Abstract
Background and Aims
Here evidence for reticulation in the pantropical orchid genus Polystachya is presented, using gene trees from five nuclear and plastid DNA data sets, first among only diploid samples (homoploid hybridization) and then with the inclusion of cloned tetraploid sequences (allopolyploids). Two groups of tetraploids are compared with respect to their origins and phylogenetic relationships.
Methods
Sequences from plastid regions, three low-copy nuclear genes and ITS nuclear ribosomal DNA were analysed for 56 diploid and 17 tetraploid accessions using maximum parsimony and Bayesian inference. Reticulation was inferred from incongruence between gene trees using supernetwork and consensus network analyses and from cloning and sequencing duplicated loci in tetraploids.
Key Results
Diploid trees from individual loci showed considerable incongruity but little reticulation signal when support from more than one gene tree was required to infer reticulation. This was coupled with generally low support in the individual gene trees. Sequencing the duplicated gene copies in tetraploids showed clearer evidence of hybrid evolution, including multiple origins of one group of tetraploids included in the study.
Conclusions
A combination of cloning duplicate gene copies in allotetraploids and consensus network comparison of gene trees allowed a phylogenetic framework for reticulation in Polystachya to be built. There was little evidence for homoploid hybridization, but our knowledge of the origins and relationships of three groups of allotetraploids are greatly improved by this study. One group showed evidence of multiple long-distance dispersals to achieve a pantropical distribution; another showed no evidence of multiple origins or long-distance dispersal but had greater morphological variation, consistent with hybridization between more distantly related parents.
Keywords: Allopolyploidy, consensus network, filtered supernetwork, low-copy nuclear genes, Orchidaceae, phylogenetic analysis, Polystachya, reticulate evolution
INTRODUCTION
The significance and extent of natural hybridization in angiosperm evolution has been widely recognized (Paun et al., 2007; Wissemann, 2007), with an estimated 25 % of vascular plants forming hybrids with other species (Mallet, 2005) and perhaps 11 % of plant species having arisen as a result of hybridization (Ellstrand et al., 1996). Outcomes of hybridization are complex and not predictable from case to case. Changes in ploidy are common, and confirmed examples in the literature of allopolyploid speciation are more common than those of homoploid hybridization, which is possibly due to easier detection and confirmation of allopolyploids in the wild compared with homoploids (Hegarty and Hiscock, 2008). Polyploidy is a common product of hybridization (Soltis and Soltis, 2000; Sang et al., 2004), usually following the union of a pair of unreduced gametes from the two parent species, although other mechanisms can also result in polyploid offspring. As well as an immediate and mostly effective barrier to introgression with their parent species due to the difference in chromosome number (even though triploid bridges still make this possible in some cases; Husband, 2004), allopolyploids express novel combinations of genes relative to both parents and often exhibit genomic and epigenetic instability and immediate plasticity in gene expression and regulatory networks (Osborn et al., 2003; Baack and Rieseberg, 2007; Chen, 2007; Leitch and Leitch, 2008). This can have an effect on colonization and dispersal abilities and allow them to occupy environmental niches unavailable to the parent species (Soltis and Soltis, 2000; Otto, 2007; Hegarty and Hiscock, 2008). Homoploid hybrids can also exhibit extreme large-scale genomic changes, such as increases in genome size due to increased retrotransposon activity (Baack and Rieseberg, 2007).
In addition to hybridization, gene flow between species via introgression is a common event, with the genomes of many species apparently permeable to alleles from related species (Baack and Rieseberg, 2007; Lexer et al., 2009). The phenomena of hybridization and introgression can confound efforts to reconstruct the phylogeny of such groups. Often, only data from the plastid genome is used in phylogeny reconstruction, and the uniparental nature of plastid DNA masks reticulation. When both plastid and biparentally inherited nuclear DNA have been used in a study they have often given conflicting phylogenetic signals (e.g. Rieseberg et al., 1996; Schilling and Panero, 1996; Oh and Potter, 2003; Kelly et al., 2010), but relatively few studies have compared plastid DNA sequences with more than one nuclear locus. Incongruence between nuclear loci or between nuclear and organellar DNA can be interpreted as a sign of interspecific hybridization, but it also can arise as a result of stochastic or population-level events causing individual gene trees to differ from the underlying species tree (McBreen and Lockhart, 2006; Holland et al., 2008). The methods used to analyse multiple loci and interpret incongruence in phylogenetic results are still under development (Linder and Rieseberg, 2004; McBreen and Lockhart, 2006).
Previous work on Polystachya (Polystachyinae; Vandeae; Orchidaceae) suggested that the genus might be well suited to the study of reticulate evolution due to variation in ploidy including some tetraploid species groups (Rupp, 2008; Russell et al., 2010). The genus comprises approx. 240 species distributed pantropically, with centres of diversity in Africa and smaller species numbers in the Indian Ocean islands, southern Asia and the Neotropics. Species radiations have occurred in the Neotropics and Madagascar, and these include polyploid clades with 2n = 4x = 80 chromosomes (Russell et al., 2010). One group of polyploid species with morphological and genetic similarity to the pantropical species, Polystachya concreta, has dispersed throughout the tropics relatively recently; another group represented, for example, by P. rosea and P. clareae has remained endemic to Madagascar and the Malagasy Islands. Some species from these two groups are illustrated in Fig. 1.
Fig. 1.
Top row: three examples of Polystachya from the pantropical tetraploid group (photos: R. Hromniak, University of Vienna Botanical Garden; left to right: P. concreta from Laos, P. masayensis from Costa Rica and P. concreta from Réunion). Bottom row: three examples of plants from the Malagasy endemic tetraploid group (photos: A. Sieder, University of Vienna Botanical Garden; left to right: P. tsaratananae, P. clareae and P. monophylla).
Previous studies on the genus have used plastid DNA sequences. Although these data were useful in constructing a well-supported phylogenetic hypothesis, as they have been in many other studies of plant evolution, the maternal inheritance of plastid DNA prevented any conclusions about the incidence of reticulate evolution. In this study, the analysis is extended to biparentally inherited nuclear DNA. The aim is to compare the results of plastid DNA analysis with those from several nuclear genes using supernetwork and consensus network analyses to gauge the extent to which hybridization has been important in Polystachya evolution. Reticulation amongst diploids is investigated using incongruence between gene trees as a potential hybridization signal. This strategy is then extended to tetraploid accessions for which homoeologous gene copies from low-copy nuclear genes can be cloned and sequenced to establish their origin. Two major groups of tetraploids are compared in terms of their morphological and biogeographical traits, but there are others in Polystachya for which sampling of species and individuals does not permit an effective study.
MATERIALS AND METHODS
Material for DNA extraction came from the collections of the Botanical Garden of the University of Vienna, the collection of Isobyl la Croix in Ross-shire, Scotland, and field collections made by the authors. DNA samples were also obtained from the DNA Bank of the Royal Botanic Gardens, Kew (http://data.kew.org/dnabank/homepage.html). See the Appendix for accession details and GenBank accessions. See Russell et al. (2010) for details of material preservation, DNA extraction and ploidy of Polystachya species. Much ploidy information was obtained using genome size measurements from Rupp (2008) and Rupp et al. (2010).
A number of nuclear genes known to be low- or single-copy in angiosperms were screened and the following loci selected, based on their ease of amplification and sequencing: PgiC between exons 11 and 15; PhyC exon 1; and Rpb2 intron 23. PgiC codes for phosphoglucose isomerase, an essential glytolytic enzyme. It has been used in phylogenetic studies in Dipterocarpaceae (Kamiya et al., 2005), Stephanomeria (Compositae; Ford et al., 2006) and Clarkia (Onagraceae), in which it is present in two copies (Thomas et al., 1993; Ford and Gottlieb, 2003). PhyC is a member of the phytochrome family of genes, which code for photoreceptive proteins in plants and regulate a wide range of flowering and developmental pathways. It has been used in a number of phylogenetic studies in Phyllanthaceae (Samuel et al., 2005), Poaceae (Mathews and Sharrock, 1996) and across monocots (M. Kinney, University of Missouri, et al., unpub. res.) and other angiosperms (Saarela et al., 2007). Rpb2 codes for the second largest subunit of the RNA polymerase enzyme and has been used in phylogenetic studies in Chamaedorea (Arecaceae; Thomas et al., 2006), Hordeum (Poaceae; Sun et al., 2009), and across angiosperm families (Oxelman et al., 2004). DNA samples were initially amplified using universal primers: for PgiC and Rpb2, primers were taken from the literature (Ronçal et al., 2005; Ford et al., 2006). For PhyC, universal monocot primers were designed from GenBank sequences. When a clean single PCR band was obtained using universal primers, the product was cloned using the pGEM-T Easy system (Invitrogen) following the manufacturer's instructions to assess copy number and amount of within-sample variation (e.g. between different alleles at the same locus). The resulting sequences were aligned and Polystachya-specific primers were designed from conserved areas using Primer3 (Rozen and Skaletsky, 1999). Primer details are given in Table 1.
Table 1.
