years seed mass may be especially critical to successful establishment in the annual ephemeral E. distans.
In considering the relationship of E. distans seedlings with other life-history stages of this species, it is important to know the number of surviving offspring from seeds at different positions (sizes) in the spikelet and the number of seeds the survivors produce (see Moles and Leishman, 2008). If only the E. distans seeds that germinated under natural soil-moisture conditions (i.e. in spring) were considered, the highest percentage survival of seedlings would be for offspring of group 1 seeds. However, since fewer group 1 than group 2 or 3 seeds germinated, there were actually more living offspring from group 2 and 3 than from group 1 seeds in the plots. Further, although plants from group 1 seeds produced more seeds per plant than those from group 2 or 3 seeds, the total number of seeds produced by plants from group 2 or 3 seeds was higher than that of plants from group 1 seeds. Of course, each plant produced by a seed from any of the three groups, regardless of when the seed germinates, will produce group 1, 2 and 3 seeds, ensuring that there will be a diversity of germination responses and survival and reproductive strategies in the next generation.
Seeds in different positions in spikelets may represent different strategies in the life history of E. distans. Distal seeds have a ‘high-risk’ strategy in which germination is fast and germination percentages are high, but the fitness of seedlings is relatively low. Basal seeds have a ‘cautious’ or ‘low-risk’ strategy in which germination is slow and germination percentages are low, but the fitness of seedlings is high. The possession of two seed strategies may represent a form of bet-hedging (Venable and Levin, 1985) which can spread risk in time and space. If all seeds in a spikelet had a fast rate and high percentage of germination strategy, it might prove to be disastrous if the rain that promoted germination was followed by a long drought or severe disturbance. In contrast, if all seeds in a spikelet had a slow rate and low percentage of germination strategy, seedlings might avoid disastrous environmental conditions but not be capable of taking advantage of relatively brief periods of favourable conditions for growth. Thus, two strategies among the offspring of a single plant help ensure that successful germination, seedling survival and seed production will occur.
Although there is a potential for both a high-risk and a cautious strategy in E. distans, the rigors of the desert environment, especially the dryness in most autumns, prevents a strong expression of the two strategies, i.e. the soil is too dry in most years for any seeds to germinate in autumn, and germination occurs only in spring. Thus, the environmentally enforced (by low soil moisture) delay of germination of seeds from all positions from autumn to spring results in a reduction in the number of seeds produced per plant and consequently a reduction in fitness. However, even if all seeds from the different positions in the spikelet germinate in spring, there is still some evidence of the two strategies, i.e. although spring germination results in a reduction of fitness of plants from group 1, 2 and 3 seeds, plants from group 1 seeds (probably due to their greater mass) produce more seeds per plant than those from group 2 or 3 seeds.
ACKNOWLEDGEMENTS
This study was supported in part by the Projects on the Research and Development of High Technology of Xinjiang Uygur Autonomous Region (200810102) and the Open Projects of Xinjiang Key Laboratory of Grassland Resources and Ecology, China (XJDX 0209-2008-01).
