Abstract
Background
Signaling by IL-4 and IL-13 via the IL-4 receptor alpha chain (IL-4Rα) plays a critical role in the pathology of allergic diseases. The IL-4Rα is endowed with an immunoreceptor tyrosine-based inhibitory motif (ITIM), centered on tyrosine 709 (Y709) in the cytoplasmic domain, that binds a number of regulatory phosphatases. The function of the ITIM in the in vivo regulation of IL-4R signaling remains unknown.
Objective
To determine the in vivo function of the IL-4Rα ITIM using mice in which the ITIM was inactivated by mutagenesis of the tyrosine Y709 residue into phenylalanine (F709).
Methods
F709 ITIM mutant mice were derived by knockin mutagenesis. Activation of intracellular signaling cascades by IL-4 and IL-13 was assessed by intracellular staining of phosphorylated signaling intermediates and by gene expression analysis. In vivo responses to allergic sensitization were assessed using models of allergic airway inflammation.
Results
The F709 mutation increased STAT6 phosphorylation by IL-4 and, disproportionately, by IL-13. This was associated with exaggerated Th2 polarization, enhanced alternative macrophage activation by IL-13, augmented basal and antigen-induced IgE responses and intensified allergen-induced eosinophilic airway inflammation and hyperreactivity.
Conclusions
These results point to a physiologic negative regulatory role for the Y709 ITIM in signaling via IL-4Rα, especially by IL-13.
Keywords: IL-4 receptor, IL-4, IL-13, ITIM, SHP-1, IgE, Allergic Airway Inflammation, Asthma
Introduction
The IL-4/IL-13 cytokine pathway plays a central role in regulating Th2 responses, including induction of IgE synthesis, modulation of lymphocyte and antigen-presenting cell function and allergic tissue responses such as airway inflammation in asthma 1, 2. IL-4 and IL-13 share a common receptor component, the IL-4Rα chain, which can pair with alternative subunits to assemble a complete receptor 1-3. IL-4Rα associates with the common gamma c chain (γc) to form a type I IL-4 receptor complex that is found predominantly in hematopoietic cells and binds exclusively IL-4 4. IL-4Rα can also pair with the IL-13Rα1 subunit to form a type II IL-4 receptor capable of binding either IL-4 or IL-13. The type II receptor is expressed on both hematopoietic and non-hematopoietic cells including airway epithelium, and is essential for allergen-induced airway hyperreactivity and mucus hypersecretion 5-7. Binding of IL-4 and IL-13 to these receptors activates receptor-associated Janus kinases, which initiate a number of intracellular signaling cascades by phosphorylating specific tyrosine (Y) residues in the cytoplasmic domain of IL-4Rα (4-6). Phosphorylation of Y575, Y603 and Y633 of human IL-4Rα mobilizes the transcription factor STAT6, which induces IL-4 and IL-13-responsive genes. Additional cell growth and regulatory functions are served by Y497, which activates phosphatidylinositol 3 (PI3)-kinase and MAP kinase (MAPK) pathways 8, 9. Once recruited to IL-4Rα, both IRS-2 and STAT6 are subject to Jak-mediated phosphorylation, which enables their effector functions.
The sequence around Y709 at the carboxyl-terminus of murine IL-4Rα (IVYSSL) is concordant with the canonical immunoreceptor tyrosine-based inhibitory motif (ITIM) sequence (consensus I/V/LxYxxL/V) 10-12. The IL-4R ITIM binds a number of regulatory phosphatases including Src homology 2 domain-containing protein tyrosine phosphatase 1 and 2 (SHP-1 and SHP-2) and Src homology 2 domain-containing inositol 5-phosphatase 1 (SHIP-1), suggesting a role for this tyrosine residue in the recruitment of these regulators to IL-4Rα 13. Global SHP-1 and SHIP-1 deficiency is associated with enhanced signaling via IL-4Rα and enhanced allergic airway inflammation 14-16. However, SHP-1, SHP-2 and SHIP-1 associate with multiple other receptors relevant to the allergic response, including Fc and mast cell inhibitory receptors, thus rendering the specific function of the ITIM in modulation of IL-4Rα signaling difficult to discern. In this report we aimed to specifically examine the role of the ITIM in IL-4Rα signaling in mice whose IL-4Rα ITIM has been inactivated by targeted knockin mutagenesis.
Methods
Derivation of mutant mice
The derivation by targeted knockin mutagenesis of mutant mice on BALB/c background carrying a homozygous Phenylalanine (F) substitution at position Y709 of IL-4Rα (C.129Il4raF709/F709) and similarly manipulated control mice that retain the native Y709 residue (C.129.Il4raY709/Y709) is detailed in the Online Repository Methods section. PCR genotyping of WT and mutant mice was carried out as detailed in the Online Repository Methods section. All protocols were in accordance with NIH guidelines and approved by the Animal Care and Use Committees at the University of California at Los Angeles and the Childrens Hospital, Boston.
Flow Cytometry and intracellular staining
Single–cell suspensions were stained with the indicated antibodies and analyzed on a FACSCalibur cytometer (Becton Dickinson). FITC- or PE-conjugated mAbs used were obtained from Pharmingen and eBioscience. Intracellular staining with anti-phospho(p)-Y641-STAT6 (pSTAT6), anti-p-Threonine (T) 308-AKT (pAKT) and anti-pT180/pY182 p38 MAP kinase (pp38) antibodies (BD Biosciences) was carried out as described 17. The cells were then stained with conjugated pSTAT6, pAKT or pp38 (Alexa fluor 647; BD Biosciences) and analyzed by flow cytometry.
Serum total and OVA-specific IgE antibodies, in vitro IgE production and Th cell differentiation
These assays were carried out as previously described 8.
