Abstract
Background
Reactive astrocytosis and microgliosis are important features of the pathophysiology of hydrocephalus, and persistent glial "scars" that form could exacerbate neuroinflammation, impair cerebral perfusion, impede neuronal regeneration, and alter biomechanical properties. The purpose of this study was to determine the efficacy of minocycline, an antibiotic known for its anti-inflammatory properties, to reduce gliosis in the H-Tx rat model of congenital hydrocephalus.
Methods
Minocycline (45 mg/kg/day i.p. in 5% sucrose at a concentration of 5-10 mg/ml) was administered to hydrocephalic H-Tx rats from postnatal day 15 to day 21, when ventriculomegaly had reached moderate to severe stages. Treated animals were compared to age-matched non-hydrocephalic and untreated hydrocephalic littermates. The cerebral cortex (both gray matter laminae and white matter) was processed for immunohistochemistry (glial fibrillary acidic protein, GFAP, for astrocytes and ionized calcium binding adaptor molecule, Iba-1, for microglia) and analyzed by qualitative and quantitative light microscopy.
Results
The mean number of GFAP-immunoreactive astrocytes was significantly higher in untreated hydrocephalic animals compared to both types of controls (p < 0.001). Minocycline treatment of hydrocephalic animals reduced the number of GFAP immunoreactive cells significantly (p < 0.001). Likewise, the mean number of Iba-1 immunoreactive microglia was significantly higher in untreated hydrocephalic animals compared to both types of controls (p < 0.001). Furthermore, no differences in the numbers of GFAP-positive astrocytes or Iba-1-positive microglia were noted between control animals receiving no minocycline and control animals receiving minocycline, suggesting that minocycline does not produce an effect under non-injury conditions. Additionally, in six out of nine regions sampled, hydrocephalic animals that received minocycline injections had significantly thicker cortices when compared to their untreated hydrocephalic littermates.
Conclusions
Overall, these data suggest that minocycline treatment is effective in reducing the gliosis that accompanies hydrocephalus, and thus may provide an added benefit when used as a supplement to ventricular shunting.
Background
Shunt failure continues to be one of the largest problems facing those who suffer with hydrocephalus. Despite the initial benefits of shunting, 40% of children with hydrocephalus undergo shunt revisions within the first year, and 50% will have a shunt revision within the first 2 years of insertion [1]. Shunts fail for numerous reasons; one likely cause is failure of the shunt valve to actuate properly due to the changing hydrostatic properties of a stiff, non-compliant brain [2]. This lack of compliance may be attributed to a proliferation of glial cells.
Gliosis is an important feature of the pathophysiology of hydrocephalus [3-10], and persistent glial "scars" that form in the shunted brain could promote neuroinflammation, impair cerebral perfusion, alter the blood-brain barrier, prevent repair of damaged neural tissue and impede neuronal plasticity, and change intracranial compliance which poses additional problems for optimal shunt function. We have shown previously that gliosis in the form of reactive astrocytosis and microgliosis increases significantly in the H-Tx rat model of congenital hydrocephalus [9,10]. These changes occur in both cortical and subcortical regions, and while ventricular shunting can reduce gliosis in this model, it does not restore glial parameters to normal levels.
Experimental and clinical studies have demonstrated that minocycline, a semi-synthetic tetracycline derivative, inhibits the proliferation of microglial cells after various types of experimental brain injuries and clinical disorders, and is an effective neuroprotective agent [11-17] (reviewed recently by Buller et al [18] and Hailer [19]). Minocycline has also been shown to be effective in reducing astrogliosis [13]. These promising results support the hypothesis that gliosis could be reduced or prevented during the progression of hydrocephalus by the administration of minocycline. This study is the first investigation of minocycline treatment in hydrocephalus-induced gliosis and therefore represents a promising new approach in the treatment of this condition.
Methods
Subjects
All procedures conducted in this study were approved by the Animal Care and Use Committee of Wayne State University. A colony of H-Tx rats, which develop congenital hydrocephalus due to a closure of their cerebral aqueduct between embryonic day 18 and postnatal day 5 [20-25], was maintained at Wayne State University. Hydrocephalus progresses rapidly in these animals, and by 21 days of age they become severely hydrocephalic. If these hydrocephalic animals are not treated with shunts, they will eventually die around post-natal day 30. Animals in this experiment were housed in a cage with their littermates and parents and were maintained in a humidity and temperature-controlled room on a 12 hour light/dark cycle. Food and water were provided ad libitum. This model provides a naturally occurring form of non-communicating, obstructive hydrocephalus that correlates with third trimester onset relative to human brain development [26].