Nuclear low-copy and ITS primers used in this study
| PgiC AA11F | TTYGCNTTYTGGGAYTGGGT | Universal primer | Ford et al. (2006) |
| PgiC AA16R | CCYTTNCCRTTRCTYTCCAT | Universal primer | Ford et al. (2006) |
| PgiC Pol E12F1 | GTTGGTGTGCTTCCKTTGTCTC | Polystachya-specific | This study |
| PgiC Pol E12F2 | CTCTCCAATATGGATTTCCAATC | Polystachya-specific | This study |
| PgiC Pol E15R | AAGTGCTTGAGARTATGGTAATATAGC | Polystachya-specific | This study |
| PgiC Pol I12F1 | AGTAATTTAAGAGTCAGTGGTGATCG | Polystachya-specific internal sequencing primer | This study |
| RPB2-INT-23F | CAACTTATTGAGTGCATCATGG | Universal primer | Ronçal et al. (2005) |
| RPB2-INT-23R | CCACGCATCTGATATCCAC | Universal primer | Ronçal et al. (2005) |
| RPB2-POL-23F1 | CTCCATTCACTGATGTTACGG | Polystachya-specific | This study |
| RPB2-POL-23F2 | GGAGATGCTACTCCATTCACTG | Polystachya-specific | This study |
| RPB2-POL-23R | GAACAGTGGTCARCCTCCAAG | Polystachya-specific | This study |
| phyc503f | TCVGGGAAGCCSTTYTAYGC | Monocot-specific | This study |
| phyc1705r | GRATWGCATCCATYTCAACATC | Monocot-specific | This study |
| phyc515f-OR | AAGCCSTTYTAYGCAATTCTACACCG | Orchid-specific | This study |
| phyc1699r-OR | ATWGCATCCATYTCAACATCKTCCCA | Orchid-specific | This study |
| phyc524f-OR | GCAATTCTACACCGTATCAATGA | Orchid-specific internal sequencing primer | This study |
| phyc1690r-OR | TCAACATCKTCCCATGGAAGGCT | Orchid-specific internal sequencing primer | This study |
| phyc974f-OR | GCTCCTCATGGMTGTCATGCTCA | Orchid-specific internal sequencing primer | This study |
| phyc1145r | CCTGMARCARGAACTCACAAGCATATC | Monocot-specific internal sequencing primer | This study |
| ITS 18s F | ACCGATTGAATGGTCCGGTGAAGTGTTCG | Universal primer | Gruenstaeudl et al. (2009) |
| ITS 26s R | CTGAGGACGCTTCTCCAGACTACAATTCG | Universal primer | Gruenstaeudl et al. (2009) |
| ITS 5·8S F | ACTCTCGGCAACGGATATCTCGGCTC | Universal internal sequencing primer | Gruenstaeudl et al. (2009) |
| ITS 5·8S R | ATGCGTGACGCCCAGGCAGACGTG | Universal internal sequencing primer | Gruenstaeudl et al. (2009) |
Plastid DNA sequences came from the rps16 intron, the rps16–trnK spacer and the trnK intron, including matK, and were already available from a previous study (Russell et al., 2010). The ITS region (ITS1-5·8S–ITS2 nuclear ribosomal DNA) was also sequenced as an additional source of data. The high copy number of ITS sequences in the nuclear genome makes the region relatively easy to amplify and sequence, and it is commonly used in plant phylogenetics. Results from ITS are often contrasted with plastid sequences to show possible reticulation (Hodkinson et al., 2002; Schwarzbach and Rieseberg, 2002; Chase et al., 2003; van den Berg et al., 2009). Although some properties of nrDNA (multiple copy number, concerted evolution, and frequent occurrence of pseudogenes) sometimes makes its use in phylogenetics problematic, especially in the study of hybrids (van den Hof et al., 2008; Alvarez and Wendel, 2003; Feliner and Rossello, 2007), it was felt that in the context of a multi-gene study, involving several plastid and nuclear gene regions, ITS sequences could provide useful additional information in this study.
There are fewer Polystachya species included in this study than in the Russell et al. (2010) paper; samples were excluded because some nuclear genes could not be amplified, directly sequenced or, in the case of tetraploids, successfully cloned, either because of deficiencies in the PCR protocols or because the DNA samples contained too little intact nuclear DNA. Taxon sampling of Polystachya tetraploids includes five groups found in the Russell et al. (2010) study, two of which comprise multiple accessions. Nine accessions belong to a pantropical group with affinities to P. concreta; five accessions belong to a group endemic to the Malagasy islands; three other accessions from mainland Africa occur separately with diploid sister species.
DNA amplification and sequencing
After initial cloning to design primers and develop PCR protocols for sequencing low-copy nuclear genes, initial analyses suggested the PgiC, PhyC and Rpb2 genes were effectively single-copy in diploids and that sequences in different individuals could be treated as orthologous. For diploid accessions, these genes were then sequenced directly from PCR products, whereas PCR products from tetraploids were cloned to amplify homoeologous gene copies separately if more than one was present. Plastid and ITS sequences were obtained directly from PCR products.
In this study, 20-μL PCR reactions used, with 18·0 µL ABGene ReddyMix PCR Master Mix, 0·5 µL of each primer at 20 µm, and 1·0 µL template DNA. Thermocycling was performed with initial denaturation at 80 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, annealing for 30 s and 72 °C for 2 min, and a final extension of 72 °C for 5 min. Annealing temperature was usually 55 °C for PgiC and 58 °C for PhyC, Rpb2 and ITS. PCR products for direct sequencing were cleaned with a mixture of 1 unit CIAP (calf intestinal alkaline phosphatase; Fermentas) and 10 units exonuclease I (Fermentas) to degrade single-stranded DNA fragments and dNTPs in the PCR product (Werle et al., 1994). The mixture was incubated at 37 °C for 45 min, then denatured at 80 °C for 15 min. PgiC, PhyC and Rpb2 PCR products from tetraploid accessions were gel-purified and cloned using the pGEM-T Easy cloning system (Invitrogen) following the manufacturer's instructions. Colonies were fixed in TE buffer, and subsequent amplification and sequencing were performed using vector primers M13F, M13R, SP6 and T7. Five to fifteen colonies were sequenced per accession – an attempt was made to always sequence the higher number, but some samples had a high sequencing failure rate even from clones.
Cycle sequencing was carried out in 10-μL reactions with 1·0 µL ABI BigDye Terminators kit, 1·0 µL sequencing primer at 3·2 µm, and 8·0 µL cleaned-up PCR product, cycling with 30 cycles of 96 °C for 10s, 50 °C for 5s, and 60 °C for 4 min. Sequencing was performed on a 48 capillary sequencer, Applied Biosystems (ABI) 3730 DNA Analyzer, following the manufacturer's protocols.
Analysis of diploids
Sequences were edited with FinchTV (Geospiza Inc.) and assembled with either AutoAssembler 1.4.0 (ABI) or LaserGene 7·1 SeqMan (DNASTAR Inc.). They were aligned initially with MUSCLE (Edgar, 2004), and these alignments were adjusted by eye in MacClade (Maddison and Maddison, 2005) following the guidelines of Kelchner (2000). Non-alignable end sequences and gap-rich sequences (>50 % missing data) were excluded from the analyses. Characteristics of sequence alignments are presented in Table 2.
Table 2.
Characteristics of the five loci used to construct individual gene trees, and parsimony scores of the equally most-parsimonious (e.m.p.) trees after analysis in PAUP*
| Locus | No. of characters included | Potentially parsimony-informative characters | No. of e.m.p. trees found | Length of e.m.p. trees | Consistency index | Retention index |
|---|---|---|---|---|---|---|
| Plastid DNA | ||||||
| Diploids only | 4422 | 346 | 72 | 1072 | 0·75 | 0·81 |
| Diploids and tetraploids | 4419 | 377 | 432 | 1177 | 0·74 | 0·83 |
| ITS | ||||||
| Diploids only | 815 | 162 | >10 000 | 499 | 0·61 | 0·82 |
| Diploids and tetraploids | 815 | 172 | >10 000 | 562 | 0·60 | 0·84 |
| PgiC | ||||||
| Diploids only | 1035 | 146 | 4014 | 490 | 0·86 | 0·88 |
| Diploids and tetraploids | 1033 | 176 | >10 000 | 535 | 0·84 | 0·91 |
| PhyC | ||||||
| Diploids only | 1183 | 81 | 1661 | 253 | 0·82 | 0·89 |
| Diploids and tetraploids | 1183 | 93 | 8222 | 313 | 0·84 | 0·93 |
| Rpb2 | ||||||
| Diploids only | 833 | 156 | >10 000 | 513 | 0·87 | 0·90 |
| Diploids and tetraploids | 833 | 184 | >10 000 | 570 | 0·85 | 0·91 |
Individual gene trees were constructed using maximum parsimony and Bayesian analyses. Parsimony analyses were conducted in PAUP*4·10b (Swofford, 2003) using a two stage heuristic search strategy with tree bisection and reconnection branch swapping, saving a maximum of 10 000 trees. Bootstrap percentages (BP) were calculated using 1000 heuristic search replicates, saving ten trees per replicate with tree bisection and reconnection branch swapping. Bayesian trees were made in MrBayes 2·1 (Huelsenbeck and Ronquist, 2001) using the facilities of the Computational Biology Service Unit at Cornell University (http://cbsuapps.tc.cornell.edu) and the University of Oslo Bioportal (https://www.bioportal.uio.no). Best-fitting nucleotide substitution models were determined beforehand using MrModeltest 2·3 (Nylander, 2004) following the Akaike information criterion in each case. Two independent sets of four metropolis-coupled Markov chain Monte Carlo runs were executed for five million generations, sampling every 500 generations, with chains heated to 0·2, a burnin of 25 % and default priors. Nucleotide models were: PgiC, GTR + G; PhyC, GTR + I + G; Rpb2, HKY + G; plastid DNA, GTR + I + G; ITS, GTR + G. The program Tracer (Rambaut and Drummond, 2007) was used to check the runs had reached stationarity, and effective sample size of all the parameters was high (>100).
To illustrate incongruities between the individual gene trees, a filtered supernetwork (Splitstree 4·10; Huson and Bryant, 2006) was constructed from the five 50 % bootstrap consensus trees from parsimony analysis, filtering the splits to show only those present in a minimum of two input trees.
Consensus networks were constructed in Dendroscope 2·3 (Huson et al., 2007) using the galled network algorithm (in which each inferred reticulation is independent of all the others; Linder and Rieseberg, 2004) with a 20 % threshold for network construction. Input trees were 50 % bootstrap consensus trees. Since five gene trees were analysed, a 20–39 % threshold effectively excluded incongruent clades unique to a single gene tree without support from any of the other trees. A threshold setting of 40–59 % would have excluded incongruent clades found in one or two gene trees, further reducing the possibility of a false positive reticulation signal but resulting in much reduced overall resolution in the consensus network. Constructing supernetworks and consensus networks without filtering in this way would generate a reticulation for each of the incongruities between the input trees, but incongruity alone does not necessarily signify reticulate evolution. It can also be due to processes such as deep coalescence, gene duplication, recombination or character homoplasy within individual genes (Linder and Rieseberg, 2004). Hybridization is hypothesized to cause more large-scale genomic changes affecting many genes, and so incongruence due to hybridization should be detectable from consistent differences in the phylogenetic signal from different genes. Filtering the clades used to construct phylogenetic networks allows only the more consistent differences in phylogenetic signal to be presented (McBreen and Lockhart, 2006; Holland et al., 2008).