Cell stimulation and Immunoblotting
Purified cell populations were serum starved for 4 hours at 37°C prior to stimulation with IL-4 or IL- 13 (100 ng/ml) for the indicated Time periods. Whole cell lysates were normalized for their protein content, resolved by SDS/PAGE then transferred to nitrocellulose filters and immunoblotted with the indicated antibody. Antibodies against the following phosho-proteins and proteins were used in immunoblotting: pY641-STAT6, STAT6, pY980-JAK3 (Santa Cruz Biotech), anti-pY1020-SHIP-1, pY580-SHP-2, pSerine (S)473-AKT, AKT, pT202,pY204-ERK1/2, ERK1/2, pT180/182-38, p38, pY1054/1055-TYK2 and Tubulin (Cell Signaling Technology), anti-pY1021/1022-JAK1 (Signal Aldrich), and anti-pY536-SHP-1 (ECM Biosciences). The blots were developed using horseradish peroxidase-conjugated secondary antibodies and enzyme-linked chemiluminescence (ECL, Amersham Pharmacia Biotech).
Macrophage and Lung Fibroblast cultures and arginase assay
Bone marrow-derived macrophages (BMDM) were derived by culturing bone marrow aspirates for 7 days with M-CSF. cultured in 24-well tissue culture plates, then stimulated with IL-4 or IL-13 at the indicated concentrations and times. The arginase assay was carried out as described 5. Primary lung fibroblasts cultures were prepared as described 18.
OVA-induced allergic airway inflammation
Mice were sensitized then boosted on day 0 and 14, respectively, with i.p. injection of 100 μg OVA mixed in aluminum potassium phosphate (alum), then challenged intranasally with 50 μg of OVA daily starting on day 28 for 3 consecutive days. Control mice were sensitized and boosted with PBS mixed with alum then challenged with OVA. Bronchoalveolar lavage was performed as described 8. Paraffin-embedded lungs sections were stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining as described 8. Peribronchiolar inflammation was graded with a score as follows: 0, normal; 1, few cells; 2, a ring of inflammatory cells 1 cell layer deep; 3, a ring of inflammatory cells 2–4 cells deep; 4, a ring of inflammatory cells of >4 cells deep 19. The abundance of PAS-positive goblet cells in the airways was scored as follows: 0, <5% goblet cells; 1, 5–25%; 2, 25–50%; 3, 50–75%; 4, >75% 19.
Induction of AHR and measurement of airway responsiveness
Mice were sensitized with 50 μg of OVA (Sigma-Aldrich) in 2mg alum administered i.p. A week later, the mice were challenged with intranasal antigen (50 μg OVA/d) given on 3 consecutive days. Control mice received i.p. injections of alum and intranasal administrations of OVA. Twenty-four hrs following the final dose, AHR was assessed as previously described 20. Briefly, mice were anesthetized with 50 mg/kg pentobarbital and instrumented for the measurement of pulmonary mechanics (BUXCO Electronics). Mice were tracheostomized, intubated, and mechanically ventilated as previously described 20. Baseline lung resistance (RL) and responses to aerosolized saline (0.9% NaCl) were measured first, followed by responses to increasing doses (0.32 to 40 mg/ml) of aerosolized acetyl-β-methylcholine chloride (methacholine; Sigma-Aldrich). The three highest values of RL obtained after each dose of methacholine were averaged to obtain the final values for each dose.
Statistical analysis
Student's t test, two way ANOVA and repeat measure ANOVA with Bonferroni post test analysis of groups, and Kruskal Wallis test with Dunn's post test analysis of groups were used to compare test groups. A p value smaller than 0.05 was considered to be statistically significant.
Results
Characterization of the IL-4Rα ITIM
To identify putative ITIMs in IL-4Rα and elucidate their function, we first analyzed the capacity of individual tyrosine motifs present in the human IL-4Rα chain cytoplasmic domain to serve as ITIMs. These studies identified the tyrosine motif at position Y713 to uniquely serve as an ITIM (Fig. E1 in the online Repository). A peptide spanning phospho-Y713, but not other phospho-Y residues, bound the carboxyl-terminal SH2 domain of SHP-1 and activated SHP-1 phosphatase activity, consistent with its function as an ITIM. In contrast, unphosphorylated Y713 peptide failed to bind to SHP-1 (Fig. E1 in the Online Repository).
The functional consequences of ITIM inactivation were analyzed in a cellular model using a chimeric receptor composed of the extracellular and transmembrane domains of the erythropoietin receptor and the cytoplasmic domain of IL-4Rα (EpoR/IL-4Rα). The chimeric receptor was expressed in the human Burkitt lymphoma cell line BJAB, which does not express erythropoietin receptor (data not shown). Treatment with erythropoietin induces dimerization of the EpoR/IL-4Rα chain and receptor activation independent of contribution of the γc chain or IL-13Rα1. When stimulated with erythropoietin, BJAB cells expressing the chimeric receptor mediated activation of JAK1 and STAT6, as evidenced by their phospho-tyrosine phosphorylation (Figure E2 in the Online Repository). Inactivation of the ITIM by mutagenesis of Y713 into F713 resulted in enhanced activation of JAK1 and STAT6, consistent with a negative regulatory function of the ITIM in receptor signaling. By using an over-expression system in HeLa cells in which the EpoR/IL-4Rα and SHP-1 were co-expressed, it could be demonstrated that the SHP-1 associated with EpoR/IL-4Rα and that mutagenesis of the ITIM Y713 to F713 abrogated this association (Figure E3 in the Online Repository).
