Abstract
Background and Aims
Plants respond to the spatial and temporal heterogeneity of a resource supply. However, their responses will depend on intraspecific competition for resource acquisition. Although plants are subject to various intensities of intraspecific competition, most studies of resource heterogeneity have been carried out under a single density so that the effects of intraspecific competition on plant responses to resource heterogeneity are largely unknown.
Methods
A growth experiment was performed to investigate plant responses to the temporal heterogeneity of water supply and nutrient levels under multiple plant densities. The annual plant Perilla frutescens was grown using different combinations of frequency of water supply, nutrient level and density, while providing the same total amount of water under all conditions. The effects of the treatments on biomass, allocation to roots and intensity of competition were analysed after 48 d.
Key Results
Biomass and allocation to roots were larger under homogeneous than under heterogeneous water supply, and the effects of water heterogeneity were greater at high density than at low density. The effects of water heterogeneity were greater at high nutrient level than at low level for biomass, while the effects were greater at low nutrient level than high level for allocation to roots. Competition was severer under homogeneous than under heterogeneous water supply.
Conclusions
Competition for water probably makes plants more sensitive to the water heterogeneity. In addition, the intensity of intraspecific competition can be affected by the temporal patterns of water supply. Because both resource heterogeneity and intraspecific competition affect resource acquisition and growth of plants, their interactive effects should be evaluated more carefully under future studies.
Keywords: Biomass allocation, competition intensity, heterogeneous water supply, intraspecific competition, nutrient level, Perilla frutescens, population density, resource heterogeneity
INTRODUCTION
In the field, plant resources exhibit spatial and temporal heterogeneity (Jackson and Caldwell, 1993a, b; Farley and Fitter, 1999; James et al., 2003), and plant responses to the resource heterogeneity are likely to depend on the intraspecific competition for resource acquisition. However, most studies have been conducted under a single population density in focusing on plant responses to resource heterogeneity. Therefore, the effects of competition on plant responses have not been evaluated, although plants are subject to various intensities of intraspecific competition (Begon et al., 2006).
Water is a plant resource with temporally variable availability (James et al., 2003). The temporal heterogeneity of water supply (hereafter, ‘water heterogeneity’) affects plant growth characteristics such as biomass and biomass allocation (Saeed and El-Nadi, 1998; Novoplansky and Goldberg, 2001; Fay et al., 2003; Maestre and Reynolds, 2007; Hagiwara et al., 2008). Plant biomass tends to increase under homogeneous water supply (low variability) compared with under heterogeneous water supply (high variability), even when the same amount of water is supplied under both regimes (Saeed and El-Nadi, 1998; Novoplansky and Goldberg, 2001, but see Heisler-White et al., 2008). Several studies have focused on the responses of biomass allocation to roots as well as biomass itself (Novoplansky and Goldberg, 2001; Fay et al., 2003; Maestre and Reynolds, 2007; Hagiwara et al., 2008), because plants can alter their allocations to roots in response to water availability (Kramer, 1983; Lambers et al., 1998). Fay et al. (2003) suggest that allocation to roots would increase under heterogeneous water supply to compensate for the intervening water deficits and that plants would prevent further reductions in shoot biomass. Thus, biomass growth can be modulated by the allocation responses to variability in water availability; large allocation to roots would lead to constant biomass growth being maintained. If intraspecific competition for water becomes severe, water is more likely to limit the plant growth and the sensitivity of plants to variability in water availability will increase.
In addition, resource heterogeneity can affect the intensity of competition. In the study of nutrient heterogeneity (Day et al., 2003), spatially heterogeneous nutrient supply increased the intensity of intraspecific competition of Briza media compared with homogeneous supply, because concentration of nutrients to a small patch would lead to intense competition. Water heterogeneity can also affect the intensity of competition, because water uptake of plants will concentrate in brief periods under heterogeneous water supply compared with homogeneous water supply. Therefore, competition may be severer under heterogeneous water supply.
The focus of this study was on the interaction between water heterogeneity and nutrient level, because the effects of the amounts and heterogeneities of multiple resources should be investigated simultaneously (Maestre and Reynolds, 2007; Hagiwara et al., 2008). Nutrient level is likely to affect plant responses to water heterogeneity, for three reasons: (1) nutrient availability depends on water availability (Lambers et al., 1998); (2) water availability is likely to limit plant growth under nutrient-rich conditions, because under these conditions plants will grow larger and require more water than under nutrient-poor conditions (Goldberg and Novoplansky, 1997; Stevens et al., 2006); and (3) the variability in water availability is likely to affect water uptake of plants under nutrient-rich conditions, because the allocation to roots will be smaller under these conditions. Therefore, plant responses to water heterogeneity should be greater under nutrient-rich conditions. In fact, the biomass of solitary plants of Perilla frutescens was greater under homogeneous than heterogeneous water supply, only under nutrient-rich conditions (Hagiwara et al., 2008).
A growth experiment was performed using an annual forb of Lamiacea, P. frutescens, to investigate the hypothesis that water heterogeneity affects plant biomass, biomass allocation and intensity of intraspecific competition, interacting with nutrient levels and population densities. Under this hypothesis, it was predicted that (a) plant biomass would be larger under homogeneous water supply than under heterogeneous water supply, particularly when nutrient levels are high and population densities are high; (b) biomass allocation to roots would be greater under heterogeneous water supply, particularly when population densities are high; and (c) intraspecific competition would be severer under heterogeneous water supply.