LITERATURE CITED
- Akpan EEJ, Bean EW. The effects of temperature upon seed development in three species of forage grasses. Annals of Botany. 1977;41:689–695. [Google Scholar]
- Baskin CC, Baskin JM. Ecology of seed dormancy and germination in grasses. In: Cheplick GP, editor. Population biology of grasses. Cambridge: Cambridge University Press; 1998. pp. 30–83. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Baskin CC, Baskin JM, Chester EW. Germination ecology of Leptochloa panicoides, a summer annual grass of seasonally dewatered mudflats. Acta Oecologia. 1993;14:693–704. [Google Scholar]
- Baskin CC, Baskin JM, El-Moursey SA. Seasonal changes in germination responses of buried seeds of the weedy summer annual grass Setaria glauca. Weed Research. 1996;36:319–324. [Google Scholar]
- Baskin JM, Baskin CC. Seasonal changes in the germination responses of buried Lamium amplexicaule seeds. Weed Research. 1981;21:299–306. [Google Scholar]
- Baskin JM, Baskin CC. Dormancy breaking and germination requirements of nimble will (Muhlenbergia schreberi Gmel.) seeds. Journal of Range Management. 1985a;38:513–515. [Google Scholar]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. BioScience. 1985b;35:492–498. [Google Scholar]
- Beneke K, Van Rooyen MW, Theron GK. Fruit polymorphism in ephemeral species of Namaqualand. IV. Growth analysis of plants cultivated from the dimorphic diaspores. Journal of Arid Environments. 1993;24:345–360. [Google Scholar]
- Cheplick GP, Clay K. Convergent evolution of cleistogamy and seed heteromorphism in two perennial grasses. Evolutionary Trends in Plants. 1989;3:127–136. [Google Scholar]
- Cheplick GP, Quinn JA. Amphicarpum purshii and the ‘pessimistic strategy’ in amphicarpic annuals. Oecologia. 1982;52:327–332. doi: 10.1007/BF00367955. [DOI] [PubMed] [Google Scholar]
- Cheplick GP, Quinn JA. The shift in aerial/subterranean fruit ratio in Amphicarpum purshii: causes and significance. Oecologia. 1983;57:374–379. doi: 10.1007/BF00377183. [DOI] [PubMed] [Google Scholar]
- Cheplick GP, Sung LY. Effects of maternal nutrient environment and maturation position on seed heteromorphism, germination, and seedling growth in Triplasis purpurea (Poaceae) International Journal of Plant Sciences. 1998;159:338–350. [Google Scholar]
- Cui NR, Cui DF, Liu GJ, et al. Poaceae. In: Cui NR, editor. Flora Xinjiangensis. Vol. 6. Urumqi: Xinjiang Science, Technology and Hygiene Publishing House; 1996. pp. 161–164. [in Chinese] [Google Scholar]
- Datta SC, Evenari M, Gutterman Y. The heteroblasty of Aegilops ovata L. Israel Journal of Botany. 1970;19:463–483. [Google Scholar]
- Ellison AM. Effect of seed dimorphism on the density-dependent dynamics of experimental populations of Atriplex triangularis (Chenopodiaceae) American Journal of Botany. 1987;74:1280–1288. [Google Scholar]
- Fedorov AA. Flora of Russia: the European part and bordering regions. Vol. 1. Rotterdam: A. A. Balkema; 1999. [translated from Russian] [Google Scholar]
- Fenner M. Seedlings. New Phytologist. 1987;106:35–47. [Google Scholar]
- Gonzalez-Rabanal R, Casal M, Trabaud L. Effects of high temperatures, ash and seed position in the inflorescence on the germination of three Spanish grasses. Journal of Vegetation Science. 1994;5:289–294. [Google Scholar]
- Greipsson S, Davy AJ. Seed mass and germination behaviour in populations of the dune-building grass Leymus arenarius. Annals of Botany. 1995;76:493–501. [Google Scholar]
- Grime JS, Mason G, Curtis AV, et al. A comparative study of germination characteristics in a local flora. Journal of Ecology. 1981;69:1017–1059. [Google Scholar]
- Gutterman Y. Maternal effects on seeds during development. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford: CABI; 2000. pp. 59–84. [Google Scholar]
- Gutterman Y, Corbineau F, Côme D. Dormancy of Hordeum spontaneum caryopses from a population on the Negev Desert highlands. Journal of Arid Environments. 1996;33:337–345. [Google Scholar]
- Imbert E, Escarré J, Lepart J. Seed heteromorphism in Crepis sancta (Asteraceae): performanace of two morphs in different environments. Oikos. 1997;79:325–332. [Google Scholar]
- Liu JW, Ding M. Morphological and cytogenetical studies on intergeneric hybrids between Triticum aestivum and Eremopyrum orientale. Acta Genetica Sinica. 1996;23:117–123. [in Chinese] [Google Scholar]