Generation of IL-4RαF709 mutant mice by targeted knock-in mutagenesis
In view of the above results, and to elucidate the function of the IL-4Rα ITIM in an in vivo murine system, we exploited the virtual identity of the extended human and murine ITIMs (GIVpY713SALTCHL and GIVpY709SSLTCHL, respectively) to mutagenize the critical Y709 residue of the murine IL-4Rα ITIM to F709 by targeted knockin mutagenesis of Il4ra. This approach, which mirrored an earlier study that analyzed the IRS-2 docking site at the Y500 residue, disabled the ITIM by preventing its phosphorylation and its association with SHP-1 (Fig. E1 and E3 in the Online Repository) 21. Homozygous mutant mice of both sexes were phenotypically indistinguishable from their wild-type (WT) and heterozygous littermates. Expression levels of the mutant receptor protein were verified by flow cytometry to be closely matched identical to WT IL-4Rα (Figure E4 in the Online Repository). Analysis revealed the T and B lymphocytes of the IL-4Rα F709 mutant mice to be normal in number and phenotype (data not shown).
The F709 mutation augments STAT6 phosphorylation upon IL-4R signaling
To elucidate signaling mechanisms by which the F709 substitution mediates its pro-allergic inflammatory effects, we examined IL-4Rα signaling in hematopoietic and airway epithelial cells of C.129.Il4raF709/F709 and control C.129.Il4raY709/Y709 mice. Treatment with IL-4 resulted in enhanced phosphorylation of STAT6 in C.129.Il4raF709/F709 B lymphocytes as compared to the C.129.Il4raY709/Y709 controls, as detected by immunoblotting with an anti-phospho-(p)STAT6 antibody (Fig.1A). While the F709 mutation augmented STAT6 phosphorylation by IL-4, it did not influence the course of STAT6 dephosphorylation following signal termination (Fig. 1B). Enhanced STAT6 phosphorylation in IL-4-treated C.129.Il4raF709/F709 B and T cells was also documented by intracellular staining with anti-pSTAT6 antibodies, with T cells exhibiting more pronounced augmentation of pSTAT6 formation as compared to B cells (Fig. 1C, D). The increase in the mean fluorescence intensity (MFI) of pSTAT6 staining in the mutant cells reflected an absolute increase in STAT6 phosphorylation as the WT and mutant cells exhibited similar basal pSTAT6 MFI. Collectively, these data established that the ITIM mutagenesis augmented the induction phase of STAT6 phosphorylation in response to IL-4 receptor signaling.
Fig. 1.
Enhanced STAT6 activation in the C.129.Il4raF709/F709 mutant mice. A. Upper and middle panels: Western blot analysis of pSTAT6 induction in B cells at different time points following treatment with IL-4 (100 ng/ml). Lower panel: densitometric measurement of pSTAT6, normalized for STAT6 content. B. Upper panel: Decay of pSTAT6 signal in B cells upon cessation of IL-4 treatment. Cells were treated for 10 min with IL-4 (100 ng/ml), then washed (arrow) and analyzed at the indicated time points for pSTAT6 content. Lower panel: densitometric measurement of pSTAT, normalized for STAT6 content. C, D. Flow cytometric analysis of pSTAT6 induction in WT (Y709) and mutant (F709) B cells (C) or T cells (D) following treatment with graded concentrations of IL-4 for 15 min. Results for panels A and B are representative of 2 independent experiments each, and for C and D the averages of 3 mice per group/experiment with similar results observed in 3 independent experiments. ‡ p<0.0001 for genotype effect (F709 versus Y709) by repeat measure two way ANOVA; *p<0.05, **p<0.01 by two way ANOVA with Bonferroni post-test analysis of groups.
The F709 mutation promotes T helper (Th) 2 polarization and IgE responses
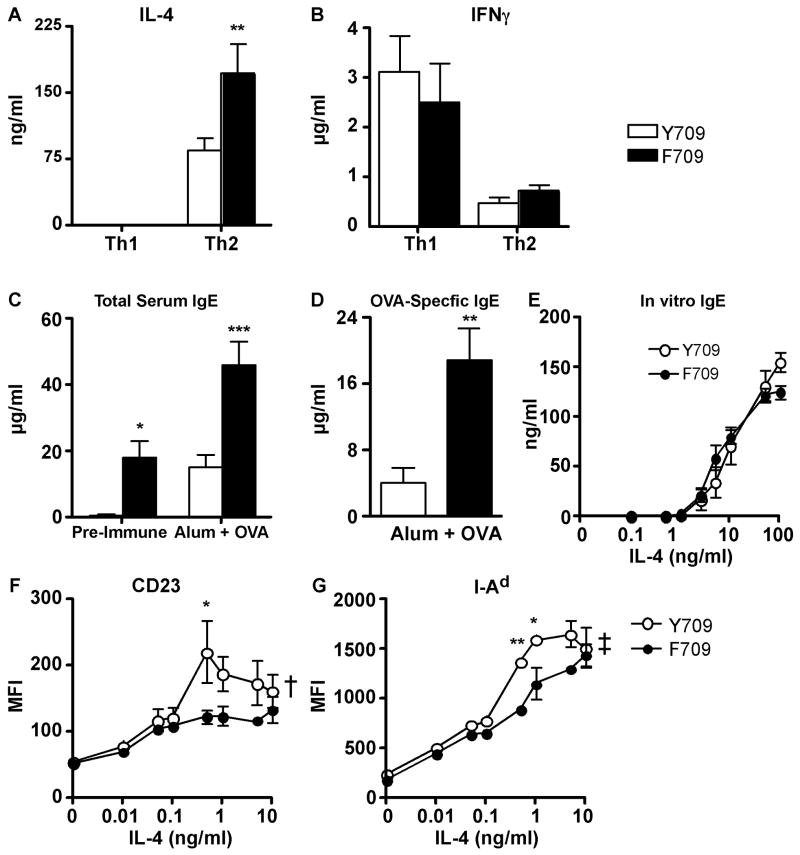
To determine whether the F709 substitution influences Th cell differentiation as a contributing factor in its promotion of allergic airway inflammation, we compared cytokine production of T cells of C.129.Il4raF709/F709 and control C.129.Il4raY709/Y709 mice that were induced to differentiate in vitro into Th1 or Th2 effector lymphocytes. C.129.Il4raF709/F709 Th2 cells exhibited a significant increase in IL-4 production as compared to C.129.Il4raY709/Y709 Th2 cells. In contrast, there was no significant difference in IFN-γ production between Th1 cells of the respective genotype. (Fig. 2A, B). These results indicated that the F709 mutation promotes IL-4 production byTh2 cells.
