Abstract
The aims of the present study were to compare matching performance between ipsilateral and contralateral finger force matching tasks and to examine the effect of handedness on finger force perception. Eleven subjects were instructed to produce reference forces by an instructed finger (index – I or little – L finger) and to reproduce the same amount force by the same or a different finger within the hand (i.e., ipsilateral matching task), or by a finger of the other hand (i.e., contralateral matching task). The results of the ipsilateral and contralateral tasks in the present study commonly showed that 1) the reference and matching forces were matched closely when the two forces were produced by the same or homologous finger(s) such as I/I task; 2) the weaker little finger underestimated the magnitude of reference force of the index finger (I/L task), even with the higher level of effort (relative force), but the two forces were matched when considering total finger forces; 3) the stronger index finger closely matched the reference force of the little finger with the lower level of relative force (i.e., L/I task); 4) when considering the constant errors, I/L tasks showed an underestimation and L/I tasks showed an overestimation compared to I/I tasks. There was no handedness effect during ipsilateral tasks. During the contralateral task, the dominant hand overestimated the force of the non-dominant hand, while the non-dominant hand attempted to match the absolute force of the dominant hand. The overall results support the notion that the absolute, rather than relative, finger force is perceived and reproduced during ipsilateral and contralateral finger force matching tasks, indicating the uniqueness of finger force perception.
Keywords: handedness, finger force perception, force matching task, ipsilateral matching task, contralateral matching task
Introduction
A contralateral force matching paradigm has been commonly used to investigate mechanisms of force perception and control (Gandevia and McCloskey 1977b; Gandevia and McCloskey 1977a; Cafarelli and Bigland-Ritchie 1979; Kilbreath et al. 1997; Carson et al. 2002; Jones 2003; Jones and Piateski 2006). In this paradigm, a reference force produced by one muscle group is matched by a matching force produced by the same (homologous) or different (non-homologous) muscle groups of the contralateral limb. A common finding is that force is perceived relative to the maximal force generating capacity. This means that, when expressed as percent of its maximal force generating capacity, the relative reference force is closely matched by the relative matching force, i.e., relative force matching. For example, the relative reference force of finger flexors is closely estimated by the relative matching force of the contralateral elbow flexors, despite of different strengths between the finger flexors and elbow flexors (Jones 2003). In another study, Carson et al. reported a significant reduction (31%) in the maximal force-generating capacity of the triceps brachii immediately after a fatiguing exercise. However, the relative matching force of the fatigued triceps estimated extremely closely the relative reference force of the contralateral non-fatigued homologous muscle (Carson et al. 2002). Despite of differences in the maximal force generating capacity between non-homologous muscles or altered maximal force generating capacity in one of the homologous muscles, the observation of relative force matching suggests that force perception is likely to be based on a “sense of effort” in contralateral matching paradigms (Gandevia and McCloskey 1977b; Gandevia and McCloskey 1977a; Gandevia 1987; Carson et al. 2002).
In contrast to relative force matching observed in the contralateral force matching paradigm, Li and colleagues have demonstrated that the absolute magnitude of isometric finger flexion forces at fingertips appears to be perceived and reproduced using an ipsilateral force matching paradigm, even though the maximal isometric flexion force-generating capacities are significantly different between index and little fingers (Li 2006; Li and Leonard 2006; Park et al. 2007). For example, when the reference force of a stronger index finger is matched by a weaker little finger within the same hand, the little finger exerts a matching force with a higher effort (greater relative force, or percent), but still underestimates the absolute magnitude of the index finger reference force. However, matching errors (the difference between matching and reference forces) are significantly minimized when the total force of all fingers (instructed and uninstructed fingers) are compared during the reference and matching periods (Li and Leonard 2006). The result indicates that both instructed and uninstructed finger forces are perceived within the central nervous system (CNS) and that the absolute magnitude of total finger force is perceived and reproduced, i.e., absolute force matching, in the ipsilateral force matching paradigm. The phenomenon of uninstructed finger forces, name enslaving (Kilbreath and Gandevia 1994; Li et al. 1998; Kilbreath et al. 2002; Li et al. 2003), is of central origin to a large degree (Danion et al. 2000; Li et al. 2000b; Latash et al. 2002; Danion et al. 2003; Li et al. 2004). Similarly, in an ipsilateral matching task of an index finger reference force to be matched by a little finger, absolute force matching of finger forces and the enslaving effect on minimization of matching errors are also observed after an index finger fatiguing exercise (Park et al. 2007).
Absolute force matching during ipsilateral matching paradigms is strikingly in contrast to relative force matching during contralateral matching paradigms. The contrasting results may lie in different matching paradigms, i.e., ipsilateral vs. contralateral matching paradigms. Loss of some information may occurs due to the interhemispheric transfer in contralateral paradigms (Gordon et al. 1994). Alternatively, the results may reflect the possibility that the CNS adopts different strategies for difference muscle groups. Relative force matching has been reported in matching tasks involving proximal muscles, while absolute force matching appears to be in finger force matching tasks. Therefore, the primary purpose of the present study was to compare matching performance between ipsilateral and contralateral matching tasks involving the same group of muscles, i.e., finger flexors. Handedness has been reported to play an important role in finger force control (Gordon et al. 1994; Henningsen et al. 1995). When producing isometric force simultaneously, the index finger of the dominant hand generates greater force than that from the opposite hand (Henningsen et al. 1995). Accordingly, the other purpose was to examine the effect of handedness on finger force perception particularly in contralateral matching tasks.