Experimental animal groups
Four groups of animals were examined; n = 5 for all groups:
Group I - Non-hydrocephalic: These rats did not develop hydrocephalus (determined by lack of a domed head on gross examination) and were sacrificed at post-natal day 21. These animals served as the untreated control group.
Group II - Minocycline-treated non-hydrocephalic: These rats were identical to Group I animals except that they received minocycline from day 15 to 21. This comprised the minocycline-treated control group.
Group III - Untreated hydrocephalic: These rats developed severe hydrocephalus (determined as described for Group I) and were left untreated. They were sacrificed at post-natal day 21.
Group IV - Minocycline-treated hydrocephalic: At postnatal day 15, when hydrocephalus is progressing to a severe state [9,27], rats received minocycline treatment for 7 days, ending at sacrifice on day 21 when ventriculomegaly had become severe. Hydrocephalus was again determined using the methods described previously.
Minocycline Treatment
Due to the small size of the young rats used in this study (20 g body weight vs. 250 g for adults), intravenous injection was not a practical method of minocycline delivery. Therefore, intraperitoneal (IP) injections were used instead. This type of administration has been shown to provide adequate delivery of minocycline to the brain across the blood brain barrier as assessed by both serum and cerebrospinal fluid (CSF) levels [28]. Minocycline-treated animals received minocycline HCl (Sigma Chemicals, St. Louis, USA) dissolved in 5% sucrose at a concentration of 10 mg/ml at a dose of 45 mg/kg/day for 7 days, beginning at postnatal day 15 and ending at day 21 with the termination of the experiment. Injection sites were rotated between the four abdominal quadrants.
Tissue Processing
At postnatal-day 21, animals were anesthetized with 4% chloral hydrate and perfused transcardially, first with 500 ml of 0.9% saline to flush the vascular system, followed by 500 ml of 4% paraformaldehyde. After perfusion was complete, the brain was carefully and rapidly extracted and post-fixed in 50 ml of 4% paraformaldehyde for 2 h. Brains were then rinsed and stored in buffered saline at 4°C until paraffin embedding. Prior to embedding, hydrocephalic brains were injected with a solution of 4% agar in dH2O in order to provide support to the dilated and fragile ventricles. Using an 18-gauge needle and syringe, warm agar was injected through the temporal cortex into the lateral ventricles. The agar-filled brain was then placed into a cool bath of buffered saline, allowing the agar to solidify. All extracted brains from all 3 experimental groups were cut along 2 coronal planes, separating the frontal, parietal, and occipital cortices. Parietal and occipital segments were put in separate, marked embedding trays and placed into the paraffin embedding machine. Tissue was dehydrated in 50, 70, 95, and 100% alcohol, cleared in toluene, and embedded in paraffin wax. Tissue sections were cut in the coronal plane at a thickness of 20 μm as an optimal dimension for stereology (the Optical Fractionator) floated on a warm water bath, and positioned onto glass slides.
Immunohistochemistry
Sections were de-paraffinized, re-hydrated and incubated in 3% H2O2. Following procedures described previously [9], astrocytes were detected by the presence of glial fibrillary acidic protein (GFAP); the primary anti-GFAP antibody (DAKO, Carpinteria, USA) was diluted 1:300 and replaced with an anti-rabbit secondary antibody (Vector Laboratories, Burlingame, USA) diluted 1:200. An avidin-biotin complex (ABC kit, Vector) was applied for 30 min and color developed using an un-enhanced diaminobenzidine (DAB) kit (Vector). Slides were dehydrated in increasing concentrations of alcohol, cleared in xylene and cover-slipped with Permount (Fisher Scientific, Pittsburgh, USA). Microglia were detected as described previously [29] using ionized calcium binding adaptor molecule (Iba-1), a marker for both resting and reactive microglia. The same procedures described for GFAP were followed with the exception that primary anti-Iba-1 antibody (Waco, Richmond, VA, USA) was diluted 1: 500.
Stereological analysis
The full thickness of the cerebral cortex (gray matter laminae and white matter) was examined using light microscopy and stereological quantitative analysis. The observer was blinded to the identity of each animal as well as to the experimental condition as much as possible; because of the obvious thinning of the hydrocephalic cortex it is often impossible to completely blind the analyst. Three regions (2 occipital and 1 parietal) from each brain were selected for analysis.