MATERIALS AND METHODS
Under natural conditions, Perilla frutescens grows on the floor of evergreen forests where there is a gap in the canopy; it reaches heights of 50–80 cm, and flowers from September to October (Iwatsuki et al., 1993). It is relatively drought-tolerant but is sensitive to water deficiency; it can therefore serve as a model species for studying plant responses to variability in water availability.
The growth experiment was conducted in a plastic film greenhouse under natural sunlight and ambient temperature at Tokyo Metropolitan University from July to September 2007. The experiment was a three-way factorial randomized block design: the factors were water heterogeneity, nutrient level and plant density. The water heterogeneity treatment had two levels of frequency of water supply: homogeneous (100 mL of water daily) and heterogeneous water supply (400 mL of water every 4 d). The maximum amount of water at any single supply was chosen so that it would not exceed the capacity of pots (18 cm in diameter, 15 cm tall, 2-L volume). Therefore, the total amount of water provided to each pot was the same in both frequencies of water supply throughout the experimental period. The amount of water provided per day was 4·1 L m−2, which was close to the ‘medium water availability’ treatment in Maestre and Reynolds (2007). The nutrient treatment had two levels: for the low nutrient level, 5 g of slow-release fertilizer [Magamp K, 6 : 40 : 6 : 15 (N-P-K-Mg); Hyponex Japan, Osaka, Japan] was provided in each pot, versus 30 g for the high nutrient level. The density treatment had four levels: one, three, six and nine plants per pot. There were thus 16 treatment combinations (two water heterogeneities × two nutrient levels × four plant densities), and each combination was replicated 18 times.
Seeds of P. frutescens were obtained from the Atariya Seed Co. (Chiba, Japan). The seeds were germinated on peat moss in July 2007. Ten days after germination, seedlings with cotyledons and two leaves were transplanted into pots filled with a homogeneous mixture of 800 mL of clay particles plus 800 mL of vermiculite, which contained the fertilizer and was placed above a 150-mL base layer of clay particles. To ensure a relatively even distribution of the plants, the seedlings were spaced evenly around the perimeter of the pots. During the subsequent week, each pot received 200 mL of water every 2 d (i.e. a level intermediate between the two water heterogeneity treatments). Seedlings in each pot were thinned to the required density 1 week after transplanting. The watering regime began on the same day and was continued for 48 d, from late July to early September; thus, the watering regime consisted of 12 repetitions of the 4-d watering cycle.
Soil moisture (volumetric water content) was measured with an ECH2O soil moisture probe (Decagon Devices, Inc., Pullman, WA, USA) in three pots in each treatment combination during the experimental period. Nutrient level and density treatments were pooled so that the soil moisture in 24 pots was measured in each water heterogeneity treatment. The measurement was carried out every day before watering, except during a 16-d period in August when technical problems prevented measurements. Relative soil moisture content was calculated as (Mm – Mmin)/(Mmax – Mmin), where Mm is the measured soil moisture, and Mmin and Mmax are the minimum and maximum values, respectively, that were recorded during the experimental period (James et al., 2003). The temporal mean value of the relative soil moisture content during the watering regime was calculated. Also the mean variance of the relative soil moisture content was calculated within each 4-d watering cycle to permit a comparison of the variability in water availability between the water heterogeneity treatments.
Plants were harvested after the 48-d watering regime. Shoots were cut at the soil surface, then all roots in each pot were carefully excavated from the soil. However, the roots could not be separated into individual plants because they were entangled. The shoots and roots were dried at 70 °C for at least 3 d and were then weighed. Shoot biomass was defined as the sum of individual shoots and total biomass was defined as the sum of shoot and root biomass of each pot. To investigate the effects of the treatments on intensity of intraspecific competition, two indices were calculated: the absolute competitive intensity (ACI) and the standardized competitive intensity. As the ACI, the reduction in average biomass of a plant per pot under intraspecific competition compared with biomass in solitary plants was used (Day et al., 2003). A formula of Day et al. (2003) was adapted to calculate ACI as: Bo – Bw, where Bo is total biomass without competition (i.e. one-plant density treatment) and Bw is the average biomass of a plant per pot with competition (i.e. three-, six- or nine-plant density treatments). Large values of ACI indicate that competition is severe. As the standardized competitive intensity, relative interaction index (RII) suggested by Armas et al. (2004) was used to standardize biomass of plants with the intraspecific competition to biomass without competition. A formula of Armas et al. (2004) was adapted to calculate RII as: (Bo – Bw)/(Bo + Bw). The values of RII range from –1 to 1; positive for competition and negative for facilitation. With ACI, the intensity of intraspecific competition is evaluated as absolute value of biomass changes. With RII, competitive intensity is evaluated as relative value; RII can be useful to compare treatments that result in quite different plant growth. ACI and RII were calculated for each block in each combination of water heterogeneity, nutrient level and plant density (three-, six- and nine-plant density).
Data analysis
The effects of the water heterogeneity treatment on the temporal mean of the relative soil moisture content and the mean variance over the 4-d cycle were analysed by means of one-way ANOVA. There were 24 replicates for each water heterogeneity treatment.