- Lv L, Tan DY. The characteristics of fruit-set and reproductive package in four species of ephemeral plants in Eremopyrum (Poaceae) Journal of Xinjiang Agricultural University. 2005;28(3):21–25. [in Chinese] [Google Scholar]
- Marañon T. Ecologia del polimorfismo somatico de semillas y la sinaptospermia en Aegilops neglecta Req. ex Bertol. Anales del Jardin Botanico de Madrid. 1987;44:97–107. [Google Scholar]
- Marañon T. Variation in seed size and germination in three Aegilops species. Seed Science & Technology. 1989;17:583–588. [Google Scholar]
- Maun MA, Payne AM. Fruit and seed polymorphism and its relation to seedling growth in the genus Cakile. Canadian Journal of Botany. 1989;67:2743–2750. [Google Scholar]
- Moles AT, Leishman MR. The seedlings as part of a plant's life history strategy. In: Leck MA, Parker VT, Simpson RL, editors. Seedling ecology and evolution. Cambridge: Cambridge University Press; 2008. pp. 217–238. [Google Scholar]
- Moravcová L, Perglová I, Pyšek P, Jitěch V, Pergl J. Effects of fruit position on fruit mass and seed germination in the alien species Heracleum mantegazzianum (Apiaceae) and the implications for its invasion. Acta Oecologica. 2005;28:1–10. [Google Scholar]
- Nikolaeva MG. Factors controlling the seed dormancy pattern. In: Khan AA, editor. The physiology and biochemistry of seed dormancy and germination. Amsterdam: North-Holland; 1977. pp. 51–74. [Google Scholar]
- Raju MVS, Ramaswamy SN. Studies on the inflorescence of wild oats (Avena fatua) Canadian Journal of Botany. 1983;61:74–78. [Google Scholar]
- Salimi H, Ghorbanli M. A study on seed germination of Avena ludoviciana and the effective factors in seed dormancy breaking. Rostaniha. 2001;2:41–55. [in Arabic with English summary and references] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. San Francisco, CA: Freeman; 1995. [Google Scholar]
- Stanton ML. Seed size and emergence time within a stand of wild radish (Raphanus raphanistrum L.): the establishment of a fitness hierarchy. Oecologia. 1985;67:524–531. doi: 10.1007/BF00790024. [DOI] [PubMed] [Google Scholar]
- Sun HZ, Lu JJ, Tan DY, Baskin JM, Baskin CC. Dormancy and germination characteristics of the trimorphic achenes of Garhadiolus papposus (Asteraceae), an annual ephemeral from the Junggar Desert, China. South African Journal of Botany. 2009;75:537–545. [Google Scholar]
- Timson J. New method of recording germination data. Nature. 1965;207:216–217. [Google Scholar]
- Vaughton G. Nonrandom patterns of fruit set in Banksia spinulosa (Proteaceae): interovary competition within and among inflorescences. International Journal of Plant Sciences. 1993;154:306–313. [Google Scholar]
- Venable DL, Lawlor L. Delayed germination and dispersal in desert annuals: escape in space and time. Oecologia. 1980;46:272–282. doi: 10.1007/BF00540137. [DOI] [PubMed] [Google Scholar]
- Venable DL, Levin DA. Ecology of achene dimorphism in Heterotheca latifolia. 1. Achene structure, germination and dispersal. Journal of Ecology. 1985;73:113–145. [Google Scholar]
- Venable DL, Burquez AM, Corral G, Morales E, Espinosa F. The ecology of seed heteromorphism in Heterosperma pinnatum in central Mexico. Ecology. 1987;68:65–76. [Google Scholar]
- Wang L, Huang ZY, Baskin JM, Baskin CC. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Annals of Botany. 2008;102:757–769. doi: 10.1093/aob/mcn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washitani I, Masuda M. A comparative study of the germination characteristics of seeds from a moist tall grassland community. Functional Ecology. 1990;4:543–557. [Google Scholar]
- Webb CJ, Bawa KS. Patterns of fruit and seed production in Bauhinia ungulata (Leguminosae) Plant Systematics and Evolution. 1985;151:55–65. [Google Scholar]
- Wyatt R. The reproductive biology of Asclepias tuberosa. 1. Flower number, arrangement, and fruit-set. New Phytologist. 1980;85:119–131. [Google Scholar]
- Wyatt R. Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. American Journal of Botany. 1982;69:585–594. [Google Scholar]
- Zhu YJ, Dong M, Huang ZY. Effects of sand burial and seed size on seed germination and seedling emergence of Psammochloa villosa. Acta Phytoecologica Sinica. 2005;29:730–739. [in Chinese with English abstract] [Google Scholar]