Glial Density: In order to quantify any glial changes, Stereo Investigator software (MicroBrightField, Inc. Williston, USA) was utilized to obtain an estimated density of both GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia present in the entire cerebral cortex of treated and untreated hydrocephalic rats. The combination of cell counting using the optical dissector with fractionator sampling is defined as the Optical Fractionator [30,31]. One main benefit of this probe is that data output is not affected by tissue shrinkage which occurs during the tissue preparation process. Using a counting frame of 200 × 200 μm, 45 areas of the neocortex were randomly probed (15 frames per coronal section × 3 sections/animal), marking all immune-labelled cells (the presence of either a soma or process was recorded as a positive occurrence). All probing was performed at 400 × using brightfield optics. By systematically sampling a known fraction of section thickness, along with a known fraction of a sectional area, it was possible to produce an unbiased estimate of the total number of astrocytes and microglia present in a given volume of cortex. Note that this type of sampling included both gray and white matter regions of the cerebral cortex and because of the extreme thinning of white matter in severely hydrocephalic brains, no attempts were made to evaluate these two regions individually. Using the estimated area calculated by the program's planimetry data-generating function, a glial density volume (glial cells/μm3) was obtained for each section viewed.
Cortical Thickness: The thickness of the cerebral cortex (pial to ventricular surface) was measured perpendicular to the pial surface using the Stereo Investigator "linear measurement" tool. At 50 × magnification, thickness was measured at the most dorsal portion of the hemisphere, the most lateral portion, and the most inferior portion of the temporal region of each section of brain.
Statistics
Analysis of glial density was carried out by light microscopic examination of three 20 μm thick coronal sections from each rat, including one parietal and two occipital sections per animal. The mean value of these three sections was determined to yield a representative value of cell density for each animal. Comparisons between groups were determined by performing an ANOVA test followed by a Bonferroni correction. No data were expunged and all values obtained were utilized in the statistical analyses.
Using the same three sections described above from each animal, a slightly different method of data analysis was used for cortical thickness. On each section, three regions of the cortex were measured for thickness. These were the dorsal, lateral, and temporal regions of the brain section, and each was analyzed separately. Data were analyzed for statistical significance by performing an ANOVA test followed by a Bonferroni correction.
Results
GFAP- immunoreactive astrocyte number
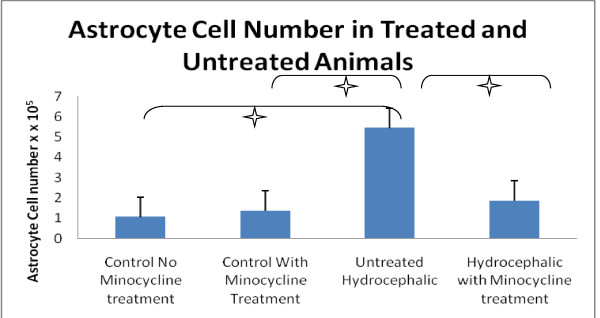
The number of GFAP-immunoreactive astrocytes was significantly increased in untreated hydrocephalic animals, but minocycline treatment significantly reduced this number (Figure 1). The number of GFAP-immunoreactive astrocytes was significantly higher (p < 0.001) in untreated hydrocephalus (mean 5.43 cells/μm3 +/- 2.04 SD) compared to both types of controls: minocycline treated mean 1.35 cells/μm3 +/- 0.28 SD and non-minocycline mean 1.05 cells/μm3 +/- 0.22 SD. A statistically significant decrease in the number of GFAP-immunoreactive astrocytes (p < 0.001) occurred in the minocycline-treated hydrocephalic group (mean 1.85 cells/μm3 +/- 0.63 SD) and this number was not significantly different compared to controls. Finally, minocycline treatment alone had no significant effect in control animals. All cell counts were multiplied by 10-5 to obtain the cell number per micrometer squared.
Figure 1.
Astrocyte density (cells/μm3) in minocycline treated and untreated animals. The number of GFAP-immunoreactive cells was significantly higher (stars) in untreated hydrocephalic animals compared to both types of control (p < 0.001). Minocycline treatment significantly reduced the number of immunoreactive cells in hydrocephalic animals (p < 0.001). No significant differences were found between the control animals receiving minocycline and those that did not receive the drug, suggesting that minocycline has no effect on the normal brain. Data are mean ± SD, n = 5, cell counts were multiplied by 105 to get the actual cell number per μm3.