The effects of the treatments on total biomass were analysed by means of three-way ANOVA. The dependent variable was total biomass, and the independent variables were water heterogeneity, nutrient level, plant density and block. This was followed by two-way ANOVA at each plant density to investigate the differences in the effects of water heterogeneity and nutrient level between plant densities. The dependent variable was total biomass, and the independent variables were water heterogeneity, nutrient level and block. Within each density, the mean total biomass was compared between the treatment combinations of water heterogeneity and nutrient level by using Bonferroni multiple-means comparison test (P < 0·05). The effects of the treatments on ACI and RII were also analysed by means of three-way ANOVA followed by two-way ANOVA at each density, as in the analysis of total biomass.
Also the effects of treatments on biomass allocation to roots were investigated by means of analysis of covariance (ANCOVA). Root biomass was treated as dependent variable and shoot biomass as a covariate, instead of using root : shoot ratios (Murphy and Dudley, 2007), because the use of ratios to test biological hypotheses has been criticized (Jasienski and Bazzaz, 1999; Müller et al., 2000). Independent variables were shoot biomass, water heterogeneity, nutrient level, plant density and block. This analysis was followed by ANCOVA at each density. The dependent variable was root biomass, and the independent variables were shoot biomass, water heterogeneity, nutrient level and block. Shoot biomass was again treated as the covariate. The least-squares mean of root biomass adjusted by the covariate was calculated as the index of allocation to roots (Murphy and Dudley, 2007). The least-squares mean indicates root biomass standardized by grand mean of shoot biomass. The interaction terms between the covariate and the treatments were considered non-significant and removed from the final model if P > 0·10.
Pots that included dead plants were removed from the analyses. Therefore, the numbers of replicates for the analyses of total biomass, allocation to roots, ACI and RII varied among the treatments. At the nine-plant density, there were 14 replicates for the high nutrient level plus homogeneous water supply and high nutrient level plus heterogeneous water supply treatments; at the six-plant density, there were 16 replicates for the low nutrient level plus heterogeneous water supply treatment; and at the three-plant density, there were 17 replicates for the high nutrient level plus homogeneous water supply and high nutrient level plus heterogeneous water supply treatments. There were 18 replicates for the other treatments. All analyses were performed with version 2.7.2 of R statistical software (R Development Core Team, 2008).
RESULTS
Soil moisture
The mean of relative soil moisture did not significantly differ between the water heterogeneity treatments (F1,46 = 1·084, P = 0·303, ANOVA). However, the variance in the relative soil moisture content for the 4-d cycle was significantly affected by the water heterogeneity (F1,46 = 214·68, P < 0·001, ANOVA). Water availability in soil varied more under heterogeneous supply (mean of the variance ± standard error: 0·0230 ± 0·0012) than under homogeneous supply (0·0056 ± 0·0003).
Biomass
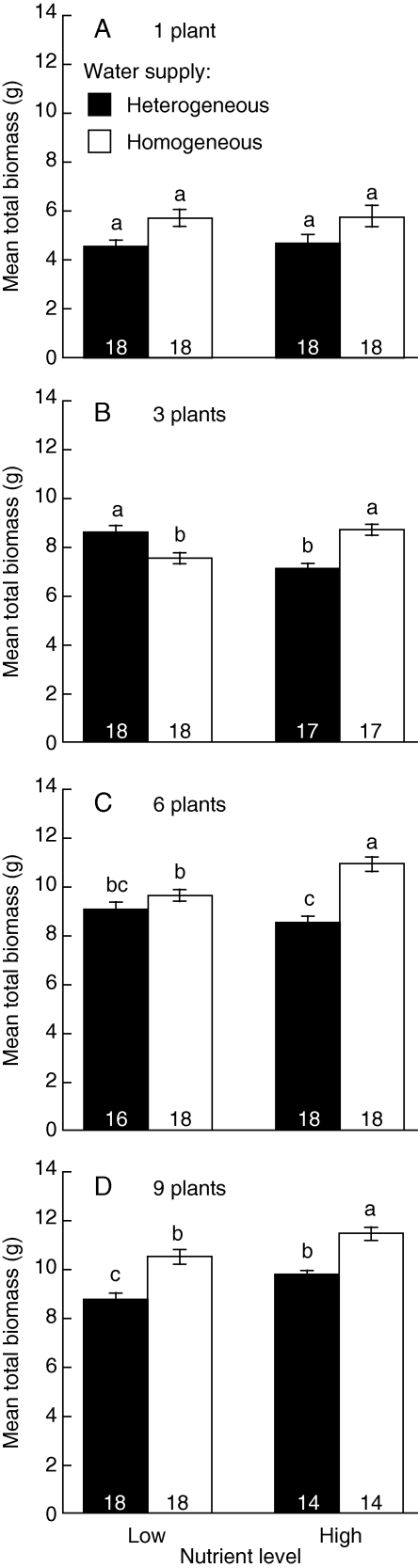
The total biomass was larger under homogeneous water supply than under heterogeneous water supply, particularly nutrient level was high and plant density was high (Fig. 1 and Table 1). The effects of water heterogeneity on total biomass differed among different combinations of nutrient level and plant density; water heterogeneity interacted with nutrient level at the three- and six-plant densities (Fig. 1 and Table 1). At the three-plant density, total biomass was greater under homogeneous supply at the high nutrient level, while total biomass was larger under heterogeneous supply at the low nutrient level (Fig. 1B and Table 1B). At the six-plant density, total biomass was significantly larger under homogeneous supply at the high nutrient level but not at the low nutrient level (Fig. 1C and Table 1B).