Fig. 2.
The F709 mutation enhances IL-4 production by Th2 cells and IgE responses in vivo. A, B. Cytokine production by Th cells. Naive WT (Y709) and F709 splenic T cells (n=3-7 mice/group) were differentiated into Th1 (A) or Th2 (B) cells, then stimulated with anti-TCRβ mAB for 48 h. Culture supernatants were analyzed for IL-4 and IFNγ production by ELISA. C-D. Total and OVA-specific serum IgE levels in F709 and WT control mice (n=6-8/group) collected prior to (pre-immune) or following immunization with OVA/alum. E. In vitro IgE production by purified splenic B cells of WT and F709 mice (n=5/group) following stimulation with anti-CD40 mAb + IL-4. There was no significant difference between the two groups (repeat measure two way ANOVA). F, G. Upregulation of CD23 and I-Ad expression in Y709 and F709 B cells treated with increasing concentrations of IL-4 (n=3/group). *P<0.05, **P<0.01, ***P<0.001; †p<0.01, ‡p<0.0001 for genotype effect (Student's t-test for panel D, and 2 way ANOVA with post-test analysis for panels A, C, F and G).
The impact of the F709 mutation on humoral immunity was examined by measuring the total and antigen-specific antibody responses following immunization with OVA mixed with alum adjuvant to promote Th2 type responses 22, or with saline/alum as immunization control. Fig. 2C shows that the C.129.Il4raF7096/F709 mice exhibited enhanced total serum concentrations of IgE following immunization with OVA/alum as compared to C.129.Il4raY709/Y709 control mice. Significantly, OVA-specific antibody responses were also increased in the F709 mutant mice as compared to controls (Fig. 2D). The heightened IgE production in the F709 mutant mice was not the result of an intrinsic alteration in the capacity of the C.129.Il4raF709/F709 B cells to produce IgE, as purified mutant and control B cells produced similar levels of IgE when stimulated in vitro with anti-CD40 mAb + increasing concentrations of IL-4 (Fig. 2E). Also, IL-4 stimulation did not superinduce the expression in B cells of CD23 and I-Ad, which proceeds in a STAT6-dependent mechanism. These results suggested that the increased IgE production in C.129.Il4raF709/F709 mice was extrinsic to the B cells, possibly involving increased IL-4 production by the C.129.Il4raF709/F709 T cells.
The F709 mutation promotes alternative macrophage activation
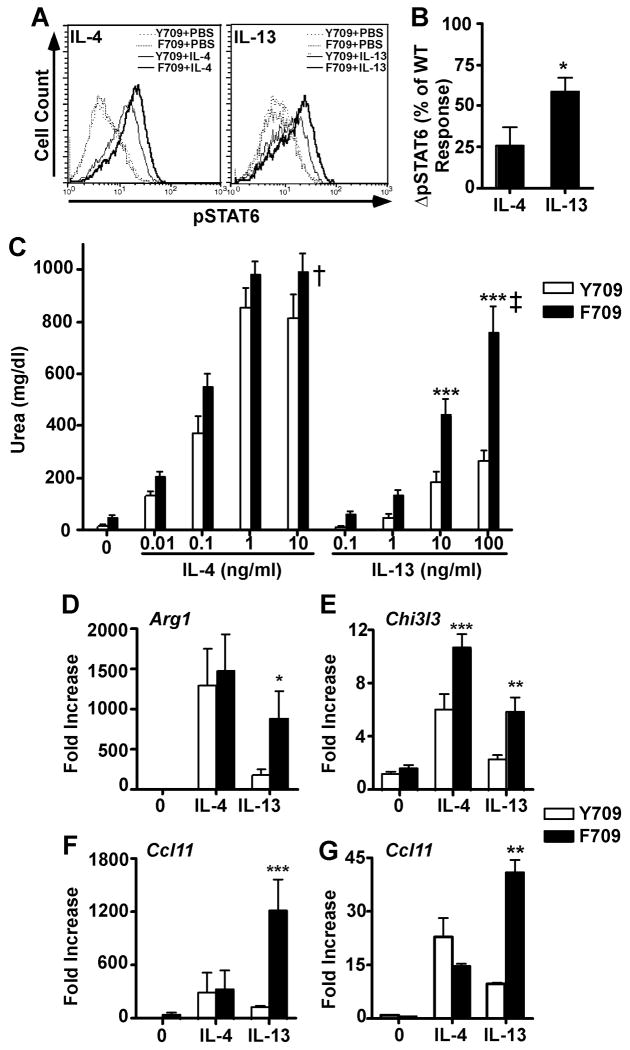
Alternative macrophage activation is regulated by IL-4Rα/STAT6 signaling, and is more effectively achieved by signaling via the type I IL-4R 5, 6, 23. To elucidate the impact of the F709 mutation on alternative macrophage activation, we examined the induction by IL-4 and IL-13 of STAT6 activation, the expression of the STAT6-regulated genes Arg1 and Chi3l3, and of arginase enzymatic activity in BMDM of C.129.Il4raF709/F709 mutant and C.129.Il4raY709/Y709 WT control mice. BMDM are normally more sensitive to IL-4 treatment as compared to IL-13 5, 6. Both IL-4 and to a lesser extent IL-13 induced increased pSTAT6 formation in Y709 BMDM, which plateaued at 10ng/ml and 100 ng/ml, respectively (Fig. 3A). While the F709 mutation upregulated pSTAT6 formation by both cytokines, it disproportionately augmented pSTAT6 formation by IL-13 as compared to similarly treated WT BMDM (Fig. 3A, B). In contrast, the F709 mutation did not alter the activation by IL-4 and IL-13 of the protein kinase AKT, a down-stream target of the IL-4R-coupled IRS2/PI3-kinase pathway (Fig. E5 in the Online Repository). Similarly, it did not affect the induction by IL-4 and, more modestly, IL-13 of p38 MAP kinase activation, whereas activation of p42/p44 ERK kinases was not induced in either WT or mutant BMDM above baseline (Fig. E5 in the Online Repository and data not shown). Overall these results indicated a measure of specificity in the impact of the F709 mutation on IL-4R-coupled signaling pathways.