Methods
Subjects
Eleven healthy subjects (25.1 ± 6.5 years old; 7 men and 4 women) participated in the experiment. Ten subjects were right-handed and one subject was left-handed according to their preferential use of the right or left hand during writing and eating. All subjects gave informed consent and all procedures were approved by the Institutional Review Board of The University of Montana and conformed to the Declaration of Helsinki.
Apparatus
The subject was seated comfortably during the experiments. The two upper limbs were symmetrical with respect to the body midline. The upper arms were at approximately 15° of abduction in the frontal plane and 45° of abduction in the sagittal plane, and elbow joints at approximately 90° of flexion. The forearms were stabilized on a wooden board using Velcro strips. Fingers of two hands were positioned on force sensors. Eight force sensors (208C02, PCB Piezotronics) were used to measure the finger flexion forces generated by individual fingers of both hands. Four sensors were mounted on each wooden board and were able to move vertically and horizontally to accommodate different hand sizes. Eight signal conditioners (484B11, PCB Piezotronics) were connected to the force sensors for amplifying signals. The amplified signals were then digitized by a 16-bit analog-to-digital converter (PCI-6229, National Instruments) and stored in a personal computer. The force signals were sampled at 1000 Hz.
Force-matching Tasks and Procedure
Maximal isometric flexion force of index and little fingers of each hand was measured three times for each subject before starting the main experiment. During MVC trials, subjects were explicitly instructed to press with the instructed finger (index or little) as hard as possible, while paying little attention to possible force production by other un-instructed fingers. Standard verbal encouragement and online real-time visual feedback were provided. The highest peak value from three trials was considered the MVC force. Based on individual MVCs, a target level of reference force was established and displayed on the computer screen as a red horizontal line.
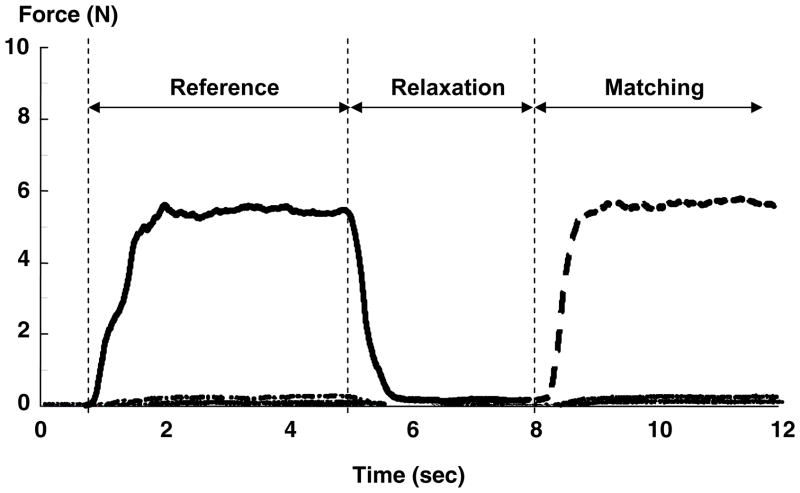
Figure 1 depicts a matching trial. When a trial began, subjects heard a beep sound, waited one second and then produced a reference force for 4 seconds following a pre-determined visual target. This was immediately followed by a 3-second relaxation period. During force relaxation (5 to 8 second time period), traces of all finger forces were displayed to ensure force relaxation. Subjects were then instructed to reproduce the reference force without visual feedback, using the same or another instructed finger. Subjects were instructed to maintain this force to the end of the trial (4 sec). Subjects received no feedback regarding force matching accuracy during the experiment. During each trial, subjects were explicitly instructed to match the magnitude of the reference force (i.e. match the absolute force rather than the level of effort) and to maintain a produced force level during the matching period.
Fig 1.
A typical force profile during the force-matching task at 15% MVC of I/I task in an ipsilateral matching task. Reference, Relaxation and Matching represent the periods of the reference force production, force relaxation, and the matching force production, respectively. During each trial, the subject was instructed to match a reference force using the same or different fingers within the hand (i.e., the ipsilateral task), or the fingers of the other hand (i.e., the contralateral task). Note that visual feedback was provided during the Reference and Relaxation periods (solid line), but not the Matching period (dash line).
The experiment had both ipsilateral and contralateral matching tasks and was categorized into the following four HAND conditions:
1) DH/DH (ipsilateral task): the reference and matching forces were produced by the instructed fingers of the dominant hand (DH); 2) NH/NH (ipsilateral task): the reference and matching forces were produced by the instructed fingers of the non-dominant hand (NH); 3) DH/NH (contralateral task): the reference force was produced by the instructed fingers of the dominant hand while the matching force was produced by the instructed fingers of the non-dominant hand; 4) NH/DH (contralateral task): the reference force was produced by the instructed fingers of the non-dominant hand while the matching force was produced by the instructed finger of the dominant hand. Half of the subjects were tested with the DH/DH and DH/NH conditions first and the NH/NH and NH/DH conditions later; and half of subjects were examined vise versa. These sessions were tested in two separate days with an interval of at least two days.