Analysis of tetraploids
Submatrices of cloned DNA sequences from each sample were aligned in MacClade, and chimeric sequences, those cloned only a single time, were removed. In vitro recombination of DNA sequences is a problem when cloning products of PCR reactions in which multiple alleles or paralogous gene copies have been amplified (Cronn et al., 2002; Anthony et al., 2007; Kelly et al., 2010). Since alignments of cloned PCR products generated by this study were small (5–15 sequences with 800–1200 sites from each cloned sample), it was most expedient to screen the sequences by eye for chimeric clones. Unrooted neighbor-joining trees of the clones from a sample were made, and these were used to find the most distant sequences, termed type 1 and type 2. This information was used as an aid to screening the submatrix of cloned sequences by eye in MacClade for evidence of recombination. Recombinant sequences were identified as those that shared characteristic mutations (single nucleotide polymorphisms or indels) with both type 1 and type 2 sequences at different points along their length (Salmon et al., 2010). The recombination detection programs included in the RDP3 package (Martin et al., 2005) were usually unable to detect chimeric sequences that were obvious to the eye, and when they did detect recombination, they gave wrong results both for identities of the parental sequences and positions of recombination breakpoints. Anthony et al. (2007) found similar problems when using these programs to detect chimeric sequences, and this might be because the programs require higher levels of sequence divergence to be effective (Posada and Crandall, 2001). Occasional single nucleotide polymorphisms and small indels in the sequences were expected as a result of the cloning procedure (Speksnijder et al., 2001) or DNA damage prior to amplification (Lindahl, 1993) and not taken as evidence of either in vitro recombination or sequence paralogy. To counteract the effect of mutations introduced in the course of cloning, consensus sequences were made for each of the parental sequences identified for each set of clones (i.e. from a given accession) to be used in subsequent analyses.
Sequences from tetraploids were aligned with directly sequenced diploid species and analysed using parsimony and MrBayes in the same way as for the diploids-only data matrices. See Table 2 for characteristics of the alignments. After constructing individual gene trees, each sequence from the tetraploid samples was assigned to a particular sequence type based on its similarity to diploid accessions, so that the taxon labels could be made consistent between gene trees. The 50 % bootstrap consensus trees were then used as input trees to construct a galled consensus network in Dendroscope. Differences in taxon sampling between the individual gene trees, due to difficulties in amplifying and cloning the available material and the occurrence of only one of the parental sequence types for some loci, required correction using the Z-closure algorithm (Huson et al., 2004). This is built into the network construction methods in Dendroscope and uses the phylogenetic information shared between the input trees to overcome gaps in the taxon sampling of individual gene trees. Holland et al. (2008) found that the effects of potential false splits introduced by the Z-closure algorithm are offset by count-based filtering of the splits during network construction. It was found that, using Z-closure, a 20–39 % threshold for network construction in Dendroscope resulted in some clades that only appeared in one of the input trees being used to calculate reticulations in the consensus network, contrary to the present purpose of filtering the incongruent clades. Therefore a more stringent 40 % threshold was used to construct a consensus network from the combined diploid and tetraploid data to avoid poorly supported reticulations at the cost of overall resolution. Tetraploid samples for which different sequence types appeared in separate clades were manually reconnected using hybridization nodes in enewick format (Cardona et al., 2008) and redrawn in Dendroscope.
RESULTS
Analysis of diploids
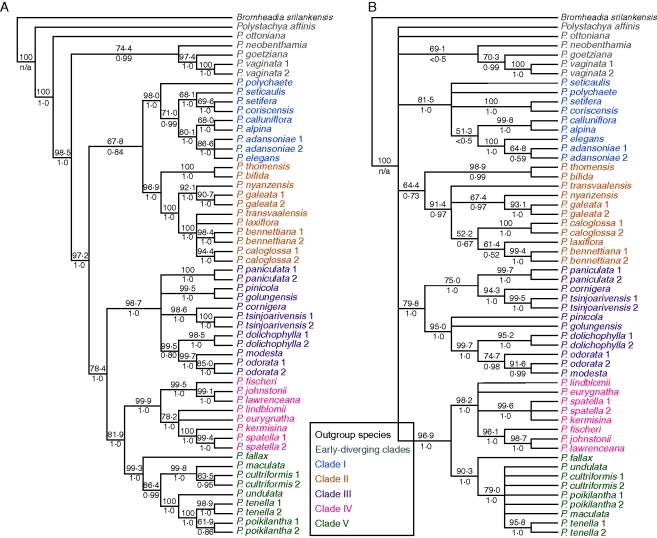
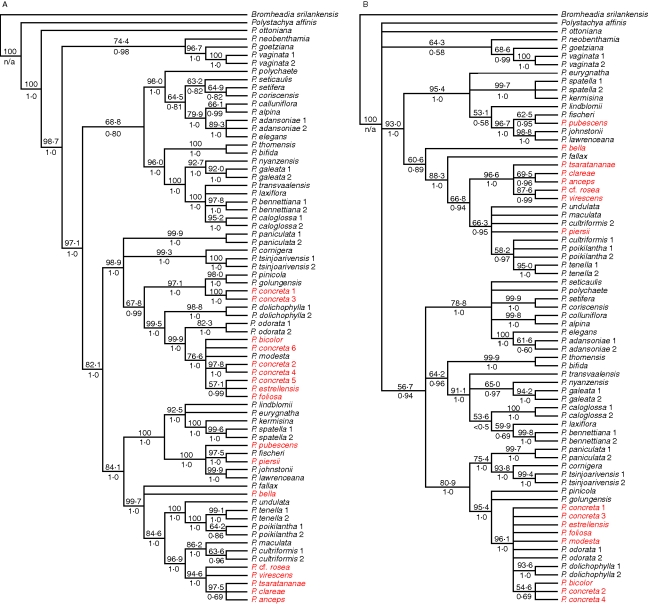
Individual gene trees for the three low-copy nuclear genes, the combined plastid DNA and ITS sequences are presented (Fig. 2) in the form of 50 % bootstrap consensus trees with the species names coloured according to their phylogenetic position in the plastid trees of Russell et al. (2010). Table 2 provides tree scores from maximum parsimony analysis of each data set in PAUP*. Each of the tree topologies is unique, although many clades are shared by more than one tree. Majority rule consensus trees from Bayesian analysis were congruent with parsimony trees but with higher resolution than the parsimony strict consensus trees. Since strict consensus trees for the nuclear genes contained clades that received low bootstrap and posterior probability support and Bayesian posterior probabilities are often unrealistically high (Simmons et al., 2004), the option was taken to present the bootstrap consensus trees here.
Fig. 2.
Fifty per cent bootstrap consensus trees from maximum parsimony analysis of diploid samples, using five loci: plastid DNA (A), ITS (B), PgiC (C), PhyC (D) and Rpb2 (E). Numbers above branches are bootstrap percentages; numbers below branches are Bayesian posterior probabilities. Species' names are coloured according to their correspondence to the main clades identified by plastid DNA analysis: clades I–V and species-poor, early diverging clades (Russell et al., 2010).
The topology of the plastid tree (Fig. 2A) agrees well with the more complete plastid trees presented in Russell et al. (2010). The previous plastid study identified five main clades, I–V, which are also found in the plastid tree in this study with high bootstrap percentages and posterior probabilities. However, not all of them are present in all of the gene trees. Plastid DNA analysis found a number of species-poor, early diverging clades sister to the larger clade containing clades I–V; in the nuclear gene trees these relationships were unresolved. Clades II, III, IV and V are not present (i.e. do not appear as monophyletic groups of accessions) in the PgiC tree; clades I and II are not present in PhyC; clades I, II and III are not present in Rpb2. However, although the trees show many differences, many of these are not strongly supported by bootstrap percentages and posterior probabilities of clades, especially in the nuclear gene trees.
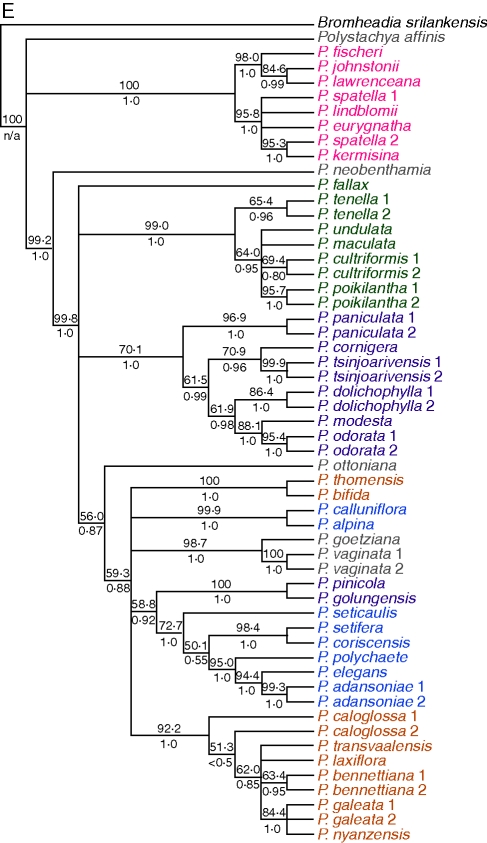
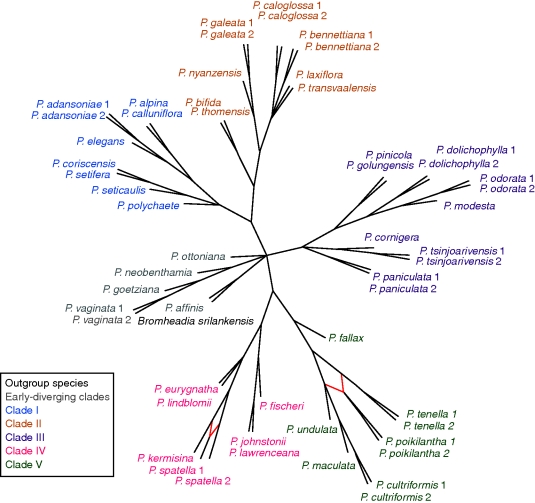
Differences between the trees are represented graphically by a filtered supernetwork (Fig. 3). This is an implicit reticulate network: cycles in the network represent conflicting phylogenetic signals rather than explicit phylogenetic hypotheses. Areas of incongruence according to Fig. 3 are at the base of the tree, the bases of clades I and III, the P. bennettiana/P. transvaalensis group in clade II and throughout clade V except for P. fallax. Despite the fact that the input trees were incongruent, the five main clades still group together in the supernetwork. A supernetwork (not shown) obtained without filtering the input trees shows a greater degree of conflict between splits at the core of the network, but the main clades are still recovered.
Fig. 3.
Filtered supernetwork using the five 50 % bootstrap consensus gene trees from parsimony analysis as input trees, with MinNumberTrees set at 2. Species' names are coloured according to their correspondence to the main clades identified by plastid DNA analysis: clades I–V and species-poor, early diverging clades (Russell et al., 2010).