Fig. 3.
The F709 mutation enhances alternative macrophage activation. A. Intracellular staining of pSTAT6 in WT (Y709) and F709 BMDM stimulated for 15 min at 37 °C with IL-4 (10 ng/ml) or IL-13 (100 ng/ml). B. Bar graph plot of the difference in the relative increase in pSTAT6 formation (ΔpSTAT6) in IL-4 and IL-13 treated F709 as compared to similarly treated Y709 BMDC (n=3 mice/group). C. Arginase activity in lysates of BMDMs treated with various concentrations of IL-4 or IL-13 and analyzed after 48 h by measurement of urea production ((n=9-12/group). D-G. Real-time PCR of genes encoding arginase 1 (Arg1), chitinase 3-like 3 (Chi3l3), and Ccl11 in BMDMs (D-F) and Ccl11 in primary lung fibroblast cultures (G) stimulated for 20 h with IL-4 or IL-13 at 10 ng/ml each (n=7-12/group). *p<0.05, **p<0.01, ***p<0.001; †p<0.01, ‡p<0.0001 for genotype effect (Student's t-test for panel B, and 2 way ANOVA with post-test analysis for panels C-G).
In Y709 BMDM, IL-4 induced markedly higher levels of Arg1, Chi3l3 and Ccl11 transcripts and arginase enzymatic activity, with a left-shifted dose response curve, as compared to IL-13 (Fig. 3C-F). The F709 mutation markedly augmented the induction by IL-13 of Arg1, Chi3l3 and Ccl11 transcripts and of arginase enzymatic activity, whereas it more modestly upregulated the IL-4 responses or left them unaffected (Fig. 3C-F). Furthermore, examination of responses to IL-4 and IL-13 in primary lung fibroblast cultures of F709 mice revealed a similar pattern of differential upregulation of Ccl11 transcription by IL-13 but not IL-4 (Figure 3G and data not shown). These results are consistent with differential enhancement by the F709 mutation of IL-13-induced alternative macrophage activation via the type II IL-4R.
To elucidate mechanisms by which the F709 substitution augmented IL-4 and IL-13 signaling, we examined the phosphorylation of the ITIM substrates: SHP-1, SHP-2 and SHIP-1, in response to IL-4 and IL-13 treatment of BMDM derived from C.129.Il4raF709/F709 and control C.129.Il4raY709/Y709 mice. Phosphorylated proteins were detected by immunoblotting using specific anti-phospho-antibodies. SHIP-1 was found constitutively phosphorylated in BDMD. Both IL-4 and IL-13 induced partial dephosphorylation of SHIP-1 in WT cells, whereas this dephosphorylation was abrogated in C.129.Il4raF709/F709 mutant BMDM (Fig.4). IL-4 treatment induced an early increase in SHP-2 phosphorylation that was, in general, of similar magnitude in WT and mutant BMDM. IL-13 induced delayed phosphorylation of SHP-2 that was observed only at high cytokine concentration and which was also similar between WT and mutant BMDM (Fig. 4). IL-4 also induced SHP-1 phosphorylation in WT BMDM, the magnitude of which was attenuated in F709 BMDM. The attenuation by the F709 mutation of SHP-1 phosphorylation was more pronounced in the case of IL-13 treatment. Examination of JAK1 revealed that it was hyperphosphorylated at baseline in F709 relative to Y709 BMDM,. It became hypo-phosphorylated upon activation in both cell types, while maintaining increased pY content in F709 cells. Both JAK3 and Tyk2 underwent activation-induced hyperphosphorylated in F709 BMDM relative to WT controls. Collectively, these data established that the ITIM mutagenesis impaired SHP-1 phosphorylation and, reciprocally, exaggerated receptor-associated kinase activation and STAT6 phosphorylation, consistent with defective recruitment of SHP-1 as one likely mechanism by which the ITIM mutagenesis exerted its effects.
Fig 4.
The F709 mutation impairs SHP-1 activation in BMDM cells. WT or F709 mutant BMDM cells were treated with IL-4 or IL-13 at 10 or 100 ng/ml and for 15 or 60 min, as indicated. Cellular lysates were derived and probed for pSHP-1, pSHP-2, pSHIP-1, pJAK1, pJAK3, and pTYK2, and β-Tubulin, the latter as a protein loading control. Results are representative of 2-3 independent experiments per immunblot panel.