Iba-1- immunoreactive microglial number
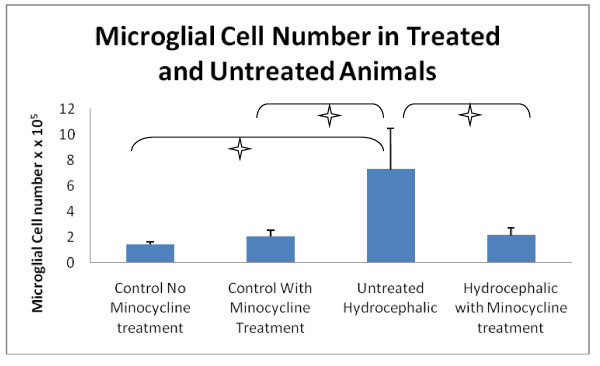
Minocycline treatment significantly reduced the number of Iba-1 immunoreactive microglial cells present in the hydrocephalic brain (Figure 2). Significant differences (p < 0.001) were present between the hydrocephalic animals receiving minocycline (mean 2.10 cells/μm3 +/- 0.60 SD) and the untreated hydrocephalic animals (mean 7.27 cells/μm3 +/- 3.21 SD), as well as both control groups. Likewise, the number of Iba-1 immunoreactive microglia in untreated hydrocephalic animals was significantly higher (p < 0.001) than in both control animals receiving minocycline (mean 2.00 cells/μm3 +/- 0.50 SD) and control animals not receiving minocycline (mean 1.41 cells/μm3 +/- 0.22 SD). It is important to note that no significant differences were found between the two control groups. All cell counts were multiplied by 105 to obtain the number of cells per micrometer squared.
Figure 2.
Microglia density (cells/μm3) in minocycline treated and untreated animals. The number of immunoreactive microglia in untreated hydrocephalic animals was significantly higher than in the minocycline-treated hydrocephalic group (p < 0.001). Minocycline treatment had no effect on control brains. There was a statistically significant increase between both control groups compared to the untreated hydrocephalic animals (p < 0.001). Data are mean ± SD, n = 5, cell counts were multiplied by 10-5 to get the actual cell number per per μm3.
Effect on cortical thickness
Overall, four outcomes were observed with regard to the thickness of the cerebral cortex (Table 1): (1) Untreated hydrocephalic animals exhibited a decreased cortical thickness compared to non-hydrocephalic controls with and without minocycline (* and + symbols in Table 1); this effect was almost always statistically significant. (2) Minocycline treatment in hydrocephalic animals significantly increased cortical thickness compared to untreated hydrocephalic animals in most regions (β symbol in Table 1). (3) This increase restored cortical thickness to control levels in some regions, but six of 9 regions remained significantly thinner compared to controls with and without minocycline (# and δ symbols in Table 1). (4) Finally, minocycline treatment had no effect on cortical thickness in non-hydrocephalic control animals.
Table 1.
Cortical thickness of all regions (parietal, occipital 1 and occipital 2) and locations (dorsal, lateral and temporal) in the four groups of experimental animals (n = 5 for all groups).
| Mean cortical thickness in mm ± SD | ||||
|---|---|---|---|---|
| Cortical Region | Control no minocycline | Control with minocycline | Untreated hydrocephalic | Minocycline-treated hydrocephalic |
| Parietal | ||||
| Dorsal | 1.44 ± 0.13 | 1.32 ± 0.27 | 0.55 ± 0.27 +++ | 0.76 ± 0.34 ββ, #, δδ |
| Lateral | 1.78 ± 0.11 | 1.41 ± 0.14 | 0.71 ± 0.32 | 1.12 ± 0.67 |
| Temporal | 1.59 ± 0.21 | 1.79 ± 0.53 | 0.33 ± 0.16 +++ | 0.58 ± 0.37 βββ, ###, δδδ |
| Occipital 1 | ||||
| Dorsal | 1.26 ± 0.13 | 1.25 ± 0.18 | 0.34 ± 0.09** | 0.61 ± 0.15 βββ, ###,δδδ |
| Lateral | 1.29 ± 0.11 | 1.23 ± 0.12 | 0.35 ± 0.12 *, +++ | 0.74 ± 0.28 |
| Temporal | 1.24 ± 0.14 | 1.17 ± 0.30 | 0.21 ± 0.12 **, +++ | 0.59 ± 0.24 |
| Occipital 2 | ||||
| Dorsal | 2.04 ± 0.27 | 1.04 ± 0.15 | 0.33 ± 0.09 *, +++ | 0.45 ± 0.11 ββ, ##, δδ |
| Lateral | 3.60 ± 0.78 | 1.29 ± 0.42 | 0.28 ± 0.14 +++ | 0.48 ± 0.24 βββ, ###, δδδ |
| Temporal | 3.47 ± 0.31 | 1.31 ± 0.64 | 0.31 ± 0.11 ++ | 0.48 ± 0.23 ββ, ###, δδ |
*, **, p < 0.05 and 0.01; Untreated hydrocephalic compared to control no minocycline.