The consensus galled network (Fig. 4), in which cycles explicitly represent alternative phylogenetic inferences between the trees, collapses relationships between the main clades to a four-way polytomy at the core of the tree (the ‘backbone’ if the tree was rooted). The accessions involved in reticulations are P. spatella 1 (but not 2), P. poikilantha 1 and P. poikilantha 2. As with the filtered supernetwork, the five main clades expected from plastid DNA analysis are all recovered, and the outer branches are generally well resolved.
Fig. 4.
Unrooted consensus galled network (20 % threshold for network construction) summarizing incongruities between the five individual gene trees of diploid species using 50 % bootstrap consensus trees as input. Branch lengths are not to scale; only the topology is shown. Red lines represent reticulations. Species names are coloured according to their correspondence to the main clades identified by plastid DNA analysis: clades I–V and species-poor, early diverging clades (Russell et al., 2010).
Analysis of tetraploids
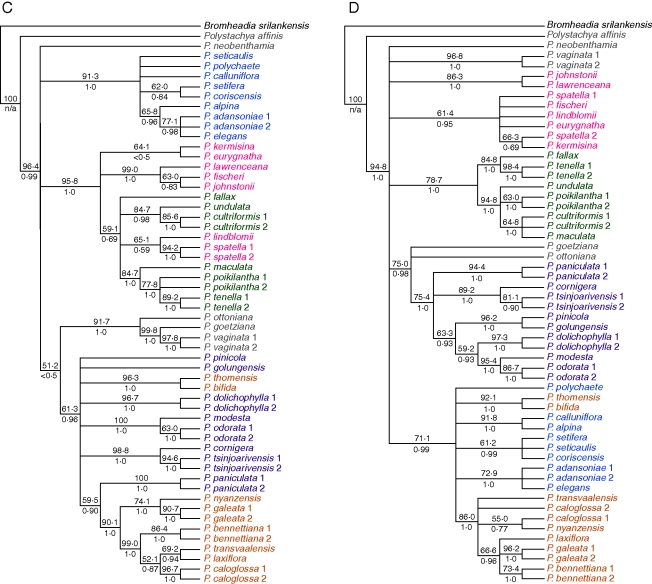
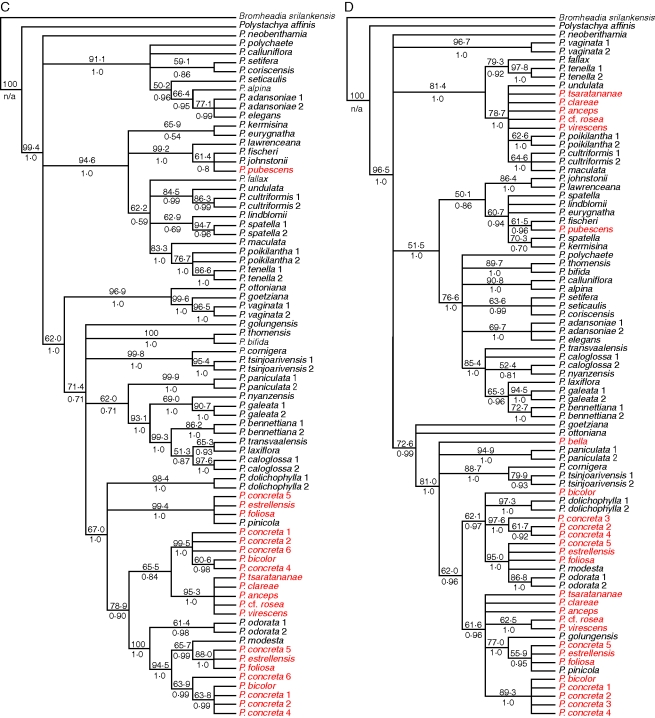
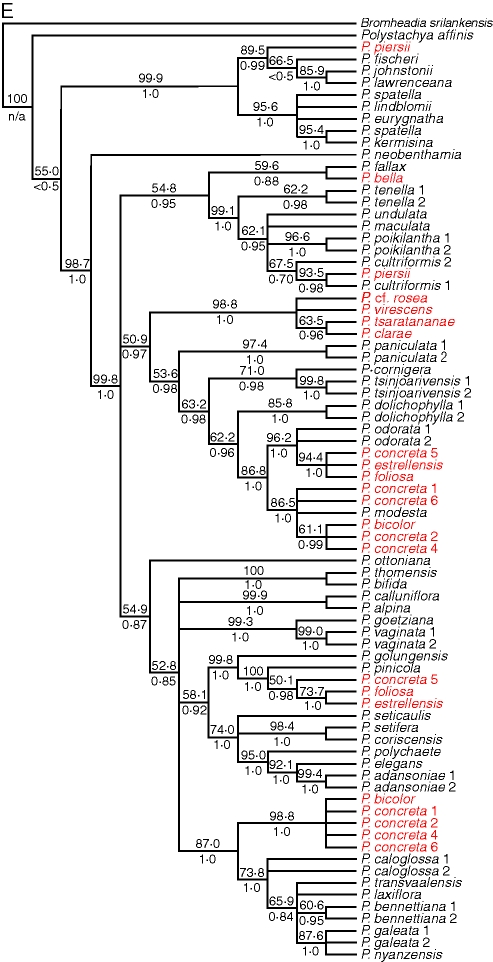
Overall, 60 out of 232 cloned sequences appeared chimeric (25·8 %) from tetraploid accessions from which two homoeologous sequences were recovered, but chimeric sequences were not distributed evenly among the three nuclear genes. The following percentages of clones were chimeric: PhyC, 36·9 %; PgiC, 22·1 %; Rpb2, 9·6 %. When cloned tetraploid sequences were included with diploid sequences in parsimony analysis, again none of the individual gene trees was congruent with any other (Fig. 5). As with the diploids-only data, Bayesian analysis agreed with the parsimony trees but with greater resolution overall. Due to the occurrence of clades in the strict consensus parsimony trees that received no bootstrap or posterior probability support, the bootstrap consensus trees are presented here, with maximum parsimony tree scores presented in Table 2.
Fig. 5.
Fifty per cent bootstrap consensus trees from maximum parsimony analysis of diploid and tetraploid samples, using five loci: A, plastid DNA; B, ITS; C, PgiC; D, PhyC; E, Rpb2. Numbers above branches are bootstrap percentages; numbers below branches are Bayesian posterior probabilities. Tetraploid accessions are shown in red.
With cloning, two distinct sequence types were found for almost all members of the pantropical tetraploid group for all three nuclear low-copy genes; only a single PhyC sequence type (Fig. 5D) was recovered in the sample P. concreta 1 (Madagascar), but it is unclear whether this is due to the loss of one copy of PhyC in some populations or PCR bias: 12 clones were sequenced, which should have easily recovered a product that was half of the PCR product. By contrast, from members of the Malagasy endemic clade only a single copy of PgiC (Fig. 5C) and Rpb2 (Fig. 5E) could be recovered, but two copies of PhyC. The two PhyC copies had sequences similar to those of P. odorata and P. cultriformis; the PgiC and Rpb2 sequences were all similar to P. odorata, whereas the plastid sequences and ITS were all similar to P. cultriformis. Since the network construction methods using the Z-closure algorithm do not require all of the parental sequences to be present in all of the samples, there was enough phylogenetic information in the five data sets to resolve the relationships of these species with confidence in spite of missing data or copy number reduction in PgiC and Rpb2. In constructing the consensus networks, an estimate had to be made of the parental haplotype of each gene copy in the tetraploids so that the terminal taxa of the individual trees could be correlated to each other. In the case of the pantropical tetraploids, each sequence could be said to have similarity to that of either P. modesta or P. golungensis (both diploids). These two species were not inferred to be the exact parental species, but rather diploid representatives of the two sequence types found in the tetraploids for each gene. Similarly, in the case of the Malagasy tetraploids, each sequence was similar to either P. cultriformis or P. modesta, and in the case of P. piersii each sequence was similar to either P. cultriformis or P. fischeri.
The consensus galled supernetwork (40 % threshold with five input gene trees using the Z-closure method to correct for differences in taxon sampling) including the tetraploid sequences is shown in Fig. 6. Relationships among the diploid species are similar to the results from analysing diploids alone, but the higher threshold for network construction has resulted in lower resolution overall. Polystachya fischeri is the one diploid species involved in a reticulation in Fig. 6; it was not involved in any reticulations in Fig. 4.
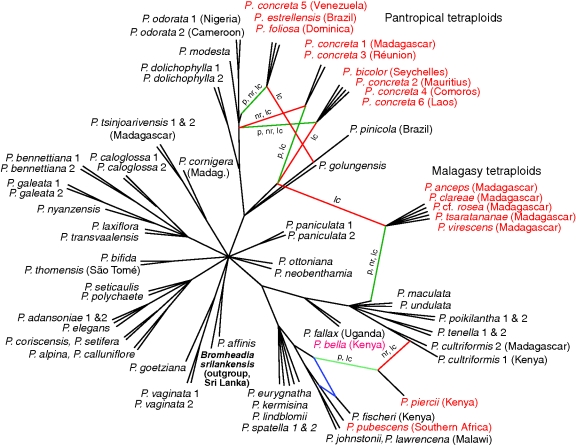
Fig. 6.
Unrooted consensus galled network (40 % threshold for network construction) summarizing incongruities and allopolyploids in the five individual gene trees of diploid and tetraploid accessions, using 50 % bootstrap consensus trees as input. Branch lengths are not to scale; only the topology is shown. Reticulations inferred solely from incongruence between the gene trees are coloured blue. Red- and green-coloured branches represent reticulations involving cloned tetraploids, for which the parental sequences were manually reconnected as hybridization nodes. Green branches represent relationships according to the plastid DNA tree; red branches are only present in nuclear DNA trees; these branches are also annotated if they are represented in the plastid (p), ITS (nr) or nuclear low-copy (lc) gene trees. Species names of the cloned tetraploid samples are coloured red. Accessions are from mainland tropical Africa unless otherwise indicated.
The parental sequences of pantropical tetraploids from Indian Ocean islands and Asia (eastern group) are closely related and unresolved. Parental sequences from the Neotropical members of the group are also closely related to each other, but they form a clade distinct from the eastern group. Among the eastern group, it was possible to differentiate between specimens that belonged to the same clade after network analysis but for which the plastid sequences corresponded to different reticulation edges. Branches corresponding to the plastid DNA sequences of the tetraploids are coloured green in Fig. 6, and each reticulate branch is labelled to indicate whether it is represented in the plastid, ITS or low-copy nuclear gene trees.