The F709 mutation results in enhanced antigen-induced allergic airway inflammation
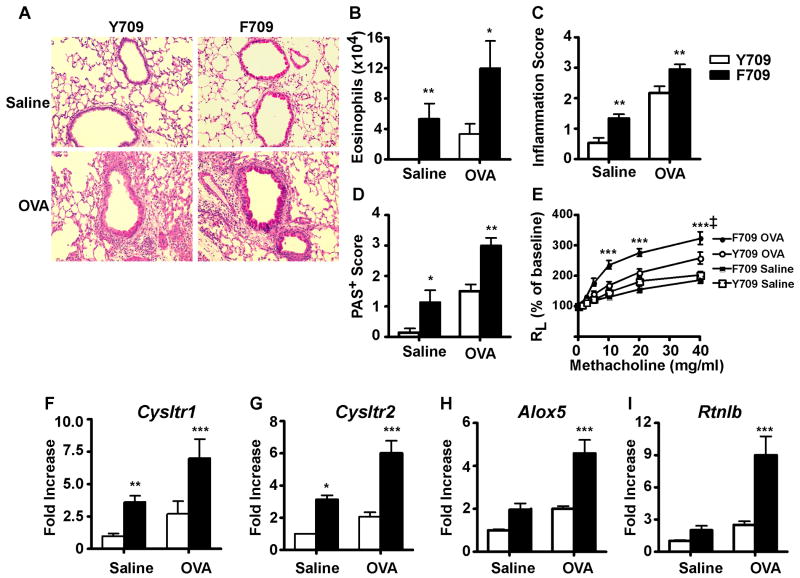
The functional consequences of the F709 mutation were analyzed in antigen-driven murine models of allergic airway inflammation. In an acute model of antigen-induced allergic airway inflammation, C.129.Il4raF709/F709 and C.129.Il4raY709/Y709 mice were sensitized on day 0 and boosted on day 14 with OVA mixed with alum, then challenged at 4 weeks post-sensitization with inhaled OVA for three consecutive days. Control mice were immunized and boosted with PBS/Alum and challenged with inhaled OVA. Microscopic examination of H&E and PAS-stained lung sections of WT and mutant mice revealed patchy peribronchial inflammatory infiltrates, composed primarily of eosinophils and lymphocytes, that were substantially more prominent in OVA sensitized and challenged C.129.Il4raF709/F709 mice as compared to C.129.Il4raY709Y709 controls (Fig. 5A and data not shown). In contrast, sham immunized mice exposed to aerosolized OVA showed normal lung histology (data not shown). Significantly, OVA-challenged C.129.Il4raF709/F709 mutants exhibited markedly increased peribronchial and perivascular inflammation and increased numbers of goblet cells compared to C.129.Il4raY709/Y709 controls 24-27 (Fig. 5B, C). Analysis of BAL fluid of OVA-sensitized and challenged mice revealed that OVA-sensitized C.129.Il4raF709/F709 mice exhibited increased accumulation of eosinophils in the airways as compared to similarly treated controls (Fig. 5D). To determine whether the F709 mutation increased AHR following antigenic sensitization and challenge, a more limited antigenic sensitization protocol was employed to better discriminate the genotype effect (see materials and methods section). Results revealed that OVA sensitized C.129.Il4raF709/F709 mice exhibited augmented AHR in response to OVA challenge as compared to similarly treated Y709 control BALB/c mice (Fig. 5E). The heightened AHR in the F709 mutants induced by the limited OVA sensitization protocol was also associated with a dramatic increase in airway eosinophilia as compared to Y709 control mice (Fig. E6A in the Online Repository). These results indicated that the F709 substitution caused an increase in allergic airway inflammation and AHR in response to allergen sensitization and challenge.
Fig. 5.
The Y709F substitution promotes acute antigen-induced allergic airway inflammation and AHR. Sham (Saline/Alum) or OVA/Alum sensitized C.129.Il4raY709/Y709 (Y709) and C.129.Il4raF709/F709 (F709) mice (n=10 mice/group) were challenged intranasally with OVA on 3 consecutive days then analyzed. A. Lung histology (PAS staining, 20×). B. Peribronchial inflammation scores. C. PAS-positive goblet cells in the airways. D. Eosinophilia in BAL fluid of sham and OVA immunized mice challenged with OVA. E. AHR measurements. Changes in lung resistance (RL) were measured in mice that had been either sham immunized or subjected to a limited protocol of OVA immunization (n=5-8/group). F-I. Real time PCR analysis of Cysltr1, Cysltr2, Alox5, Alox15, and Rtnlb mRNA levels in total lung extracts of OVA sensitized and challenged mice versus controls (n=3-6/group). *p<0.05, **p<0.01, ***p<0.001; ‡p<0.0001 for genotype effect (B: Kruskal Wallis test; C-I: 2 way ANOVA with post-test analysis).
To determine the impact of the F709 mutation on the transcription of STAT6-responsive genes induced during allergic airway inflammation, we measured the relative transcript levels of a battery of STAT6-responsive genes in OVA and sham sensitized C.129.Il4raF709/F709 and C.129.Il4raY709/Y709 mice following challenge with OVA. Results revealed that the F709 mutation upregulated the expression of a number of STAT6-responsive gene transcripts both at baseline and following OVA challenge, including transcripts of the cysteinyl leukotriene receptor 1 and 2 genes (Cysltr1 and Cysltr2, respectively), 5-lipoxygenase (Alox5), and resistin like b (Retnlb) (Fig. 5F-I). Transcripts encoded by the Th2 cytokine genes Il4 and Il9 were also significantly increased in lung tissues of allergen-challenged C.129.Il4raF709/F709 mutant mice as compared to WT controls, while those encoded by Il5 and Il13 were increased but did not achieve significance (Figure E6B-E). These results indicated that the F709 mutation upregulated the expression of a number of STAT6-responsive genes involved in allergic airway inflammation.
Discussion
Our findings reveal a significant regulatory function of the IL-4Rα chain ITIM in receptor signaling in vivo. Inactivation of the ITIM site disrupted the normal homeostasis of allergic effector pathways, giving rise to spontaneously elevated IgE levels, goblet cell metaplasia of the bronchial epithelium and influx of eosinophils into the airways of allergen-naïve mice. Isolated T, B and BMDM cells evidenced enhanced receptor signaling in response to IL-4 and, for BMDM, IL-13. Furthermore, the F709 mutation resulted in amplification of the allergic response to the allergen, OVA, as evidenced both by increased total and antigen specific IgE production and by exaggerated airway inflammation, mucus metaplasia and AHR. These results identify a negative regulatory function for the IL-4Rα ITIM in controlling allergic tone both in the unchallenged basal setting and following challenge with allergens.