Fig. 1.
Mean total biomass (±s.e.) at the treatment combination of water heterogeneity (homogeneous or heterogeneous supply, as indicated) and nutrient level (Low and High) at each plant density. At each density, columns labelled with different letters differ significantly (P < 0·05, Bonferroni multiple-means comparison test). Numbers inside each column give the sample size for each treatment combination.
Table 1.
Effects of water heterogeneity (W), nutrient level (N), plant density (D) and block on total biomass
| (A) Three-way ANOVA | ||||
|---|---|---|---|---|
| Source | d.f. | SS | F-value | P |
| W | 1 | 91·45 | 78·09 | <0·001 |
| N | 1 | 6·59 | 5·62 | 0·018 |
| D | 3 | 1021·57 | 290·78 | <0·001 |
| W × N | 1 | 21·98 | 18·77 | <0·001 |
| W × D | 3 | 25·33 | 7·21 | <0·001 |
| N × D | 3 | 12·03 | 3·42 | 0·018 |
| W × N × D | 3 | 22·92 | 6·52 | <0·001 |
| Block | 17 | 84·28 | 4·23 | <0·001 |
| Error | 243 | 284·57 | ||
| (B) Two-way ANOVA at each density | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-plant density | 3-plant density | 6-plant density | 9-plant density | |||||||||
| Source | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P |
| W | 1 | 14·08 | <0·001 | 1 | 0·76 | 0·387 | 1 | 40·80 | <0·001 | 1 | 51·23 | <0·001 |
| N | 1 | 0·08 | 0·774 | 1 | 0·48 | 0·492 | 1 | 3·30 | 0·075 | 1 | 15·83 | <0·001 |
| W × N | 1 | 0·01 | 0·912 | 1 | 36·85 | <0·001 | 1 | 13·45 | <0·001 | 1 | 0·02 | 0·885 |
| Block | 17 | 2·69 | 0·003 | 17 | 1·59 | 0·103 | 17 | 2·09 | 0·023 | 17 | 1·14 | 0·354 |
| Error | 51 | 49 | 49 | 43 | ||||||||
Biomass allocation to roots versus shoots
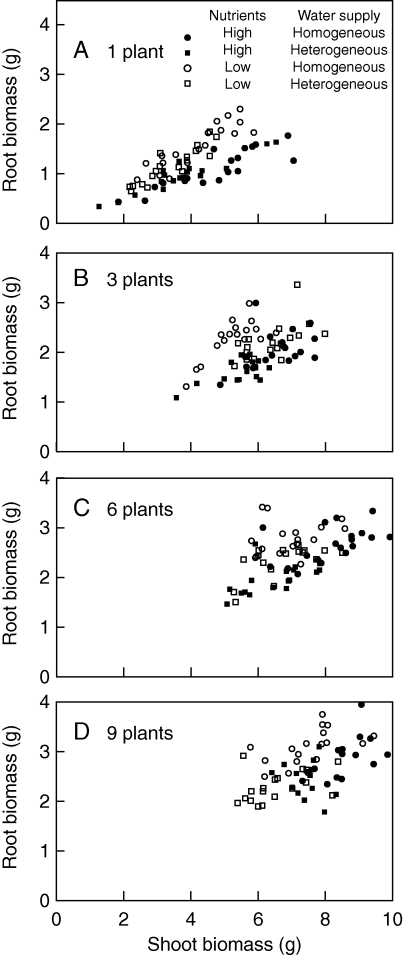
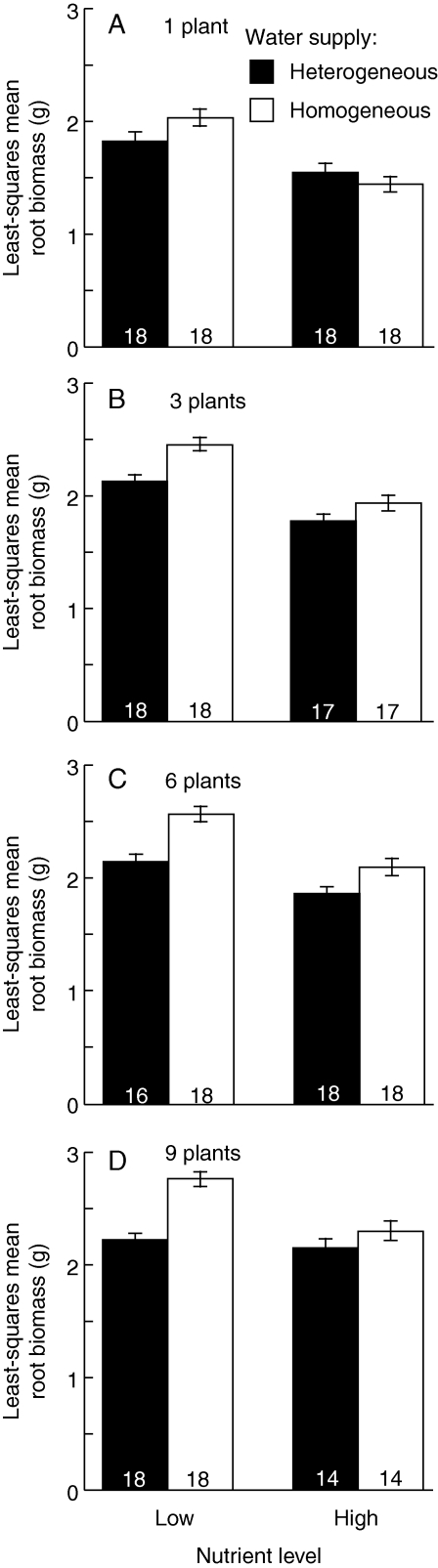
Root biomass was significantly correlated with shoot biomass (Table 2, F1,242 = 193·09, P < 0·001). The relationship between root biomass and shoot biomass, which means allocation to roots versus shoots, differed between the treatments (Fig. 2 and Table 2). The allocation to roots tended to be larger under homogeneous water supply, particularly at the low nutrient level and at high density, because the least-squares mean root biomass was larger (Fig. 3 and Table 2). The allocation to roots was also larger at the low nutrient level and at high density (Fig. 3 and Table 2). Water heterogeneity interacted with nutrient level at the one- and nine-plant densities (Table 2B). At the one-plant density, the allocation to roots was larger under homogeneous supply at the low nutrient level, but larger under heterogeneous supply at the high nutrient level (Fig. 3A and Table 2B). At the nine-plant density, the allocation to roots was larger under homogeneous water supply, particularly at the low nutrient level (Fig. 3D and Table 2B).
