Abstract
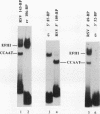
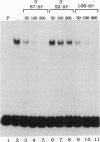
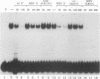
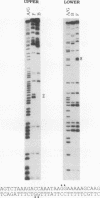
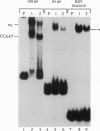
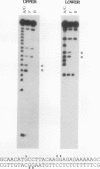
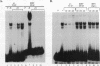
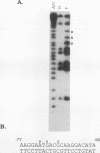
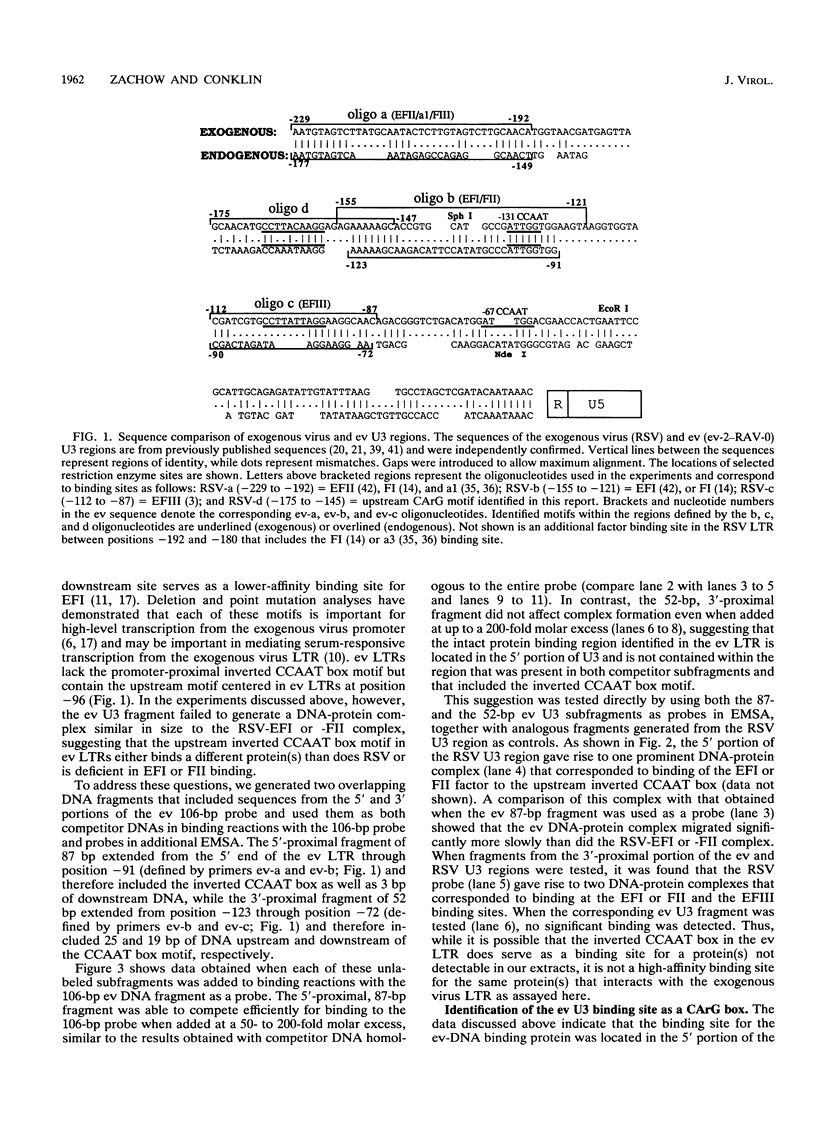
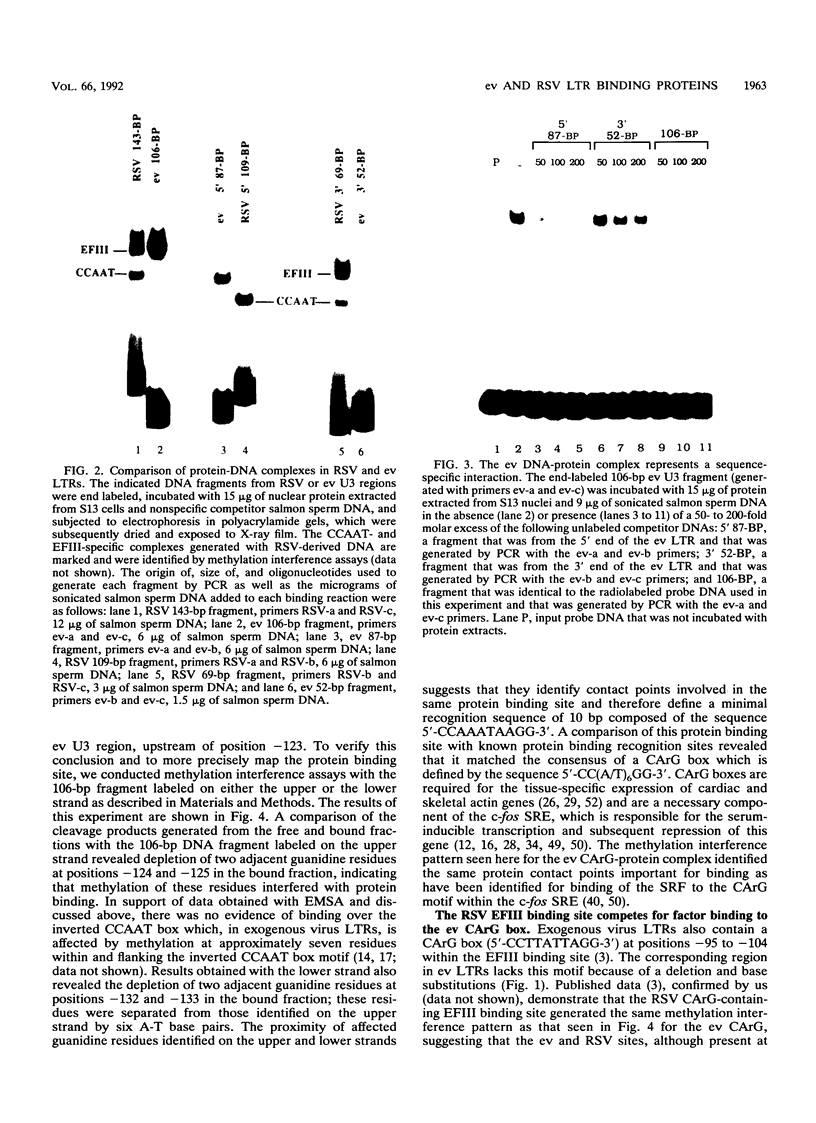
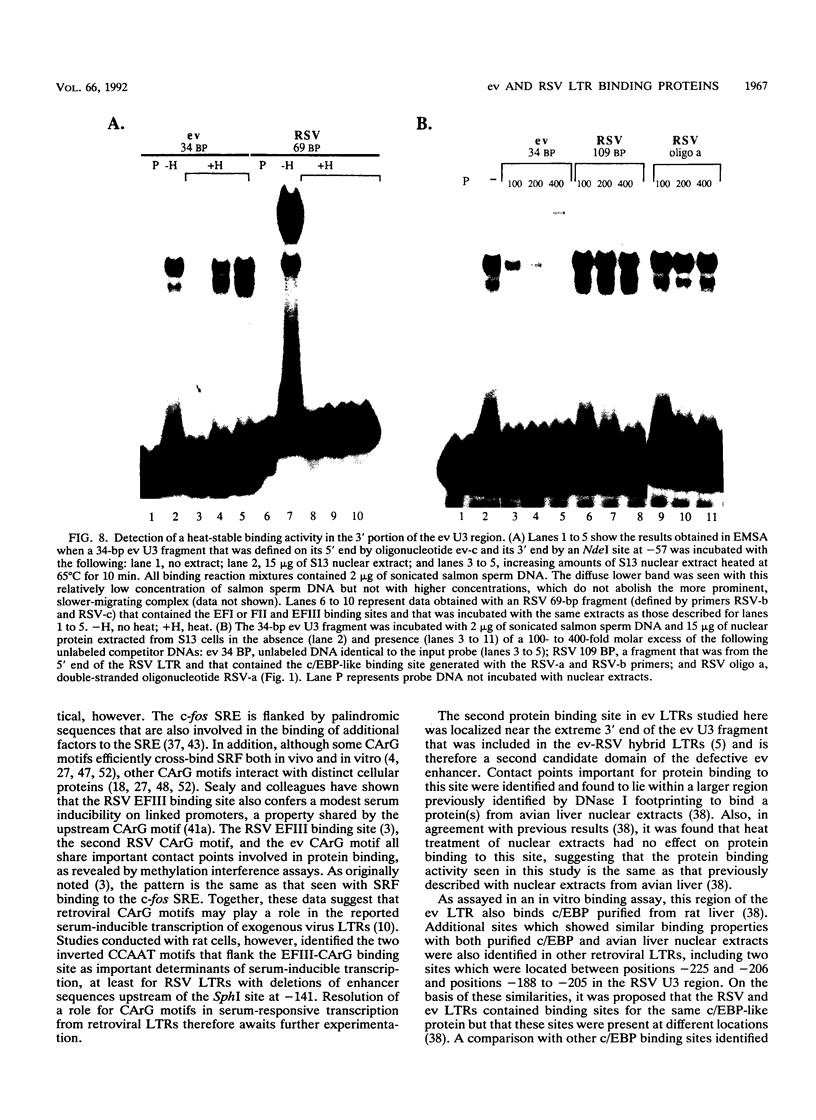
A strong enhancer element is located within the long terminal repeats (LTRs) of exogenous, oncogenic avian retroviruses, such as Rous sarcoma virus (RSV) and the avian leukosis viruses. The LTRs of a second class of avian retroviruses, the endogenous viruses (evs), lack detectable enhancer function, a property that correlates with major sequence differences between the LTRs of these two virus groups. Despite this lack of independent enhancer activity, we previously identified sequences in ev LTRs that were able to functionally replace essential enhancer domains from the RSV enhancer with which they share limited sequence similarity. To identify candidate enhancer domains in ev LTRs that are functionally equivalent to those in RSV LTRs, we analyzed and compared ev and RSV LTR-specific DNA-protein interactions. Using this approach, we identified two candidate enhancer domains and one deficiency in ev LTRs. One of the proposed ev enhancer domains was identified as a CArG box, a motif also found upstream of several muscle-specific genes, and as the core sequence of the c-fos serum response element. The RSV LTR contains two CArG motifs, one at a previously identified site and one identified in this report at the same relative location as the ev CArG motif. A second factor binding site that interacts with a heat-stable protein was also identified in ev LTRs and, contrary to previous suggestions, appears to be different from previously described exogenous virus enhancer binding proteins. Finally, a deficiency in factor binding was found within the one inverted CCAAT box in ev LTRs, affirming the importance of sequences that flank CCAAT motifs in factor binding and providing a candidate defect in the ev enhancer.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Atchison M. L. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Boulden A., Sealy L. Identification of a third protein factor which binds to the Rous sarcoma virus LTR enhancer: possible homology with the serum response factor. Virology. 1990 Jan;174(1):204–216. doi: 10.1016/0042-6822(90)90069-4. [DOI] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin K. F. Activation of an endogenous retrovirus enhancer by insertion into a heterologous context. J Virol. 1991 May;65(5):2525–2532. doi: 10.1128/jvi.65.5.2525-2532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Transcriptional activity of avian retroviral long terminal repeats directly correlates with enhancer activity. J Virol. 1985 Feb;53(2):515–521. doi: 10.1128/jvi.53.2.515-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Skalka A. M., Ju G. Endogenous avian retroviruses contain deficient promoter and leader sequences. Proc Natl Acad Sci U S A. 1983 May;80(10):2946–2950. doi: 10.1073/pnas.80.10.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Stoeckle M. Y., Hanafusa H. Serum and v-src increase the level of a CCAAT-binding factor required for transcription from a retroviral long terminal repeat. Genes Dev. 1990 Feb;4(2):243–254. doi: 10.1101/gad.4.2.243. [DOI] [PubMed] [Google Scholar]
- Faber M., Sealy L. Rous sarcoma virus enhancer factor I is a ubiquitous CCAAT transcription factor highly related to CBF and NF-Y. J Biol Chem. 1990 Dec 25;265(36):22243–22254. [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Parsons J. T. Identification of transcriptional elements within the long terminal repeat of Rous sarcoma virus. Mol Cell Biol. 1983 Oct;3(10):1834–1845. doi: 10.1128/mcb.3.10.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H. Identification of three sequence-specific DNA-binding proteins which interact with the Rous sarcoma virus enhancer and upstream promoter elements. J Virol. 1988 Jun;62(6):2186–2190. doi: 10.1128/jvi.62.6.2186-2190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greuel B. T., Sealy L., Majors J. E. Transcriptional activity of the Rous sarcoma virus long terminal repeat correlates with binding of a factor to an upstream CCAAT box in vitro. Virology. 1990 Jul;177(1):33–43. doi: 10.1016/0042-6822(90)90457-3. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Kedes L. Identification of multiple proteins that interact with functional regions of the human cardiac alpha-actin promoter. Mol Cell Biol. 1989 Aug;9(8):3269–3283. doi: 10.1128/mcb.9.