Induction of STAT6 phosphorylation in ITIM mutant cells was enhanced at different concentrations of IL-4 and IL-13. Signal enhancement started at the earliest time points examined, and it persisted for the duration of cytokine treatment. However, the course of STAT6 dephosphorylation once the cytokine treatment was terminated remained unaltered. In contrast to the conclusions of a previous report, our results suggest that the ITIM regulates the induction, rather than termination, phase of IL-4R signaling, at least in the cell types under study 28. Earlier studies on other receptor systems and on the IL-4R itself indicate that the mechanism of action of ITIMs in regulating receptor signaling involves recruitment of phosphatases SHP-1, SHP-2 and SHIP-1 13. SOCS proteins also bind at ITIMs, and may thus may contribute to signal regulation at the IL-4Rα chain ITIM 29. The identity of the respective intermediate recruited to the IL-4R ITIM in different cell types may vary depending on the level of expression. It is also likely that, in addition to signal regulation, the IL-4Rα ITIM may mediate distinct effector functions that are dependent on identity of the bound intermediate. The balance of intermediates bound at the IL-4Rα ITIM in different tissues remains to be established, as is the nature of their distinct effector functions in response to IL-4 and/or IL-13 signaling.
The cytoplasmic domain of the IL-4Rα chain possesses three STAT6 binding sites in tandem, which ensures amplification of the receptor signaling by means of STAT6 activation, and a single ITIM site, whose function is demonstrably inhibitory 1, 2, 30. While at baseline the receptor did not signal constitutively, the F709 mutation amplified IL-4R-related responses in vivo. Many of the phenotypes associated with the F709 mutation, including strikingly elevated baseline IgE levels, differential augmentation of alternative macrophage activation by IL-13, and enhanced goblet cell metaplasia and allergic airway inflammation are attributes associated with increased signaling via type II IL-4R 5, 6. These results indicate an important function of the ITIM would be to increase the threshold for receptor activation, especially by the type II IL-4R, so as to suppress exaggerated in vivo responses to sub-threshold immunogenic stimuli.
Both in vitro and in vivo studies revealed that mechanism by which the IL-4R ITIM promotes signaling involves upregulation of JAK kinase activation and STAT6 phosphorylation. This mechanism does not relate to an influence on IL-4 and IL-13 binding to the IL-4R extracellular domain, as it remains operative when examined in a chimeric receptor composed of the Erythropoietin receptor extracellular domain and the IL-4R intracellular domain (Figure E2 in the online repository). Rather, it appears to involve defective recruitment of SHP-1 to the mutant receptor. SHP1 phosphorylation was decreased upon IL-4, and more so IL-13, signaling, in mutant BMDM, indicative of impaired recruitment and activation of SHP-1 by the ITIM. The mechanism for the disproportionate impact of the ITIM mutation on signaling via the IL-4R type II receptor (by IL-13) versus type I receptor (by IL-4) may involve residual recruitment of SHP-1 by the other components of IL-4R type I, as evidenced by decreased though not absent phosphorylation of SHP-1 by IL-4 in ITIM mutant cells (Figure 4). A recent study has linked the enhanced efficacy of the type I IL-4R in inducing alternative macrophage activation to its differential capacity to activate the IRS/PI3-kinase pathway 31. However, there was no evidence that the ITIM mutant resulted in enhanced IRS-PI3-kinase activation, as monitored by the activation of the downstream kinase AKT.
While the F709 mutation augmented STAT6 phosphorylation in B cells, it was not associated with enhanced IgE production by isolated B cells induced to switch to IgE production in a T cell-independent manner. Similarly, it did not upregulate the expression of in B cells of the STAT6-responsive gene products CD23 and I-Ad. These results suggest that in addition to regulating STAT6 phosphorylation, intermediates recruited at the ITIM may provide additional signals that promote STAT6 function. The loss of such signals upon ITIM mutagensis may counterbalance the effects of upregulated STAT6 phosphorylation. Thus, The striking increase in the level of IgE production in the ITIM mutant mice is likely to reflect increased IL-4 production secondary to favored Th2 responses.
The Y709F mutation also mediated allergic airway inflammation both at baseline and in an antigen-dependent manner (OVA sensitization). In both cases the inflammation was associated with increased airway eosinophilia. Given that airway eosinophilia plays a critical role in unfolding the acute and chronic changes associated with asthma, including AHR, mucous production and subepithelial fibrosis, the increased airway eosinophil load would provide one mechanism by which the F709 allele promotes allergic airway inflammation 32, 33. Gene expression analysis identified additional mechanisms by which the F709 allele may exert pro-inflammatory functions. In particular, the Y709F mutation upregulated the expression of components of the leukotriene pathway, which plays a critical role in allergic airway inflammation: 5 lipoxygenase (Alox5), cysteinyl leukotriene receptor 1 and 2 (Cysltr1; Cysltr2) 34. It also upregulated the expression of resistin-like beta (Retnlb), which promotes allergic airway inflammation and the induction of subepithelial fibrosis 35. Together, these findings reinforce intervention strategies at these pathways aimed to interrupt the evolution of airway inflammation associated with a strongly pro-atopic phenotype like that of the Y709F substitution 35.
Despite the potentiation by the F709 mutation of STAT6 phosphorylation in B cells, there was no observed difference in the intrinsic capacity of the mutant B cells to produce IgE in response to IL-4. These results suggest that the ITIM transmits STAT6-independent signals that promote IgE production in B cells, and that this function of the ITIM is lost upon its mutagenesis. They also indicate that the increase in IgE production observed in vivo reflects the increased supply of IL-4, in line with the observed Th2 bias in the F709 mice both in vitro (Figure 2A) and in vivo (Figure E6B).