Table 2.
Analysis of covariance to test the effects of shoot biomass (S), water heterogeneity (W), nutrient level (N), plant density (D) and block on root biomass
| (A) ANCOVA for all densities | ||||
|---|---|---|---|---|
| Source | d.f. | SS | F-value | P |
| S | 1 | 9·87 | 193·09 | <0·001 |
| W | 1 | 4·23 | 82·76 | <0·001 |
| N | 1 | 8·41 | 164·58 | <0·001 |
| D | 3 | 3·94 | 25·66 | <0·001 |
| W × N | 1 | 0·94 | 18·44 | <0·001 |
| W × D | 3 | 1·17 | 7·64 | <0·001 |
| N × D | 3 | 0·35 | 2·30 | 0·078 |
| W × N × D | 3 | 0·09 | 0·57 | 0·632 |
| Block | 17 | 7·61 | 8·76 | <0·001 |
| Error | 242 | 12·37 | ||
| (B) ANCOVA at each density | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-plant density | 3-plant density | 6-plant density | 9-plant density | |||||||||
| Source | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P |
| S | 1 | 119·12 | <0·001 | 1 | 31·84 | <0·001 | 1 | 28·65 | <0·001 | 1 | 19·46 | <0·001 |
| W | 1 | 0·85 | 0·361 | 1 | 17·87 | <0·001 | 1 | 48·03 | <0·001 | 1 | 31·39 | <0·001 |
| N | 1 | 117·85 | <0·001 | 1 | 59·63 | <0·001 | 1 | 37·69 | <0·001 | 1 | 6·62 | 0·014 |
| W × N | 1 | 7·28 | 0·010 | 1 | 2·39 | 0·129 | 1 | 0·89 | 0·349 | 1 | 11·72 | 0·001 |
| S × W | – | – | – | – | – | – | – | – | – | 1 | 5·37 | 0·026 |
| S × N | 1 | 5·41 | 0·024 | – | – | – | – | – | – | – | – | – |
| Block | 17 | 1·59 | 0·104 | 17 | 3·47 | <0·001 | 17 | 4·01 | <0·001 | 17 | 3·81 | <0·001 |
| Error | 49 | 48 | 48 | 41 | ||||||||
Interaction terms between the covariate and the treatments were removed if P > 0·10.
Fig. 2.
Relationships between root and shoot biomass at the four combinations of nutrient level plus water heterogeneity at each plant density, as indicated.
Fig. 3.
Least-squares mean root biomass (±s.e.) at the treatment combination of water heterogeneity (homogeneous and heterogeneous supply, as indicated) and nutrient level (Low and High) at each plant density. Numbers inside each column give the sample size for each treatment combination.