++, +++, p < 0.01 and 0.001; Untreated hydrocephalics compared to control with minocycline.
ββ, βββ, p < 0.01 and 0.001; Minocycline-treated hydrocephalic compared to untreated hydrocephalic.
#, ##, ###, p < 0.05, 0.01 and 0.001; Minocycline-treated hydrocephalic compared to control no minocycline.
δ δ, δ δ δ, p < 0.01 and 0.001; Minocycline-treated hydrocephalic compared to control with minocycline.
Discussion
The major findings of this study are that systemic treatment with minocycline (1) significantly reduced the numbers of cortical GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia in hydrocephalic brains, (2) partially restored cortical mantle thickness to control levels, and (3) had no effect on control brains. These effects are impressive because in the cortex of untreated hydrocephalic brains the numbers of GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia increase significantly about 5-fold and the cerebral cortex is reduced to about 1/3 of its normal thickness. From a clinical perspective, these results are interesting because minocycline was administered during relatively late stages of ventriculomegaly, and thus the treatment effects apply specifically to astrocytes and microglia in advanced phases of reactivity.
Role of glia in the pathophysiology of hydrocephalus
In hydrocephalus, gliosis is known to occur, especially in the periventricular white matter [4,5,7,32-36] but also in cortical gray matter [10]. Glial scar formation could play a major role in creating the problems that chronically plague hydrocephalic children. It has been suggested by many investigators [37], including ourselves [10], that gliosis is a permanent fixture in hydrocephalic brains, even those that have been shunted successfully. Our previous studies have shown that GFAP RNA levels increase with the progression of hydrocephalus in both a congenital model of rodent hydrocephalus (the H-Tx rat) [9] and a kaolin model of induced hydrocephalus in kittens [8]. Furthermore, Yoshida found that GFAP-labeled reactive astrocytes were present surrounding cystic lesions in severely hydrocephalic H-Tx animals, but was not able to detect a significant increase in GFAP labeled astrocytes in the white matter surrounding the ventricles [38,39]. Clinically, increased levels of GFAP protein have been found in the CSF of patients with normal pressure hydrocephalus, and patients who developed secondary hydrocephalus due to subarachnoid hemorrhage [40-43], and the possibility of using GFAP protein levels as a diagnostic tool for hydrocephalus is currently being explored [44,45]. Finally, previous studies in our laboratory on a kitten model of kaolin-induced hydrocephalus demonstrated that shunting could reduce the amount of GFAP protein and RNA present in the cerebral cortex, but the results were quite variable and GFAP levels began to rise over time (unpublished observations).
Microglia play a major role in the response to brain injury (reviewed in [46-50]), including hydrocephalus [9,10,51-54]. Mangano et al [10] illustrated that microglial cell proliferation and activation increased in regions of the sensorimotor cortex and auditory cortex during the progression of hydrocephalus in moderately affected H-Tx rats. When assessing microglial changes in pediatric hydrocephalus, it is important to remember that normal microglia change morphology during differentiation. The typical maturation process of a microglial cell in rats has been demonstrated impressively by Orlowski et al. [55], and these features were used for maturation comparisons in the current study. Immature microglia have an amoeboid shape with large somata and very few short processes with growth-cone-like varicosities, but as these cells mature cellular processes become longer and more numerous and by postnatal day 30 in rats, cells have acquired the fully ramified fine long processes with extensive branching typical of resting microglia.
It is also important to note that while the present study involved counts of GFAP- and Iba-1-immunoreactive cells, the analysis does not measure proliferation of astrocytes and microglia directly. Since it is possible that less reactive cells expressing low or undetectable levels of GFAP and Iba-1 would not be counted, a more definitive labeling study with BrdU or Ki67 should be performed to assess cellular proliferation.
The role of minocycline
Minocycline, a derivative of the well-known antibiotic tetracycline, has recently shown promise as a specific inhibitor of microglial cells, one of the main elements of glial scar formation in hydrocephalus. Although its mechanism of action is still unknown [18,56-60], minocycline's promise as a neuroprotective agent is illustrated by the recent initiation of clinical trials in Parkinson's disease [61]. Since its initial demonstration as an anti-inflammatory agent [62], minocycline has been shown to have multiple benefits in brain injury (reviewed in Buller et al [18]). Minocycline can inhibit microglial activation, prevent glutamate toxicity, prevent caspase-1 and caspase-3 activated apoptosis, decrease the activity of inducible nitric oxide synthetase and p-38 mitogen activated protein kinase, inhibit matrix metalloproteinase-2 activity, and impair cytokine production. Additionally, the mechanism of action for minocycline involves not only microglia directly but also T cells and their subsequent activation of microglia. These actions of minocycline appear to be responsible for reducing brain injury in animal models of Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, traumatic brain injury, excitotoxicity, intracerebral hemorrhage, spinal cord injury, focal and global cerebral ischemia, and hypoxia.