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. H. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus 0. J Virol. 1982 Jul;43(1):191–200. doi: 10.1128/jvi.43.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Groudine M. Transcription of three c-myc exons is enhanced in chicken bursal lymphoma cell lines. Proc Natl Acad Sci U S A. 1985 Jan;82(1):53–57. doi: 10.1073/pnas.82.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Manley J. L., Guntaka R. V. Localization of active promoters for eucaryotic RNA polymerase II in the long terminal repeat of avian sarcoma virus DNA. Mol Cell Biol. 1983 May;3(5):811–818. doi: 10.1128/mcb.3.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human alpha-cardiac actin gene. Mol Cell Biol. 1987 Aug;7(8):2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Chambers A. E., Towers N., Taylor M. V. Expression of genes encoding the transcription factor SRF during early development of Xenopus laevis: identification of a CArG box-binding activity as SRF. EMBO J. 1991 Apr;10(4):933–940. doi: 10.1002/j.1460-2075.1991.tb08027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Taylor M. V., Garrett N., Gurdon J. B. The CArG promoter sequence is necessary for muscle-specific transcription of the cardiac actin gene in Xenopus embryos. EMBO J. 1989 Apr;8(4):1153–1161. doi: 10.1002/j.1460-2075.1989.tb03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T., Garrett N., Treisman R. Xenopus cytoskeletal actin and human c-fos gene promoters share a conserved protein-binding site. EMBO J. 1987 Mar;6(3):667–673. doi: 10.1002/j.1460-2075.1987.tb04806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Characterization of Rous sarcoma virus sequences essential for viral gene expression. J Virol. 1987 Apr;61(4):1171–1179. doi: 10.1128/jvi.61.4.1171-1179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer J., Faber M., Chalkley R., Sealy L. Isolation and characterization of a cDNA clone for the CCAAT transcription factor EFIA reveals a novel structural motif. J Biol Chem. 1990 Dec 25;265(36):22143–22152. [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Ruddell A., Linial M. L., Groudine M. Tissue-specific lability and expression of avian leukosis virus long terminal repeat enhancer-binding proteins. Mol Cell Biol. 1989 Dec;9(12):5660–5668. doi: 10.1128/mcb.9.