Intensity of competition
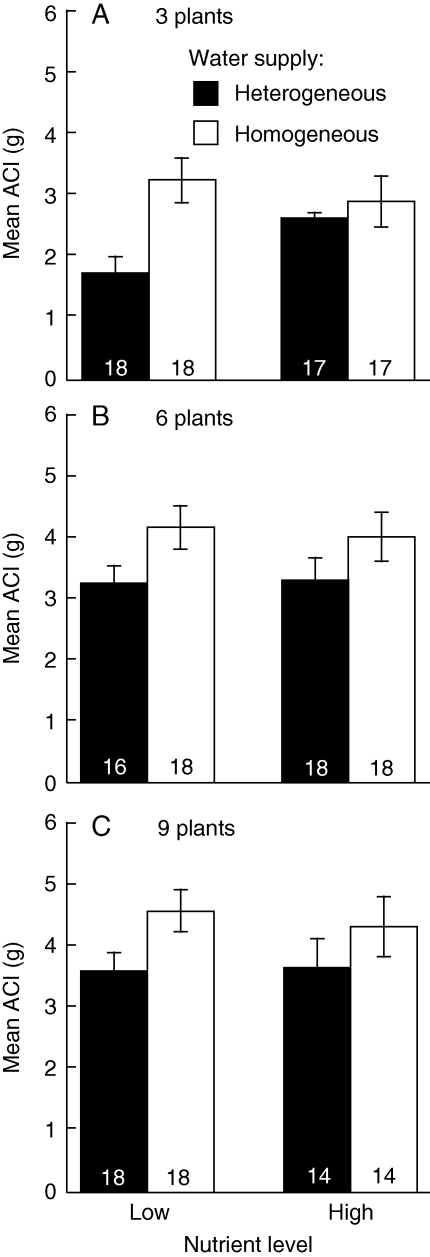
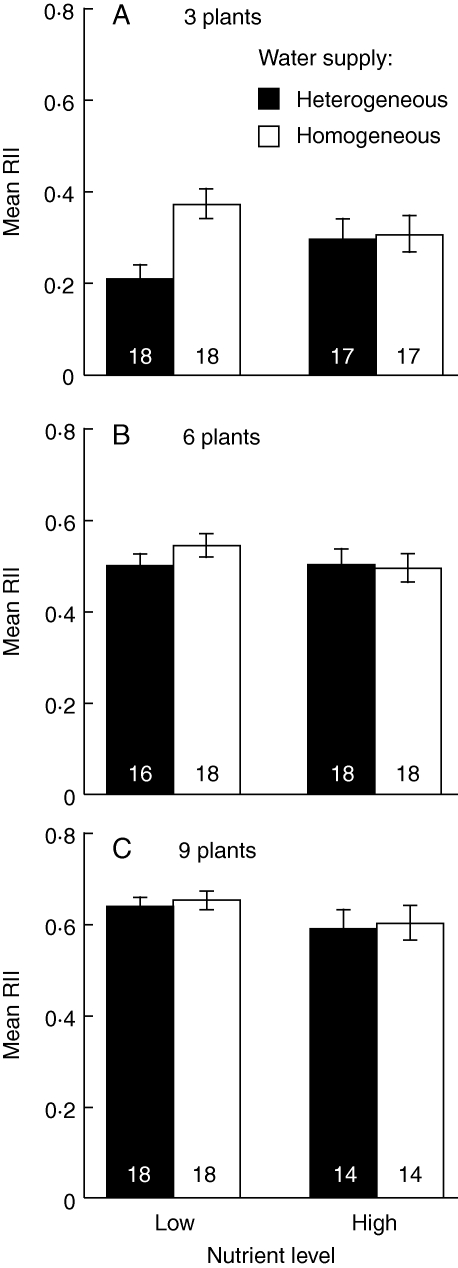
The ACI was greater under homogeneous water supply than under heterogeneous water supply (Fig. 4 and Table 3). At high density, ACI was also greater than at low density, while the effects of nutrient level were not significant (Fig. 4 and Table 3). The RII had positive value under all treatments and was greater under homogeneous supply than heterogeneous supply, particularly at the low nutrient level (Fig. 5 and Table 4). In the analysis for each density, the effects of water heterogeneity were significant only in three-plant density (Table 4). At high density, RII was also greater than at low density (Fig. 5 and Table 4). The results of indices of competitive intensity mean competition was severer under homogeneous water supply and at high density.
Fig. 4.
Mean absolute competitive intensity (ACI, ±s.e.) at the treatment combination of water heterogeneity (homogeneous and heterogeneous supply, as indicated) and nutrient level (Low and High) at each plant density. Numbers inside each column give the sample size for each treatment combination.
Table 3.
Effects of water heterogeneity (W), nutrient level (N), plant density (D) and block on ACI
| (A) Three-way ANOVA | ||||
|---|---|---|---|---|
| Source | d.f. | SS | F-value | P |
| W | 1 | 46·48 | 31·98 | <0·001 |
| N | 1 | 0·00 | 0·00 | 0·978 |
| D | 2 | 80·34 | 27·64 | <0·001 |
| W × N | 1 | 2·92 | 2·01 | 0·158 |
| W × D | 2 | 0·26 | 0·09 | 0·915 |
| N × D | 2 | 0·56 | 0·19 | 0·824 |
| W × N × D | 2 | 1·96 | 0·67 | 0·511 |
| Block | 17 | 199·12 | 8·06 | <0·001 |
| Error | 175 | 254·36 | ||
| (B) Two-way ANOVA at each density | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-plant density | 6-plant density | 9-plant density | |||||||
| Source | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P |
| W | 1 | 10·48 | 0·002 | 1 | 7·63 | 0·008 | 1 | 7·97 | 0·007 |
| N | 1 | 0·15 | 0·698 | 1 | 0·00 | 0·969 | 1 | 0·18 | 0·673 |
| W × N | 1 | 2·31 | 0·135 | 1 | 0·25 | 0·616 | 1 | 0·00 | 0·979 |
| Block | 17 | 2·08 | 0·024 | 17 | 2·42 | 0·008 | 17 | 2·26 | 0·016 |
| Error | 49 | 49 | 43 | ||||||
Fig. 5.
Mean relative interaction index (RII, no units; ±s.e.) at the treatment combination of water heterogeneity (homogeneous and heterogeneous supply, as indicated) and nutrient level (Low and High) at each plant density. Numbers inside each column give the sample size for each treatment combination.
Table 4.