The potential use of minocycline in hydrocephalus
To date, one study has attempted to protect the hydrocephalic brain by infusion of nimodipine, a calcium channel antagonist, into the ventricles of juvenile hydrocephalic rats in order to reduce white matter damage. Unfortunately, these interventions have produced only limited short-term success [63-65]. Another study has demonstrated that GFAP can be reduced in the hydrocephalic brain by administration of magnesium sulphate [66]. Thus the current study represents a relatively novel effort that, based on the previous successes achieved in other brain injury models, has a strong likelihood of promoting supplemental treatments for hydrocephalus.
One important caveat is worth noting: tetracycline (from which minocycline is a derivative) administration can impair bone and tooth development, as well as cause discoloration of teeth [18] in children. Whether minocycline also exerts these effects is not known, but this potential problem must be taken into consideration before clinical applications can be adopted.
Conclusions
Our data suggest that minocycline treatment is effective in reducing the gliosis that accompanies ventriculomegaly, and thus may provide an added benefit when used as a supplement to ventricular shunting. With additional pre-clinical data, one would anticipate that this new avenue of research would eventually culminate in a clinical study using minocycline as a supplemental treatment to shunting in human patients.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JMM participated in the design of the study, carried out the histologic work, supervised technicians in the laboratory helping with histology, gathered all data, performed the statistical analysis and helped to draft the manuscript. JPM participated in the design of the study, acquired funding for the project, and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
James P McAllister, II, Email: pat.mcallister@hsc.utah.edu.
Janet M Miller, Email: mille16j@cmich.edu.
Acknowledgements
We would like to thank Kelley E. Deren (University of Utah and Wayne State University) for her assistance in cell count analysis and Mr. Alexander G. Shanku (Wayne State University). We would like to thank STARS (Seeking Techniques Advancing Research in Shunts, Livonia MI) and the BrainChild Foundation (Carefree Arizona) for providing funding for this project.
References
- Lo P, Drake JM. Shunt malfunctions. Neurosurg Clin N Am. 2001;12:695–701. [PubMed] [Google Scholar]
- Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975;43:523–534. doi: 10.3171/jns.1975.43.5.0523. [DOI] [PubMed] [Google Scholar]
- Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200:311–320. doi: 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317–324. doi: 10.1111/j.1750-3639.2004.tb00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639–649. [PubMed] [Google Scholar]
- Del Bigio MR, McAllister JP. , II. In: Pediatric Neurosurgery. 4. Choux M, DiRocco R, Hockley AD, Walker ML, editor. Philadelphia: Churchill Livingstone; 1999. Pathophysiology of Hydrocephalus; pp. 217–236. [Google Scholar]
- Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- McAllister JP II, Chovan P. Neonatal hydrocephalus. Mechanisms and consequences. Neurosurg Clin N Amer. 1998;9:73–93. [PubMed] [Google Scholar]
- Miller JM, McAllister JP. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. doi: 10.1186/1743-8454-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano FT, McAllister JP, Jones HC, Johnson MJ, Kriebel RM. The microglial response to progressive hydrocephalus in a model of inherited aqueductal stenosis. Neurol Res. 1998;20:697–704. doi: 10.1080/01616412.1998.11740586. [DOI] [PubMed] [Google Scholar]
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Franciosi S, Sattayaprasert P, Kim SUMJ. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48:85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol Appl Neurobiol. 2008;34:379–393. doi: 10.1111/j.1365-2990.2007.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 2008;26:477–485. doi: 10.1016/j.ijdevneu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: A neuroprotective agent for hypoxic-ischemic brain injury in the neonate? J Neurosci Res. 2009;15:599–607. doi: 10.1002/jnr.21890. [DOI] [PubMed] [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kohn DF, Chinookoswong N, Chou SM. A new model of congenital hydrocephalus in the rat. Acta Neuropathol. 1981;54:211–218. doi: 10.1007/BF00687744. [DOI] [PubMed] [Google Scholar]