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell A., Linial M., Schubach W., Groudine M. Lability of leukosis virus enhancer-binding proteins in avian hematopoeitic cells. J Virol. 1988 Aug;62(8):2728–2735. doi: 10.1128/jvi.62.8.2728-2735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. A., Jr, Franza B. R., Jr, Gilman M. Z. Two distinct cellular phosphoproteins bind to the c-fos serum response element. EMBO J. 1989 Jun;8(6):1785–1792. doi: 10.1002/j.1460-2075.1989.tb03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden T. A., Beemon K. Avian retroviral long terminal repeats bind CCAAT/enhancer-binding protein. Mol Cell Biol. 1989 Mar;9(3):1155–1164. doi: 10.1128/mcb.9.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl D. R., Kahn S., Malavarca R., Astrin S., Skalka A. M. Nucleotide sequence of the long terminal repeat and flanking cellular sequences of avian endogenous retrovirus ev-2: variation in Rous-associated virus-0 expression cannot be explained by differences in primary sequence. J Virol. 1983 Feb;45(2):868–871. doi: 10.1128/jvi.45.2.868-871.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Shaw P. E., Nordheim A. Purification of intercalator-released p67, a polypeptide that interacts specifically with the c-fos serum response element. Nucleic Acids Res. 1987 Dec 23;15(24):10145–10158. doi: 10.1093/nar/15.24.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Taylor A., Erba H. P., Muscat G. E., Kedes L. Nucleotide sequence and expression of the human skeletal alpha-actin gene: evolution of functional regulatory domains. Genomics. 1988 Nov;3(4):323–336. doi: 10.1016/0888-7543(88)90123-1. [DOI] [PubMed] [Google Scholar]
- Taylor M. V. A family of muscle gene promoter element (CArG) binding activities in Xenopus embryos: CArG/SRE discrimination and distribution during myogenesis. Nucleic Acids Res. 1991 May 25;19(10):2669–2675. doi: 10.1093/nar/19.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Treisman R., Garrett N., Mohun T. Muscle-specific (CArG) and serum-responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989 May;106(1):67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Enhancer activity correlates with the oncogenic potential of avian retroviruses. EMBO J. 1985 Apr;4(4):949–956. doi: 10.1002/j.1460-2075.1985.tb03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]