The Malagasy endemic group also comprises allotetraploids, with one parent from clade III and the other from clade V. The genetic divergence between the parents is greater in the Malagasy tetraploids than in the pantropical group, and the genetic variation within the group is higher. However, the species appear to have originated from the same pair of parents (one from section Cultriformes and one from section Polystachya), despite the relatively high morphological variation compared with the pantropical clade.
Polystachya piersii from Kenya is revealed as an allotetraploid arising from distantly related parent species, one in clade IV and the other in clade V. Its clade V parent is more closely related to the accession P. cultriformis 1 (also from Kenya) than P. cultriformis 2 (from Madagascar). It was not possible to distinguish different parental sequences from another two tetraploid accessions, P. bella and P. pubescens; they appear in the consensus network in an unresolved position at the base of clade V and sister to P. fischeri, respectively.
DISCUSSION
The results presented here provide a significant modification to our understanding of Polystachya phylogenetics and illustrate the utility of low-copy nuclear genes in resolving reticulate relationships in angiosperms. Although the number of species included in this study is lower than in a previous phylogenetic study of Polystachya using plastid data alone (Russell et al., 2010), the inclusion in this study of DNA sequences from multiple loci provides a qualitative test of the accuracy of the plastid DNA results and allows information on hybridization and reticulation to be added to our hypothesis of Polystachya evolution. The importance of using multiple gene trees instead of inferring reticulations from comparison between, say plastid DNA and a single nuclear locus, is highlighted by Linder and Rieseberg (2004). This is because stochastic and population-level events can lead to misleading results in individual gene trees. Although in discarding incongruities that are unique to single gene trees some evidence for reticulation in the genus has inevitably been discarded, the reticulations retained are more likely to have accompanied large-scale genomic changes affecting multiple genes. Those affecting only one gene tree could be due to introgression or lineage sorting and thus do not affect large portions of the genomes of these taxa.
Despite apparently high levels of incongruence between diploid gene trees, supernetwork and consensus network analysis revealed the incongruence to occur mainly at deeper phylogenetic levels. The main clades identified by Russell et al. (2010) using only plastid sequences, with greater taxon sampling, are not found in all the gene trees produced for this study, but are recovered by the filtered supernetwork and consensus network methods. Relationships between the main clades are not resolved, and support for deeper-level phylogenetic structure in any of the individual trees is not reproduced by any of the other trees except for the position of P. affinis as sister to the remainder of the genus. This is similar to the findings of Murphy et al. (2008) for Braconidae (Hymenoptera) using a filtered supernetwork approach.
The results question the relationship of P. pokilantha as a sister species to P. tenella as found by plastid, ITS and PgiC trees and the monophyly of P. spatella with respect to P. kermisina as found by plastid DNA data. This could be interpreted as possible homoploid hybridization between ancestors of these species, but phenomena other than hybridization could account for differences observed in the trees, especially given the low bootstrap and posterior probability support for many of the incongruent clades. Heterogeneous rates of sequence divergence between and within genes could be confounding the tree-building algorithms or the differences could simply result from sampling error (not enough variation to obtain a clear answer). Reticulation events inferred between diploid species were not found to be consistent between analyses. When the taxon sampling was changed to include cloned tetraploids and the analysis changed to include Z-closure and a higher threshold for network construction, the above-mentioned reticulations were not recovered but instead P. fischeri was represented as involved in a reticulation, sister to both P. pubescens and P. piersii. The fact that these are both polyploid accessions and not present in all of the input gene trees makes it likely that this reticulation is the result of a lack of information in the input trees (Holland et al., 2008), especially for the clade IV parent of P. piersii, which is only present in the plastid and Rpb2 data. Homoploid hybrids are likely to lose one parental copy fairly soon after their formation, and thus homoploid hybridization is best detected by looking for linkage disequilibrium, for which large numbers of loci are needed (Chase et al., 2010). Homoploid hybridization in angiosperms is clearly difficult to detect (Hegarty and Hiscock, 2008), and more than three loci would be needed to document this robustly.
More direct evidence of hybrid origins comes from cloning and sequencing the duplicated nuclear genes present in tetraploids (e.g. Petersen and Seberg, 2009). Polyploidy is present in at least eight Polystachya clades (Russell et al., 2010), but nuclear loci were often difficult to amplify and sequence. In this study, the cloning efforts were focused on five groups of polyploids including two groups comprising multiple accessions and three comprising single accessions. The proportion of recombinant sequences among tetraploid clones (25·9 %) was higher than would be expected if these were natural recombinants (e.g. 2·4 % among homoeologous expressed sequence tags in Gossypium; Salmon et al., 2010), supporting the present interpretation of these sequences as chimeric and the result of PCR-mediated recombination. Identifying the chimeric and parental sequences by eye based on single nucleotide polymorphisms and indels characteristic to each homoeologous sequence was possible with matrices with fewer cloned sequences produced in this study. For matrices with more sequences, an automated detection technique would be desirable, such as that used by Salmon et al. (2010).
The pantropical group, including P. concreta, mostly appears in the plastid trees (Russell et al., 2010) in a clade within which there is no resolution due to low levels of divergence; this is sister to P. dolichopylla, along with P. odorata and P. modesta, also with low levels of sequence variation between samples as far apart as Laos, Madagascar and Brazil. A second, smaller group of P. concreta samples occurs in a separate clade sister to P. golungensis. Analysis of low-copy nuclear genes gives greater resolution for this group and reveals it to comprise allotetraploid species. The two clades of P. concreta found by plastid DNA are hybrids between the same parent species; in Fig. 5 they are drawn as separate groups because the accessions within the two parents contributing their plastid genome are different in each group, providing evidence of independent origins of some populations. The Neotropical tetraploids of the P. concreta group have different origins from those in Asia and the Indian Ocean, so we can deduce at least three independent origins of the pantropical Polystachya tetraploid group, all of which have dispersed rapidly and recently from the centre of Polystachya distribution in Africa (there may potentially be more than three independent origins of the pantropical group accessions in this study, but we are unable to infer more than three from these data). From the diploid taxa included in this study, P. modesta is morphologically the most similar to pantropical tetraploid accessions and could be one of the parent species. Some Neotropical species including P. foliosa bear similarity to P. golungensis in flower size, shape and colour, and could be considered intermediate in morphology between, for example, P. golungensis and P. modesta or P. odorata. From their nuclear DNA sequences, the eastern group of pantropical tetraploids could share one parent species with the Malagasy tetraploids. These hypotheses of parental species are speculative; confident identification of the parent species would require broader taxon sampling and detailed morphological analysis.
Increased dispersal capability is commonly found in allopolyploids (Chase et al., 2003; Hegarty and Hiscock, 2008), and in Polystachya the capability for long-distance dispersal has arisen repeatedly among a certain set of hybrid offspring from relatively closely related parents. The presence of the Neotropical diploid P. pinicola as a sister to the Neotropical tetraploids (Fig. 6) suggests that dispersal of diploids might have been followed by allotetraploidy. Dierschke et al. (2009) found evidence for bicontinental hybrid origins of New Zealand Lepidium (Brassicaceae); the present results suggest a similar scenario is possible for Neotropical Polystachya, although greater taxon sampling would be required to confirm this. The wide distribution of Polystachya is unusual in Orchidaceae; only ten other genera have a comparable pantropical distribution (Dressler, 1993). Although orchid seeds appear adapted for wind dispersal due to their small size and internal air spaces, most seeds do not travel more than a few metres from their parent plant (Carey, 1998; Murren and Ellison, 1998). However, there are several recorded occurrences of long-distance dispersal in orchids (Arditti and Ghani, 2000), and it is not surprising given the large numbers of seeds produced by each capsule that over the course of time some of them are transported much further than most. Reasons for the apparently greater dispersal capacity of the pantropical tetraploids compared with the rest of the genus are unknown but could include a greater ability to be transported long distances and/or greater ability for seeds to germinate and establish populations in new areas. The particular adaptations that have allowed this would be worth further investigation.
The Malagasy tetraploids are also shown to be hybrids, and with parental species both genetically and morphologically more distant from each other than is the case with the pantropical group. Plastid DNA from all five accessions (representing five species) indicated a maternal parent from section Cultriformes, suggesting a single ancestral hybrid species from which the clade has subsequently diversified, although wider sampling would be necessary to rule out multiple independent origins. The fact that only one copy each of Rpb2 and PgiC could be sequenced in these species is probably due to preferential PCR amplification, but Feldman et al. (1997) found that low-copy DNA sequences can be eliminated from allopolyploid genomes rapidly in a non-random manner.
In contrast to the pantropical group, the Malagasy allotetraploids have not shown any remarkable long-distance dispersal capability, with the species remaining endemic to Madagascar and the Comoros (located approx. 340 km from Madagascar). The morphological variation within the group, however, is greater than within the pantropical species, which is consistent with hybridization between genetically more distant parents and subsequent speciation (Paun et al., 2009). Conversely, lower morphological variation among members of the pantropical group is consistent with hybrid origins from more closely related parents (Hegarty and Hiscock, 2005).
Members of the Malagasy allotetraploids have floral morphology (texture and shape of perianth segments; shape of inflorescence) consistent with members of section Cultriformes from clade V. The vegetative morphology (with multiple leaves on each shoot and an ovoid basal pseudobulb obscured by leaf bases) is more similar to members of section Polystachya in clade III (which includes the pantropical group). In the previous study based on plastid DNA sequences (Russell et al., 2010), the apparent transition from the single-leaved habit of section Cultriformes to a section Polystachya-type habit with foliose shoots in the Malagasy species was interpreted as a loss of the single-leaved character. The results of this study allow that conclusion to be modified and suggest that if the clade originated with hybrids that were morphologically intermediate between the parents, then the single-leafed character was probably not ‘lost’ in the Malagasy group as a result of selection but was not among the characters inherited by members of the clade when it originated. Diploid P. cultriformis (clade V) is extant in Madagascar, and this species or one of its ancestors is likely to be one of the parent species; from clade III the only members of section Polystachya, for which ploidy data are available, are tetraploids, but it is possible that diploid members of this group also occur there. Morphology of the Malagasy tetraploids is consistent with hybrid origins between P. cultriformis and a P. concreta relative. Field observations of Madagascan Polystachya have led workers to believe that hybridization is a common and ongoing process (G. Fischer, University of Salzburg, and A. Sieder, University of Vienna Botanical Garden, pers. comms.), and further investigations into the role of hybridization in evolutionary processes on the island would be rewarding. This would require increased taxon sampling, more detailed morphological and geographical studies, and data from more genes and population markers.