- Jones HC, Bucknall RM. Inherited prenatal hydrocephalus in the H-Tx rat: a morphological study. Neuropathol Appl Neurobiol. 1988;14:263–274. doi: 10.1111/j.1365-2990.1988.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen L, Miyan JA. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain. 2002;125:1859–1874. doi: 10.1093/brain/awf182. [DOI] [PubMed] [Google Scholar]
- Jones HC. Aqueduct stenosis in animal models of hydrocephalus. Child's Nerv Syst. 1998;13:503–504. doi: 10.1007/s003810050124. [DOI] [PubMed] [Google Scholar]
- Nojima Y, Enzan H, Hayashi Y, Nakayama H, Kiyoku H, Hiroi M, Mori K. Neuroepithelial and ependymal changes in HTX rats with congenital hydrocephalus: an ultrastructural and immunohistochemical study. Pathol Int. 1998;48:115–125. doi: 10.1111/j.1440-1827.1998.tb03880.x. [DOI] [PubMed] [Google Scholar]
- Jones HC, Yehia B, Chen GF, Carter BJ. Genetic analysis of inherited hydrocephalus in a rat model. Exp Neurol. 2004;190:79–90. doi: 10.1016/j.expneurol.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Yager JY, Ashwal S. Animal Models of Perinatal Hypoxic-Ischemic Brain Damage. Pediatr Neurol. 2009;40:156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Harris NG, Jones HC, Williams SC. MR imaging for measurements of ventricles and cerebral cortex in postnatal rats (H-Tx strain) with progressive inherited hydrocephalus. Exp Neurol. 1992;118:1–6. doi: 10.1016/0014-4886(92)90016-J. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, Hess DC. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp Neurol. 2004;186:248–251. doi: 10.1016/j.expneurol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, DeLeo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley CJ, Bennett GW, Mayhew TM, Parker TL. Stereological analysis of regional brain volumes and neuron numbers in rats displaying a spontaneous hydrocephalic condition. Exp Neurol. 2001;168:88–95. doi: 10.1006/exnr.2000.7578. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optial fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Bruni JE. Periventricular pathology in hydrocephalic rabbits before and after shunting. Acta Neuropathol. 1988;77:186–195. doi: 10.1007/BF00687430. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Bruni JE, Fewer HD. Human neonatal hydrocephalus. An electron microscopic study of the periventricular tissue. J Neurosurg. 1985;63:56–63. doi: 10.3171/jns.1985.63.1.0056. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Cardoso ER, Halliday WC. Neuropathological changes in chronic adult hydrocephalus: cortical biopsies and autopsy findings. Can J Neurol Sci. 1997;24:121–126. doi: 10.1017/s0317167100021442. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol. 1998;154:157–169. doi: 10.1006/exnr.1998.6922. [DOI] [PubMed] [Google Scholar]
- Glees P, Hasan M. Ultrastructure of human cerebral macroglia and microglia: maturing and hydrocephalic frontal cortex. Neurosurg Rev. 1990;13:231–242. doi: 10.1007/BF00313025. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, da Silva MC, Drake JM, Tuor UI. Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci. 1994;21:299–305. doi: 10.1017/s0317167100040865. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Koya G, Tamayama K, Kumanishi T, Abe S. Development of GFAP-positive cells and reactive changes associated with cystic lesions in HTX rat brain. Neurol Med Chir. 1990;30:445–450. doi: 10.2176/nmc.30.445. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Koya G, Tamayama K, Kumanishi T, Abe S. Histopathology of cystic cavities in the cerebral white matter of HTX rats with inherited hydrocephalus. Neurol Med Chir. 1990;30:229–233. doi: 10.2176/nmc.30.229. [DOI] [PubMed] [Google Scholar]
- Albrechtsen M, Sorensen PS, Gjerris F, Bock E. High cerebrospinal fluid concentration of glial fibrillary acidic protein (GFAP) in patients with normal pressure hydrocephalus. J Neurol Sci. 1985;70:269–274. doi: 10.1016/0022-510X(85)90168-6. [DOI] [PubMed] [Google Scholar]