Effects of water heterogeneity (W), nutrient level (N), plant density (D) and block on RII
| (A) Three-way ANOVA | ||||
|---|---|---|---|---|
| Source | d.f. | SS | F-value | P |
| W | 1 | 0·09 | 7·79 | 0·006 |
| N | 1 | 0·02 | 1·76 | 0·187 |
| D | 2 | 3·59 | 150·21 | <0·001 |
| W × N | 1 | 0·07 | 5·63 | 0·019 |
| W × D | 2 | 0·05 | 2·18 | 0·116 |
| N × D | 2 | 0·02 | 0·98 | 0·377 |
| W × N × D | 2 | 0·06 | 2·45 | 0·089 |
| Block | 17 | 1·35 | 6·64 | <0·001 |
| Error | 175 | 2·09 | ||
| (B) Two-way ANOVA at each density | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-plant density | 6-plant density | 9-plant density | |||||||
| Source | d.f. | F-value | P | d.f. | F-value | P | d.f. | F-value | P |
| W | 1 | 6·77 | 0·012 | 1 | 0·57 | 0·453 | 1 | 0·46 | 0·500 |
| N | 1 | 0·01 | 0·917 | 1 | 0·73 | 0·397 | 1 | 3·47 | 0·069 |
| W × N | 1 | 5·55 | 0·023 | 1 | 1·21 | 0·278 | 1 | 0·03 | 0·865 |
| Block | 17 | 1·93 | 0·038 | 17 | 2·09 | 0·023 | 17 | 2·04 | 0·030 |
| Error | 49 | 49 | 43 | ||||||
DISCUSSION
Intraspecific competition should affect plant responses to resource heterogeneity and has been under-appreciated in most previous studies of resource heterogeneity. The results clearly illustrated that the response of plant biomass and its allocation to water heterogeneity differed significantly between plant densities (Tables 1 and 2). In addition, water heterogeneity should affect intensity of competition (Tables 3 and 4). Competition for resource acquisition may be intensified depending on the patterns of resource supply. Because most studies of resource heterogeneity have been performed under a single plant density, it is not fully understood how resource heterogeneity interacts with intraspecific competition. Since both resource heterogeneity and competition affect resource acquisition and growth of plants, their interaction should be evaluated more carefully under the fields of resource heterogeneity and intraspecific competition studies.
Biomass growth and allocation
Particularly at high densities, plant biomass and allocation to roots were affected by water heterogeneity (Tables 1 and 2). As densities increased, plants were more likely to encounter water deficits and exhibit sensitivity to water heterogeneity. In addition, plants at the higher densities may have increased their responses to the variability in water availability because plants have to absorb water before competing neighbours cause the reduction the water availability. Under intraspecific competition, plants may have received competitive cues from neighbouring individuals such as root exudates and may have altered their responses to water heterogeneity (Novoplansky, 2009).
The plants under homogeneous supply tended to have greater biomass than those under heterogeneous supply (Fig. 1), which is consistent with the results of previous studies (Saeed and El-Nadi, 1998; Novoplansky and Goldberg, 2001; Maestre and Reynolds, 2007; but see Heisler-White et al., 2008). The low variability in water availability under homogeneous supply is likely to have allowed the plants to take up water steadily, thereby allowing the plants to increase their growth continuously. In addition, the plants under homogeneous supply allocated more biomass to roots versus shoots (Fig. 3). Under homogeneous supply, a large allocation to roots may have allowed plants to absorb water efficiently and improve biomass growth.
In this study, the allocation to roots was affected by patterns of variability in water availability, rather than mean water availability, because the mean availability did not differ between water heterogeneity treatments. Under heterogeneous supply, water availability was regularly replenished by the large quantity of water at each watering event, which would have prevented increase of allocation to the roots. However, plants under homogeneous supply led to the increase of allocation to roots because of stable water availability. These results contrast with those of Fay et al. (2003), who reported that allocation to roots became greater under heterogeneous supply in a rainfall manipulation experiment. Differences in species involved and/or frequencies and amount of water supply could cause the inconsistent results. Despite the differences in results, both studies clearly illustrated that water heterogeneity can affect the allocation to roots.
Plant responses to nutrient level would affect the sensitivity to water heterogeneity. Water heterogeneity affected total biomass particularly at the high nutrient level (Fig. 1 and Table 1), because allocation to roots was smaller at the high nutrient level than at the low nutrient level (Fig. 3 and Table 2). Plants tend to increase allocation to roots under nutrient-poor conditions so that nutrients are taken up efficiently (Lambers et al., 1998) and the large root systems also allow plants to exploit a larger volume of soil to absorb water (Kramer, 1983). Because both nutrients and water are absorbed by roots and affect biomass growth and allocation, the effects of nutrients and water should be investigated simultaneously in resource heterogeneity studies (Maestre and Reynolds, 2007; Hagiwara et al., 2008).
The current experimental design allowed the effects of water heterogeneity and its interactions that are difficult to extract from complex environments, especially in the field, to be elucidated. The results suggest that water heterogeneity and its interaction can be one of factors explaining the complex phenomena in the fields. It is true that the term of the experiment seemed relatively short (48 d). However, the experiment included the total growth period of P. frutescens from seedling establishment to flowering. Therefore, this experiment is considered as the snapshot of typical responses of an annual plant species. In natural environments, P. frutescens plants are found on the floor of evergreen forests where there is a gap in the canopy. Because a plant rarely grows alone, it is important to reveal the effects of intraspecific competition in populations with various densities.