Allotetraploid P. piersii appears to be a hybrid between P. cultriformis and a relative of the P. fischeri/P. johnstonii/P. lawrenceana group from clade IV. Kenyan P. cultriformis is more closely related to P. piersii than to Madagascan P. cultriformis. Its morphology also appears intermediate between the two groups. Polystachya piersii has similar floral morphology to P. cultriformis, but its habit and vegetative morphology are more similar to clade IV members of section Affines. It is still not possible to say whether another two accessions, P. bella and P. pubescens, are allo- or autotetraploid; although we were unable to distinguish two parental sequences from any of the genes sequenced from either accession this could be because they lack hybrid origins or because, if hybrids, their parental species are too closely related for consistent sequence differences to be discerned or homoeologous gene copies from one of the parents have been lost or failed to amplify.
As well as the five groups represented by tetraploid accessions in this study, polyploidy occurs in several other groups in Polystachya; here the focus has been on taxa for which nuclear DNA could be amplified and cloned. At least two species occur as both diploids and tetraploids so, although this study has focused on hybrid clades, autopolyploidy might also be an important process in the genus. Further study on other tetraploid groups would contribute to our understanding of the significance of polyploidy in the evolution and biogeography of the genus in the African mainland.
ACKNOWLEDGEMENTS
We thank Tod Stuessy, head of the Department of Systematic and Evolutionary Botany, Vienna University for his support of this research. The following people also contributed time, expertise or materials: Elfriede Grasserbauer, Gudrun Kohl, Hanna Weiß-Schneeweiß and, especially, Cordula Blöch in the Department of Systematic and Evolutionary Botany in Vienna; Manfred Speckmaier and Anton Sieder at the Vienna University Botanic Garden; Gunter Fischer at Salzburg University; Isobyl La Croix in Ross-shire, Scotland; Laszlo Csiba and Laura Kelly at the Jodrell Laboratory, Kew; Marko Šafran and Visnja Besendorfer at Zagreb University; and Joanna Mytnik-Ejsmont at Gdansk University. Thanks go to the Annals of Botany editors and two anonymous referees who helped considerably to improve the manuscript. This work was supported by the Austrian Science Fund (FWF) [AP19108]
APPENDIX
Accession list: species name; country of origin when known; herbarium voucher when present; accession number of the living collection of the Royal Botanic Gardens, Kew or the HBV (University of Vienna Botanical Garden) where applicable; accession number for the DNA bank of the Jodrell Laboratory, Royal Botanic Gardens, Kew, where applicable; GenBank accession numbers for DNA sequences.
| Accession | Country | Herbarium voucher | Living collection (Kew/HBV) | Kew DNA bank | GenBank accession numbers |
||||
|---|---|---|---|---|---|---|---|---|---|
| Plastid | ITS | PgiC | PhyC | Rpb2 | |||||
| Bromheadia srilankensis Kruiz. & de Vogel | Sri Lanka | Chase 15746 (K) | NA | 15746 | GQ145086 | HM018544 | HM018560 | HM018513 | HM018526 |
| Polystachya adansoniae Rchb.f. 1 | Nigeria | Bytebier 429/94/469 (EA) | NA | 17957 | GQ145088 | GU556632 | GU556782 | GU556701 | GU556852 |
| Polystachya adansoniae 2 | Cameroon | A. Russell 92 (YA) | NA | NA | GQ145089 | HM018545 | HM018561 | HM018514 | HM018527 |
| Polystachya affinis Lindl. | Nigeria | Chase 21165 (K) | Kew 1981-4996 | 21165 | GQ145090 | GU556633 | GU556783 | GU556702 | GU556853 |
| Polystachya alpina Lindl. | Cameroon | A. Russell 67 (YA) | NA | NA | GQ145092 | GU556634 | GU556784 | GU556703 | GU556854 |
| Polystachya anceps Ridl. | Madagascar | Fischer & Sieder FS4068 (WU) | NA | NA | GQ145094 | GU556692 | HM018562 | GU556755-GU556756 | NA |
| Polystachya bella Summerh. | Kenya | Beatrice 783 (EA) | NA | 17950 | GQ145095 | HM018546 | NA | HM018515 | HM018528 |
| Polystachya bennettiana Rchb.f. 1 | Kenya | Beatrice 338/94/418 (EA) | NA | 17958 | GQ145096 | HM018547 | HM018563 | HM018516 | HM018529 |
| Polystachya bennettiana 2 | Unknown | Mughambi & Odhiambo 81/01 (EA) | NA | 19186 | GQ145097 | HM176598 | HM018564 | HM018517 | HM018530 |
| Polystachya bicolor Rolfe (=P. concreta (Jacq.) Garay & H.R.Sweet) | Seychelles | A. Russell Kew-2003-406 (WU) | Kew 2003-406 | 25884 | GQ145120 | GU556686 | GU556836-GU556837 | GU556760-GU556761 | GU556907-GU556908 |
| Polystachya bifida Lindl. | São Tomé | NA | Kew 2001-3989 | 25885 | GQ145100 | GU556636 | GU556787 | GU556706 | GU556857 |
| Polystachya calluniflora Kraenzl. | Cameroon | A. Russell 63 (YA) | NA | NA | GQ145104 | GU556638 | GU556788 | GU556708 | GU556859 |
| Polystachya caloglossa Rchb.f. 1 | Cameroon | A. Russell 41 (YA) | NA | NA | GQ145105 | HM018548 | HM018565 | HM018518 | HM018531 |
| Polystachya caloglossa 2 | Cameroon | A. Russell 104 (YA) | NA | NA | GQ145106 | GU556639 | GU556789 | GU556709 | GU556860 |
| Polystachya clareae Hermans | Madagascar | Fischer & Sieder s.n. 27/1/2007 (WU) | NA | NA | GQ145109 | GU556684 | GU556833 | GU556757-GU556758 | GU556904 |
| Polystachya concreta (Jacq.) Garay & H.R.Sweet 1 | Madagascar | Chase 17854 (K) | Kew 1997-4474 | 17854 | GQ145110 | GU556685 | GU556834-GU556835 | GU556759 | GU556905-GU556906 |
| Polystachya concreta 2 | Mauritius | NA | HBV ORCH07278 | NA | GQ145118 | GU556687 | GU556840-GU556841 | GU556764-GU556765 | GU556913-GU556914 |
| Polystachya concreta 3 | Réunion | NA | HBV ‘Reunion 1’ | NA | GQ145117 | GU556688 | NA | GU556766-GU556767 | GU556914-GU556915 |
| Polystachya concreta 4 | Comoros | Photograph | HBV ORCH07417 | NA | GQ145119 | GU556698 | GU556842-GU556843 | GU556768-GU556769 | GU556915-GU556916 |
| Polystachya concreta 5 | Venezuela | NA | HBV ORCH06361 | NA | GU556925 | NA | GU556846-GU556847 | GU556770-GU556771 | GU556917-GU556918 |
| Polystachya concreta 6 | Laos | A. Russell ORCH06415 (WU) | HBV ORCH06415 | NA | GU556926 | NA | GU556848-GU556849 | NA | GU556919-GU556920 |
| Polystachya coriscensis Rchb.f. | Unknown | A. Russell ORCH07314 (WU) | HBV ORCH07314 | NA | GQ145122 | GU556641 | GU556791 | GU556711 | GU556862 |
| Polystachya cornigera Schltr. | Madagascar | Fischer & Sieder FS3208 (WU) | NA | NA | GQ145123 | GU556642 | GU556792 | GU556740 | HM018532 |
| Polystachya cultriformis (Thouars) Lindl. ex Spreng. 1 | Unknown | Mugambi & Odhiambo 054/98/1607 (EA) | NA | 19182 | GQ145124 | GU556643 | GU556793 | GU556713 | GU556863 |
| Polystachya cultriformis 2 | Madagascar | Fischer & Sieder FS1045 (WU) | HBV FS1045 | NA | GQ145125 | GU556644 | GU556794 | GU556714 | GU556864 |
| Polystachya dolichophylla Schltr. 1 | Cameroon | Chase 25886 (K) | Kew 1989-1745 | 25886 | GQ145128 | GU556646 | GU556796 | GU556716 | GU556865 |
| Polystachya dolichophylla 2 | Unknown | Photograph | HBV ORCH03072 | NA | GU556927 | GU556647 | GU556797 | GU556712 | GU556866 |
| Polystachya elegans Rchb.f. | Cameroon | A. Russell 74 (YA) | NA | GQ145129 | GU556648 | GU556798 | GU556718 | GU556867 | |
| Polystachya estrellensis Rchb.f. (=P. concreta (Jacq.) Garay & H.R.Sweet) | Brazil | A. Russell ORCH06604 (WU) | HBV ORCH06604 | NA | GQ145114 | GU556693 | GU556838-GU556839 | GU556762-GU556763 | GU556909-GU556910 |
| Polystachya eurygnatha Summerh. | Unknown | Photograph | NA | NA | GQ145131 | GU556649 | GU556799 | GU556719 | GU556868 |
| Polystachya fallax Kraenzl. | Uganda | Chase 17922 (K) | Kew 2001-4022 | 17922 | GQ145132 | GU556650 | HM018566 | GU556720 | GU556869 |
| Polystachya fischeri Rchb.f. ex Kraenzl. | Kenya | Pearce 616/94/607 (EA) | NA | 17964 | GQ145133 | GU556651 | GU556800 | GU556721 | GU556870 |
| Polystachya foliosa (Hook.) Rchb.f. | Dominican Republic | NA | Kew 2001-3986 | 25887 | GQ145135 | GU556690 | HM018567-HM018568 | GU556772-GU556773 | GU556921-GU556922 |
| Polystachya galeata (Sw.) Rchb.f. 1 | Unknown | Chase O-1496 (K) | Kew 1972-1958 | O-1496 | GQ145139 | GU556652 | GU556801 | GU556722 | GU556871 |
| Polystachya galeata 2 | Unknown | ‘C283’ (K) | NA | 9041 | GU556928 | GU556653 | GU556802 | GU556723 | GU556872 |
| Polystachya goetziana Kraenzl. | Kenya | Bytebier 1772 (EA) | NA | 17955 | GQ145141 | GU556654 | GU556803 | GU556724 | GU556873 |
| Polystachya golungensis Rchb.f. | Unknown | A. Russell ORCH05170 (WU) | HBV ORCH05170 | NA | GQ145143 | GU556655 | GU556804 | GU556725 | GU556874 |
| Polystachya johnstonii Rolfe | Unknown | Photograph | HBV ORCH06241 | NA | GQ145149 | GU556657 | GU556806 | GU556727 | GU556876 |
| Polystachya kermisina Kraenzl. | Rwanda | Photograph | HBV ORCH07240 | NA | GQ145150 | GU556658 | GU556807 | GU556728 | GU556877 |
| Polystachya lawrenceana Kraenzl. | Malawi | Photograph | NA | NA | GQ145152 | HM018549 | HM018569 | HM018519 | HM018533 |
| Polystachya laxiflora Lindl. | Unknown | A. Russell ORCH07315 (WU) | HBV ORCH07315 | NA | GQ145153 | GU556659 | GU556808 | GU556729 | GU556878 |
| Polystachya lindblomii Schltr. | Kenya | Bytebier 1142/98/1695 (EA) | NA | 17967 | GQ145154 | GU556660 | GU556809 | GU556730 | GU556879 |
| Polystachya maculata P.J.Cribb | Burundi | Photograph | HBV ORCH07263 | NA | GQ145156 | GU556696 | GU556810 | GU556731 | GU556880 |