Epidemiological surveys have linked different IL-4Rα polymorphisms to phenotypes of atopy and asthma both individually and in combination. 36-39. It is remarkable that a mutation that results in a dramatic pro-allergic responses such as the F709 was nevertheless associated with a relatively modest increase in IL-4/IL-13-induced STAT6 phosphorylation (20-50% increase over WT controls). These findings indicate that even subtle changes in receptor signaling associated with some IL-4R polymorphisms may results in a profoundly atopic phenotype. The contribution of altered ITIM function to the pro-atopic and disease promoting effects of different IL-4Rα polymorphisms merits further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Raif Geha for his gift of EpoR/IL-4Rα cDNA construct, James J. Booth for animal care, and Maria Garcia-Lloret and Brigitte Gomperts for helpful discussions.
This work was supported by National Institutes of Health grant 2R01AI065617 (to T. A. Chatila), R01AI054471 (to H. C. Oettgen) and R01 AI026322 (to D. T. Umetsu), and by a March of Dimes grant (to T. A. Chatila). R. Tachdjian was supported by the Human and Molecular Development Training Grant NIH/NICHD T32 HD007512.
Abbreviations
- AHR
airway hyperresponsiveness
- Alum
aluminum potassium sulfate
- BAL
bronchoalveolar lavage
- IL-4R
IL-4 receptor
- IRS
insulin receptor substrate
- ITIM
Immuno Tyrosine Inhibitory Motif
- JAK
Janus kinase
- mAb
monoclonal antibody
- OVA
ovalbumin
- SHP-1/2
Src homology 2 domain-containing protein tyrosine phosphatase 1/2
- STAT
signal transducer and activator of transcription
- Th
T helper
Footnotes
- -The IL-4Rα ITIM negatively regulates signaling by IL-4 and especially by IL-13 in vivo.
- -Polymorphisms that subtly promote IL-4R signaling amplitude may disproportionately skew the allergic response in vivo.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 2.Chatila TA. Interleukin-4 receptor signlaing pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. quiz 91. [DOI] [PubMed] [Google Scholar]
- 4.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–3. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–5. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaeser F, Bryce PJ, Ho N, Raman V, Dedeoglu F, Donaldson DD, et al. Targeted Inactivation of the IL-4 Receptor {alpha} Chain I4R Motif Promotes Allergic Airway Inflammation. J Exp Med. 2003;198:1189–200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–20. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 10.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, et al. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweeney MC, Wavreille AS, Park J, Butchar JP, Tridandapani S, Pei D. Decoding protein-protein interactions through combinatorial chemistry: sequence specificity of SHP-1, SHP-2, and SHIP SH2 domains. Biochemistry. 2005;44:14932–47. doi: 10.1021/bi051408h. [DOI] [PubMed] [Google Scholar]
- 12.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwada M, Giallourakis CC, Pan PY, Rothman PB. Immunoreceptor tyrosine-based inhibitory motif of the IL-4 receptor associates with SH2-containing phosphatases and regulates IL-4-induced proliferation. J Immunol. 2001;167:6382–7. doi: 10.4049/jimmunol.167.11.6382. [DOI] [PubMed] [Google Scholar]
- 14.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–6. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- 15.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, et al. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–19. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SY, Zheng T, Bailey ML, Barber DL, Schroeder JT, Kim YK, et al. Src homology 2 domain-containing inositol 5-phosphatase 1 deficiency leads to a spontaneous allergic inflammation in the murine lung. J Allergy Clin Immunol. 2007;119:123–31. doi: 10.1016/j.jaci.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004;110:206–21. doi: 10.1016/j.clim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol. 2007;37:424–30. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, et al. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–77. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 20.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–93. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrighton N, Campbell LA, Harada N, Miyajima A, Lee F. The murine interleukin-4 receptor gene: genomic structure, expression and potential for alternative splicing. Growth Facors. 1992;6:103–18. doi: 10.3109/08977199209011014. [DOI] [PubMed] [Google Scholar]
- 22.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 23.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 24.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–7. [PubMed] [Google Scholar]
- 25.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 26.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 28.Hanson EM, Dickensheets H, Qu CK, Donnelly RP, Keegan AD. Regulation of the dephosphorylation of Stat6. Participation of Tyr-713 in the interleukin-4 receptor alpha, the tyrosine phosphatase SHP-1, and the proteasome. J Biol Chem. 2003;278:3903–11. doi: 10.1074/jbc.M211747200. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci U S A. 2000;97:6493–8. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan JJ, McReynolds LJ, Huang H, Nelms K, Paul WE. Characterization of a mobile Stat6 activation motif in the human IL-4 receptor. J Immunol. 1998;161:1811–21. [PubMed] [Google Scholar]
- 31.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, et al. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 33.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 35.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, et al. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L305–13. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 36.Ober C, Leavitt SA, Tsalenko A, Howard TD, Hoki DM, Daniel R, et al. Variation in the Interleukin 4-Receptor alpha Gene Confers Susceptibility to Asthma and Atopy in Ethnically Diverse Populations. Am J Hum Genet. 2000;66:517–26. doi: 10.1086/302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel SE, Balzar S, Ampleford E, Hawkins GA, Busse WW, Calhoun WJ, et al. IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med. 2007;175:570–6. doi: 10.1164/rccm.200607-909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hytonen AM, Lowhagen O, Arvidsson M, Balder B, Bjork AL, Lindgren S, et al. Haplotypes of the interleukin-4 receptor alpha chain gene associate with susceptibility to and severity of atopic asthma. Clin Exp Allergy. 2004;34:1570–5. doi: 10.1111/j.1365-2222.2004.02069.x. [DOI] [PubMed] [Google Scholar]
- 39.Risma KA, Wang N, Andrews RP, Cunningham CM, Ericksen MB, Bernstein JA, et al. V75R576 IL-4 receptor alpha is associated with allergic asthma and enhanced IL-4 receptor function. J Immunol. 2002;169:1604–10. doi: 10.4049/jimmunol.169.3.1604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.