Intensity of competition
Homogeneous water supply can cause more intense competition of biomass growth than heterogeneous supply (Figs 4 and 5). Greater biomass growth under homogeneous supply may have led to severer competition because of an increase in demand for resources (Figs 1 and 4), and even with the index standardized by plant size (RII), homogeneous supply significantly increased the intensity of competition (Table 4). Therefore, there can be other causes for increasing competitive intensity under homogeneous supply in addition to greater biomass. Under a homogeneous supply, water uptake of plants may be concentrated in a small amount of water at each supply. However, under a heterogeneous supply, plants may share a large amount of water at each supply with competing neighbours. Homogeneous supply can cause not only greater biomass growth (Fig. 1), but also severer competition for water (Figs 4 and 5).
Day et al. (2003) reported that nutrient heterogeneity also affected intensity of competition. Competition under a spatially heterogeneous nutrient supply can concentrate on small nutrient-rich patch and become severer than a homogeneous supply. In this study, competition was severer under a temporally homogeneous water supply than under a heterogeneous supply (Figs 4 and 5). Patterns of resource supply that would cause a concentration of resources will increase intensity of competition, although the effects of resource heterogeneity on intensity of competition would differ among spatial and temporal heterogeneities.
Conclusions
This study revealed that plants respond differently to water heterogeneity at different plant densities. In addition, water heterogeneity can affect intensity of competition. Temporally homogeneous water supply can cause a concentration of resources and severer competition than a heterogeneous water supply. The interactive effects of resource heterogeneity and intraspecific competition should be evaluated more carefully in further studies.
ACKNOWLEDGEMENTS
We thank Mr E. Sugiyama and Ms T. Yasuki of Tokyo Metropolitan University (TMU) and members of the Plant Ecology Laboratory of TMU for their help during the experiment. The editor, Professor Bill Shipley, and two anonymous reviewers made very constructive comments on our earlier manuscript. This work was partly supported by the Japan Society for the Promotion of Science (00291237 and 18570027 to J.-I.S., and 09J07211 to Y.H.).
LITERATURE CITED
- Armas C, Ordiales R, Pugnaire EI. Measuring plant interactions: a new comparative index. Ecology. 2004;85:2682–2686. [Google Scholar]
- Begon M, Townsend CR, Harper JL. Ecology. 4th edn. Oxford: Blackwell; 2006. [Google Scholar]
- Day KJ, John EA, Hutchings MJ. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Functional Ecology. 2003;17:454–463. [Google Scholar]
- Farley RA, Fitter AH. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology. 1999;87:688–696. [Google Scholar]
- Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL. Productivity responses to altered rainfall patterns in a C4-dominated grassland. Oecologia. 2003;137:245–251. doi: 10.1007/s00442-003-1331-3. [DOI] [PubMed] [Google Scholar]
- Goldberg DE, Novoplansky A. On the relative importance of competition in unproductive environments. Journal of Ecology. 1997;85:409–418. [Google Scholar]
- Hagiwara Y, Kachi N, Suzuki J-I. Effects of temporal heterogeneity of watering on size of an annual forb, Perilla frutescens (Lamiaceae), depend on soil nutrient levels. Botany. 2008;86:1111–1116. [Google Scholar]
- Heisler-White JL, Knapp AK, Kelly EF. Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland. Oecologia. 2008;158:129–140. doi: 10.1007/s00442-008-1116-9. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Yamazaki T, Boufford DE, Ohba H. Flora of Japan. IIIa. Tokyo: Kodansha; 1993. [Google Scholar]
- Jackson RB, Caldwell MM. Geostatistical patterns of soil heterogeneity around individual perennial plants. Journal of Ecology. 1993a;81:683–692. [Google Scholar]
- Jackson RB, Caldwell MM. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology. 1993b;74:612–614. [Google Scholar]
- Jasienski M, Bazzaz FA. The fallacy of ratios and the testability of models in biology. Oikos. 1999;84:321–326. [Google Scholar]
- James SE, Pärtel M, Wilson SD, Peltzer DA. Temporal heterogeneity of soil moisture in grassland and forest. Journal of Ecology. 2003;91:234–239. [Google Scholar]
- Kramer PJ. Water relations of plants. New York, NY: Academic Press; 1983. [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Plant physiological ecology. New York, NY: Springer; 1998. [Google Scholar]
- Maestre FT, Reynolds JF. Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology. 2007;88:501–511. doi: 10.1890/06-0421. [DOI] [PubMed] [Google Scholar]
- Müller I, Schmid B, Weiner J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:115–127. [Google Scholar]
- Murphy GP, Dudley SA. Above- and below-ground competition cues elicit independent responses. Journal of Ecology. 2007;95:261–272. [Google Scholar]
- Novoplansky A. Picking battles wisely: plant behaviour under competition. Plant, Cell & Environment. 2009;32:726–741. doi: 10.1111/j.1365-3040.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- Novoplansky A, Goldberg DE. Effects of water pulsing on individual performance and competitive hierarchies in plants. Journal of Vegetation Science. 2001;12:199–208. [Google Scholar]
- R Development Core Team. Vienna: R Foundation for Statistical Computing; 2008. R: a language and environment for statistical computing. URL: http://www.R-project.org . (26 December 2009) [Google Scholar]
- Saeed IAM, El-Nadi AH. Forage sorghum yield and water use efficiency under variable irrigation. Irrigation Science. 1998;18:67–71. [Google Scholar]
- Stevens MHH, Shirk R, Steiner CE. Water and fertilizer have opposite effects on plant species richness in a mesic early successional habitat. Plant Ecology. 2006;183:27–34. [Google Scholar]