| Polystachya modesta Rchb.f. | Unknown | NA | HBV ORCH05165 | NA | GQ145159 | GU556662 | GU556812 | GU556733 | GU556882 |
| Polystachya neobenthamia Schltr. | Unknown | Photograph | HBV ORCH07214 | NA | GQ145087 | GU556663 | GU556813 | GU556734 | GU556883 |
| Polystachya nyanzensis Rendle | Unknown | A. Russell ORCH06425 (WU) | HBV ORCH06425 | NA | GQ145163 | HM018550 | HM018570 | HM018520 | HM018534 |
| Polystachya odorata Lindl. 1 | Nigeria | Chase 17857 (K) | Kew 1970-2771 | 17857 | GQ145164 | GU556664 | GU556814 | GU556735 | GU556884 |
| Polystachya odorata 2 | Cameroon | A. Russell 42 (YA) | NA | NA | GQ145165 | GU556665 | GU556815 | GU556736 | GU556885 |
| Polystachya ottoniana Rchb.f. | Unknown | NA | Kew 2005-964 | 25888 | GQ145168 | GU556666 | GU556816 | GU556737 | GU556886 |
| Polystachya paniculata (Sw.) Rolfe 1 | Ethiopia | NA | Kew 1984-4977 | 25889 | GQ145170 | GU556667 | GU556818 | GU556739 | GU556888 |
| Polystachya paniculata 2 | Unknown | Photograph | HBV O99B26-1 | NA | HM018557-HM018559 | HM018551 | HM018571 | HM018521 | HM018535 |
| Polystachya piersii P.J.Cribb | Kenya | Beatrice 101/95/1186 (EA) | NA | 17948 | GQ145172 | HM018552 | NA | NA | HM018536-HM018537 |
| Polystachya pinicola Barb.Rodr. | Brazil | NA | HBV ORCH06606 | NA | GQ145174 | GU556668 | GU556819 | GU556717 | GU556889 |
| Polystachya poikilantha Kraenzl. 1 | Kenya | Bytebier 956/97/524 (EA) | NA | 19261 | GQ145176 | GU556669 | GU556820 | GU556741 | GU556890 |
| Polystachya poikilantha 2 (var. leucorhoda (Kraenzl.) P.J.Cribb & Podz.) | Unknown | Photograph | HBV ORCH06272 | NA | GQ145177 | HM018553 | HM018572 | HM018522 | HM018538 |
| Polystachya polychaete Kraenzl. | Kenya | NA | Kew 2001-3987 | 25890 | GQ145178 | GU556670 | GU556821 | GU556742 | GU556891 |
| Polystachya pubescens Rchb.f. | Unknown | Kurzweil 1849 (K) | NA | O-700 | GQ145179 | HM018554 | HM018573 | HM018523 | NA |
| Polystachya cf. rosea Ridl. | Madagascar | Fischer & Sieder FS796 (WU) | HBV FS796 | NA | GQ145185 | GU556689 | GU556850 | GU556774-GU556775 | GU556923 |
| Polystachya seticaulis Rendle | Congo | Chase 17924 (K) | Kew 2001-3981 | 17924 | GQ145186 | GU556671 | GU556822 | GU556743 | GU556892 |
| Polystachya setifera Lindl. | Unknown | Chase O-1493 (K) | Kew 1983-2403 | O-1493 | GQ145187 | GU556672 | HM018574 | GU556744 | GU556893 |
| Polystachya spatella Kraenzl. 1 | Kenya | Bytebier 949 (EA) | NA | 17951 | GQ145188 | GU556673 | GU556823 | GU556745 | GU556894 |
| Polystachya spatella 2 | Kenya | Khayota 381 (EA) | NA | 19263 | GQ145189 | HM018555 | HM018575 | HM018524 | HM018539 |
| Polystachya tenella Summerh. 1 | Kenya | Bytebier 955/97/1524 (EA) | NA | 17952 | GQ145193 | GU556674 | GU556824 | GU556746 | GU556895 |
| Polystachya tenella 2 | Kenya | Bytebier 955/97/1523 (EA) | NA | 19262 | GQ145194 | GU556675 | GU556825 | GU556747 | GU556896 |
| Polystachya thomensis Summerh. | São Tomé | Chase 17858 (K) | Kew 2001-3989 | 17858 | GQ145196 | GU556677 | GU556827 | GU556748 | GU556898 |
| Polystachya transvaalensis Schltr. | Kenya | Bytebier 951/97/1519 (EA) | NA | 17969 | GQ145197 | GU556678 | GU556828 | GU556749 | GU556899 |
| Polystachya tsaratananae H.Perrier | Madagascar | Chase 17861 (K) | Kew 2001-2413 | 17861 | GQ145199 | GU556691 | GU556851 | GU556776-GU556777 | HM018540 |
| Polystachya tsinjoarivensis H.Perrier 1 | Madagascar | Fischer & Sieder FS3209 (WU) | NA | NA | GQ145201 | HM018556 | HM018576 | HM018525 | HM018541 |
| Polystachya tsinjoarivensis 2 | Madagascar | Photograph | HBV FS4182 | NA | GQ145202 | GU556679 | GU556829 | GU556750 | HM018542 |
| Polystachya undulata P.J.Cribb & Podz. | Unknown | Chase 17862 (K) | Kew 2001-3975 | 17862 | GQ145203 | GU556680 | GU556830 | GU556751 | GU556900 |
| Polystachya vaginata Summerh. 1 | Kenya | Bytebier 566/95/1140 (EA) | NA | 17949 | GQ145204 | GU556681 | GU556831 | GU556752 | GU556901 |
| Polystachya vaginata 2 | Kenya | Bytebier 452/97/1587 (EA) | NA | 19265 | GQ145205 | GU556682 | GU556832 | GU556753 | GU556902 |
| Polystachya virescens Ridl. | Madagascar | Fischer & Sieder FS1002 (WU) | HBV FS1002 | NA | GQ145206 | GU556697 | HM018577 | GU556778-GU556779 | HM018543 |
LITERATURE CITED
- Alvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Molecular Phylogenetics and Evolution. 2003;29:417–434. doi: 10.1016/s1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Anthony NM, Clifford SL, Bawe-Johnson M, Abernethy KA, Bruford MW, Wickings EJ. Distinguishing gorilla mitochondrial sequences from nuclear integrations and PCR recombinants: guidelines for their diagnosis in complex sequence databases. Molecular Phylogenetics and Evolution. 2007;43:553–566. doi: 10.1016/j.ympev.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Arditti J, Ghani AKA. Tansley review no. 110. New Phytologist. 2000;145:367–421. doi: 10.1046/j.1469-8137.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- Baack EJ, Rieseberg LH. A genomic view of introgression and hybrid speciation. Current Opinion in Genetics and Development. 2007;17:513–518. doi: 10.1016/j.gde.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg C, Higgins WE, Dressler RL, Whitten WM, Soto-Arenas MA, Chase MW. A phylogenetic study of Laeliinae (Orchidaceae) based on combined nuclear and plastid DNA sequences. Annals of Botany. 2009;104:417–430. doi: 10.1093/aob/mcp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona G, Rossello F, Valiente G. Extended Newick: it is time for a standard representation of phylogenetic networks. BMC Bioinformatics. 2008;9:532. doi: 10.1186/1471-2105-9-532. doi: 10.1186/1471-2105-9-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey PD. Modelling the spread of Himantoglossum hircinum (L.) Spreng. at a site in the south of England. Botanical Journal of the Linnean Society. 1998;126:159–172. [Google Scholar]
- Chase MW, Knapp S, Cox AV, et al. Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae) Annals of Botany. 2003;92:107–127. doi: 10.1093/aob/mcg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Paun O, Fay MF. Hybridization and speciation in angiosperms: a role for pollinator shifts? BMC Biology. 2010;8:45. doi: 10.1186/1741-7007-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annual Review of Plant Biology. 2007;58:377–406. doi: 10.1146/annurev.arplant.58.032806.103835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronn R, Cedroni M, Haselkorn T, Grover C, Wendel JF. PCR-mediated recombination in amplification products derived from polyploid cotton. Theoretical and Applied Genetics. 2002;104:482–489. doi: 10.1007/s001220100741. [DOI] [PubMed] [Google Scholar]
- Dierschke T, Mandakova T, Lysak MA, Mummenhoff K. A bicontinental origin of polyploid Australian/New Zealand Lepidium species (Brassicaceae)? Evidence from genomic in situ hybridization. Annals of Botany. 2009;104:681–688. doi: 10.1093/aob/mcp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Whitkus R, Rieseberg LH. Distribution of spontaneous plant hybrids. Proceedings of the National Academy of Sciences of the USA. 1996;93:5090–5093. doi: 10.1073/pnas.93.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Liu B, Segal G, Abbo S, Levy AA, Vega JM. Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics. 1997;147:1381–1387. doi: 10.1093/genetics/147.3.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliner GN, Rossello JA. Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants. Molecular Phylogenetics and Evolution. 2007;44:911–919. doi: 10.1016/j.ympev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Ford VS, Gottlieb LD. Reassessment of phylogenetic relationships in Clarkia sect. Sympherica. American Journal of Botany. 2003;90:284–292. doi: 10.3732/ajb.90.2.284. [DOI] [PubMed] [Google Scholar]
- Ford VS, Lee J, Baldwin BG, Gottlieb LD. Species divergence and relationships in Stephanomeria (Compositae): PgiC phylogeny compared to prior biosystematic studies. American Journal of Botany. 2006;93:480–490. doi: 10.3732/ajb.93.3.480. [DOI] [PubMed] [Google Scholar]
- Gruenstaeudl M, Urtubey E, Jansen RK, Samuel R, Barfuss MHJ, Stuessy TF. Phylogeny of Barnadesioideae (Asteraceae) inferred from DNA sequence data and morphology. Molecular Phylogenetics and Evolution. 2009;51:572–587. doi: 10.1016/j.ympev.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Hiscock SJ. Hybrid speciation in plants: new insights from molecular studies. New Phytologist. 2005;165:411–423. doi: 10.1111/j.1469-8137.2004.01253.x. [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Current Biology. 2008;18:R435–R444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Hodkinson TR, Chase MW, Takahashi C, Leitch IJ, Bennett MD, Renvoize SA. The use of DNA sequencing (ITS and trnL-F), AFLP, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae) American Journal of Botany. 2002;89:279–286. doi: 10.3732/ajb.89.2.279. [DOI] [PubMed] [Google Scholar]
- van den Hof K, van den Berg RG, Gravendeel B. Chalcone synthase gene lineage diversification confirms allopolyploid evolutionary relationships of European rostrate violets. Molecular Biology and Evolution. 2008;25:2099–2108. doi: 10.1093/molbev/msn157. [DOI] [PubMed] [Google Scholar]