- Petzold A, Keir G, Kerr M, Kay A, Kitchen N, Smith M, Thompson EJ. Early identification of secondary brain damage in subarachnoid hemorrhage: a role for glial fibrillary acidic protein. J Neurotrauma. 2006;23:1179–1184. doi: 10.1089/neu.2006.23.1179. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Rosengren L, Blomsterwall E, Karlsson JE, Wikkelso C. CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology. 1998;50:1122–1127. doi: 10.1212/wnl.50.4.1122. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Blennow K, Mansson JE, Fredman P, Tisell M, Wikkelso C. Ventricular cerebrospinal fluid neurofilament protein levels decrease in parallel with white matter pathology after shunt surgery in normal pressure hydrocephalus. Eur J Neurol. 2007;14:248–254. doi: 10.1111/j.1468-1331.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- Beems T, Simons KS, Van Geel WJ, De Reus HP, Vos PE, Verbeek MM. Serum- and CSF-concentrations of brain specific proteins in hydrocephalus. Acta Neurochir (Wien) 2003;145:37–43. doi: 10.1007/s00701-002-1019-1. [DOI] [PubMed] [Google Scholar]
- Petzold A, Keir G, Green AJE, Giovannoni G, Thompson EJ. An ELISA for glial fibrillary acidic protein. J Immunol Meth. 2004;287:169–177. doi: 10.1016/j.jim.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW. Reactive microglia in cerebral ischaemia: an early mediator of tissue damage? Neuropathol Appl Neurobiol. 1995;21:277–289. doi: 10.1111/j.1365-2990.1995.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Bonnekoh P, Miyazawa T, Hossmann KA, Kreutzberg GW. Immunocytochemical study of an early microglial activation in ischemia. J Cereb Blood Flow Metab. 1992;12:257–269. doi: 10.1038/jcbfm.1992.36. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. TINS. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- SoLtys Z, Ziaja M, Pawlinski R, Setkowicz Z, Janeczko K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res. 2001;63:90–97. doi: 10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Miller JM, Kumar R, McAllister JP, Krause GS. Gene expression analysis of the development of congenital hydrocephalus in the H-Tx rat. Brain Res. 2006;1075:36–47. doi: 10.1016/j.brainres.2005.12.094. [DOI] [PubMed] [Google Scholar]
- Weller RO, Mitchell J, Griffin RL, Gardner MJ. The effects of hydrocephalus upon the developing brain. Histological and quantitative studies of the ependyma and subependyma in hydrocephalic rats. J Neurol Sci. 1978;36:383–402. doi: 10.1016/0022-510X(78)90046-1. [DOI] [PubMed] [Google Scholar]
- Lu J, Kaur C, Ling EA. An immunohistochemical study of the intraventricular macrophages in induced hydrocephalus in prenatal rats following a maternal injection of 6-aminonicotinamide. J Anat. 1996;188:491–495. [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Murase SI, Horwitz AF, Mandell JW. Infiltrative microgliosis: activation and long-distance migration of subependymal microglia following periventricular insults. J Neuroinflammation. 2005;2:5. doi: 10.1186/1742-2094-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski D, SoLtys Z, Janeczko K. Morphological development of microglia in the postnatal rat brain. A quantitative study. Int J Dev Neurosci. 2003;21:445–450. doi: 10.1016/j.ijdevneu.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase 1 and caspase 3 expression and delays mortality in a transgenic mouse model of Huntington's disease. Nat Med. 2000;6:797–801. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, Gonzalez-Garcia C, Cena V, Aguirre N, Jordan J. Involvement of mitochondrial potential and calcium buffering capacity in minocycline cytoprotective actions. Neuroscience. 2005;133:959–967. doi: 10.1016/j.neuroscience.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1401. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. The Journal of Neuroscience. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Ravina BM, Fagan SC, Hart RG, Hovinga CA, Murphy DD, Dawson TM, Marler JR. Neuroprotective agents for clinical trials in Parkinson's disease: a systematic assessment. Neurology. 2003;60:1234. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. PNAS. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, Massicotte EM. Protective effect of nimodipine on behavior and white matter of rats with hydrocephalus. J Neurosurg. 2001;94:788–794. doi: 10.3171/jns.2001.94.5.0788. [DOI] [PubMed] [Google Scholar]
- Khan OH, McPhee LC, Moddemann LN, Del Bigio MR. Calcium antagonism in neonatal rats with kaolin-induced hydrocephalus. J Child Neurol. 2007;22:1161–1166. doi: 10.1177/0883073807306259. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Wang X, Wilson MJ. Sodium channel-blocking agents are not of benefit to rats with kaolin-induced hydrocephalus. Neurosurgery. 2002;51:460–466. doi: 10.1097/00006123-200208000-00029. [DOI] [PubMed] [Google Scholar]
- Khan OH, Enno T, Del Bigio MR. Magnesium sulfate therapy is of mild benefit to young rats with kaolin-induced hydrocephalus. Pediatr Res. 2003;53:970–976. doi: 10.1203/01.PDR.0000061561.42921.5B. [DOI] [PubMed] [Google Scholar]