- Holland BR, Benthin S, Lockhart PJ, Moulton V, Huber KT. Using supernetworks to distinguish hybridization from lineage-sorting. BMC Evolutionary Biology. 2008;8:202. doi: 10.1186/1471-2148-8-202. doi:10.1186/1471-2148-8-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Husband BC. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biological Journal of the Linnean Society. 2004;82:537–546. [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Huson DH, Dezulian T, Klöpper T, Steel MA. Phylogenetic super-networks from partial trees. In: Jonassen I, Kim J, editors. Algorithms in bioinformatics. Heidelberg: Springer-Verlag; 2004. [DOI] [PubMed] [Google Scholar]
- Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. doi:10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Harada K, Tachida H, Ashton PS. Phylogeny of PgiC gene in Shorea and its closely related genera (Dipterocarpaceae), the dominant trees in Southeast Asian tropical rain forests. American Journal of Botany. 2005;92:775–788. doi: 10.3732/ajb.92.5.775. [DOI] [PubMed] [Google Scholar]
- Kelchner SA. The evolution of non-coding chloroplast DNA and its application in plant systematics. Annals of the Missouri Botanical Garden. 2000;87:482–498. [Google Scholar]
- Kelly LJ, Leitch AR, Clarkson JJ, Hunter RB, Knapp S, Chase MW. Intragenic recombination events and evidence for hybrid speciation in Nicotiana (Solanaceae) Molecular Biology and Evolution. 2010;27:781–799. doi: 10.1093/molbev/msp267. [DOI] [PubMed] [Google Scholar]
- Leitch AR, Leitch IJ. Genomic plasticity and the diversity of polyploid plants. Science. 2008;320:481–483. doi: 10.1126/science.1153585. [DOI] [PubMed] [Google Scholar]
- Lexer C, Joseph J, van Loo M, et al. The use of digital image-based morphometrics to study the phenotypic mosaic in taxa with porous genomes. Taxon. 2009;58:349–364. [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Linder CR, Rieseberg LH. Reconstructing patterns of reticulate evolution in plants. American Journal of Botany. 2004;91:1700–1708. [PMC free article] [PubMed] [Google Scholar]
- McBreen K, Lockhart PJ. Reconstructing reticulate evolutionary histories of plants. Trends in Plant Science. 2006;11:398–404. doi: 10.1016/j.tplants.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4: analysis of phylogeny and character evolution. Sunderland, MA: Sinauer Associates; 2005. [DOI] [PubMed] [Google Scholar]
- Mallet J. Hybridization as an invasion of the genome. Trends in Ecology and Evolution. 2005;20:229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Martin DP, Williamson C, Posada D. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics. 2005;21:260–262. doi: 10.1093/bioinformatics/bth490. [DOI] [PubMed] [Google Scholar]
- Mathews S, Sharrock R. The phytochrome gene family in grasses (Poaceae): a phylogeny and evidence that grasses have a subset of the loci found in dicot angiosperms. Molecular Biology and Evolution. 1996;13:1141–1150. doi: 10.1093/oxfordjournals.molbev.a025677. [DOI] [PubMed] [Google Scholar]
- Murphy N, Banks JC, Whitfield JB, Austin AD. Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera: Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. Molecular Phylogenetics and Evolution. 2008;47:378–395. doi: 10.1016/j.ympev.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Murren C, Ellison A. Seed dispersal characteristics of Brassavola nodosa (Orchidaceae) American Journal of Botany. 1998;85:675–680. [PubMed] [Google Scholar]
- Nylander JAA. MrModeltest. Uppsala: program distributed by the author; 2004. v2. [Google Scholar]
- Oh SH, Potter D. Phylogenetic utility of the second intron of LEAFY in Neillia and Stephanandra (Rosaceae) and implications for the origin of Stephanandra. Molecular Phylogenetics and Evolution. 2003;29:203–215. doi: 10.1016/s1055-7903(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Osborn TC, Pires JC, Birchler JA, et al. Understanding mechanisms of novel gene expression in polyploids. Trends in Genetics. 2003;19:141–147. doi: 10.1016/s0168-9525(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Otto SP. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Oxelman B, Yoshikawa N, McConaughy BL, Luo J, Denton AL, Hall BD. RPB2 gene phylogeny in flowering plants, with particular emphasis on asterids. Molecular Phylogenetics and Evolution. 2004;32:462–79. doi: 10.1016/j.ympev.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Paun O, Fay MF, Soltis DE, Chase MW. Genetic and epigenetic alterations after hybridization and genome doubling. Taxon. 2007;56:649–656. [PMC free article] [PubMed] [Google Scholar]
- Paun O, Forest F, Fay MF, Chase MW. Hybrid speciation in angiosperms: parental divergence drives ploidy. New Phytologist. 2009;182:507–518. doi: 10.1111/j.1469-8137.2009.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen G, Seberg O. On the origin of the tetraploid species Hordeum capense and H. secalinum (Poaceae) Systematic Botany. 2009;29:862–873. [Google Scholar]
- Posada D, Crandall KA. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proceedings of the National Academy of Sciences of the USA. 2001;98:13757–13762. doi: 10.1073/pnas.241370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Edinburgh, UK: Institute of Evolutionary Botany, University of Edinburgh; 2007. Tracer v1.4. available from http://beast.bio.ed.ac.uk/Tracer . [Google Scholar]
- Rieseberg LH, Whitton J, Linder CR. Molecular marker incongruence in plant hybrid zones and phylogenetic trees. Acta Botanica Neerlandica. 1996;45:243–262. [Google Scholar]
- Ronçal J, Francisco-Ortega J, Asmussen CB, Lewis CE. Molecular phylogenetics of tribe Geonomeae (Arecaceae) using nuclear DNA sequences of phosphoribulokinase and RNA polymerase II. Systematic Botany. 2005;30:275–283. [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz SA, editors. Bioinformatics methods and protocols. Totowa, NJ: Humana Press; 1999. [Google Scholar]
- Rupp B. Genome size and molecular phylogeny of selected Polystachya species (Orchidaceae) 2008 Diploma Thesis, University of Vienna, Austria. [Google Scholar]
- Rupp B, Samuel R, Russell A, Temsch EM, Chase MW, Leitch IJ. Genome size in Polystachya (Orchidaceae) and its relationship to epidermal characters. Botanical Journal of the Linnean Society. 2010 in press. [Google Scholar]
- Russell A, Samuel MR, Rupp B, et al. Phylogenetics and cytology of a pantropical orchid genus Polystachya (Polystachyinae; Vandeae; Orchidaceae): evidence from plastid DNA sequence data. Taxon. 2010;59:389–404. [Google Scholar]
- Saarela JM, Rai HS, Doyle JA, et al. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature. 2007;446:312–315. doi: 10.1038/nature05612. [DOI] [PubMed] [Google Scholar]
- Salmon A, Flagel L, Ying B, Udall JA, Wendel JF. Homoeologous nonreciprocal recombination in polyploid cotton. New Phytologist. 2010;186:123–134. doi: 10.1111/j.1469-8137.2009.03093.x. [DOI] [PubMed] [Google Scholar]
- Samuel R, Kathriarachchi H, Hoffmann P, et al. Molecular phylogenetics of Phyllanthaceae: evidence from plastid matK and nuclear PHYC sequences. American Journal of Botany. 2005;92:132–141. doi: 10.3732/ajb.92.1.132. [DOI] [PubMed] [Google Scholar]
- Sang T, Pan J, Zhang DM, et al. Origins of polyploids: an example from peonies (Paeonia) and a model for angiosperms. Biological Journal of the Linnean Society. 2004;82:561–571. [Google Scholar]
- Schilling EE, Panero JL. Phylogenetic reticulation in subtribe Helianthinae. American Journal of Botany. 1996;83:939–948. [Google Scholar]
- Schwarzbach AE, Rieseberg LH. Likely multiple origins of a diploid hybrid sunflower species. Molecular Ecology. 2002;11:1703–1715. doi: 10.1046/j.1365-294x.2002.01557.x. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Pickett KM, Miya M. How meaningful are Bayesian support values? Molecular Biology and Evolution. 2004;21:188–199. doi: 10.1093/molbev/msh014. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speksnijder A, Kowalchuk GA, De Jong S, Kline E, Stephen JR, Laanbroek HJ. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Applied and Environmental Microbiology. 2001;67:469–472. doi: 10.1128/AEM.67.1.469-472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Pourkheirandish M, Komatsuda T. Molecular evolution and phylogeny of the RPB2 gene in the genus Hordeum. Annals of Botany. 2009;103:975–983. doi: 10.1093/aob/mcp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sunderland, MA: Sinauer Associates; 2003. Version 4. [Google Scholar]
- Thomas BR, Ford VS, Pichersky E, Gottlieb LD. Molecular characterization of duplicate cytosolic phosphoglucose isomerase genes in Clarkia and comparison to the single-gene in Arabidopsis. Genetics. 1993;135:895–905. doi: 10.1093/genetics/135.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MM, Garwood NC, Baker WJ, et al. Molecular phylogeny of the palm genus Chamaedorea, based on the low-copy nuclear genes PRK and RPB2. Molecular Phylogenetics and Evolution. 2006;38:398–415. doi: 10.1016/j.ympev.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissemann V. Plant evolution by means of hybridization. Systematics and Biodiversity. 2007;5:243–253. [Google Scholar]