For each HAND condition, there were three matching tasks with different finger combinations (FINGER) as follows: 1) I/I tasks: both reference and matching forces were produced by the index finger(s); 2) I/L tasks: the reference force was produced by an index finger while the matching force was produced by a little finger; 3) L/I tasks: the reference force was produced by a little finger while the matching force was produced by an index finger. Note that, for an I/L task, the index reference force of the dominant hand was instructed to be matched by the little finger matching force of the dominant hand in the DH/DH condition, while it was instructed to be matched by the little finger matching force of the non-dominant hand in the DH/NH condition. Each task (I/I, I/L and L/I) had three levels of reference force: 15%, 25%, and 35% MVCs. Four trials were recorded for each matching task at each reference force level.
A practice session was allowed for subjects to get familiarized with the experimental setting and instructions. During the practice session, ten trials of ipsilateral and contralateral I/I tasks at 15% MVC were presented. During the experiment, an interval of at least 20 seconds was given between consecutive trials to avoid possible finger fatigue. The order of experimental conditions was randomized, and each condition was conducted in a block of four trials.
Data Analysis
To standardize data analysis as in previous studies (Li and Leonard 2006; Park et al. 2007), we selected a 1-s period of force production for both reference and matching forces when forces were most stable. The mean force from 3.5 – 4.5 seconds was calculated for the reference force, and the mean force from 10.5 – 11.5 seconds was calculated for the matching force, i.e., 0.5 seconds prior to the end of the reference and matching periods. Based on the 1-s force data, the following dependent variables were analyzed.
Absolute forces generated by both the instructed fingers (i.e., instructed finger forces) and all fingers (i.e., total finger forces) were recorded in Newtons (N). Relative forces of the instructed fingers were calculated as percentage of corresponding MVC of the finger.
To evaluate matching performance, constant errors were calculated from instructed finger forces (iCE) and from total finger forces (tCE). iCE was defined as the difference between matching and reference forces of the instructed fingers. Similarly, tCE was calculated to account for contributions of the uninstructed finger forces (i.e., enslaving forces) to the force matching performance. Positive values indicated that the reference forces were overestimated by the matching forces, and negative values indicated the reference forces were underestimated by the matching forces.
Statistics
Descriptive statistics was used. Repeated-Measures ANOVAs were used to evaluate matching performances, with factors of HAND (2 levels, depending on comparison), PERIOD (2 levels: reference and matching periods), FL (3 levels: 15%, 25% and 35% MVCs), FINGER (3 levels: I/I, I/L, and L/I), and ERROR (2 levels: iCE and tCE). Whenever necessary, Tukey HSD post-hoc test was performed. Detailed description of comparisons was provided in the Results session. The level of significance was set at p≤0.05.
Results
In the present experiment, a visual target of the reference was created based on MVCs of the instructed index and little fingers. The index finger MVC of the dominant hand was greater than that of the non-dominant hand (42.8 N vs. 37.1 N, p<0.001), while MVCs produced by the little finger of the dominant and non-dominant hands were similar (20.2 N vs. 19.0 N).
Absolute Finger Force
The instructed finger forces and the total forces of instructed and non-instructed fingers were summarized in Table 1. Two-way ANOVAs were performed, with factors of PERIOD and FL, to compare absolute reference and matching forces in each experimental condition.
Table 1.
Mean and standard error (SE) of the reference and matching forces in all conditions. DH/NH represents the task in which the finger of the dominant hand (DH) produces the reference force and the finger of the non-dominant hand (NH) produces the matching force. Similar coding for DH/DH, NH/NH, and NH/DH. I/L represents the task in which the index (I) finger produces the reference force and the little (L) finger produces the matching force. Similar coding for I/I and L/I. There are three reference force levels, 15%, 25% and 35% MVC. Ref and Mat represent reference and matching forces, respectively. Inst and Total are the forces produced by the instructed finger (I or L) and by all fingers (sum of the instructed and uninstructed finger forces), respectively. Values in ( ) represent standard errors. Units are in Newton.
| Inst | Total | Inst | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Mat | Ref | Mat | Ref | Mat | Ref | Mat | ||||||
| DH/DH | I/I | 15 | 6.5 (1.8) | 6.3 (2.2) | 6.7 (1.9) | 6.5 (2.2) | DH/NH | I/I | 15 | 6.5 (1.8) | 6.3 (2.2) | 6.9 (2.1) | 6.6 (2.4) |
| 25 | 10.7 (3.0) | 10.1 (2.7) | 11.4 (3.4) | 10.6 (3.0) | 25 | 10.6 (2.9) | 9.2 (3.1) | 11.5 (3.4) | 10.1 (3.3) | ||||
| 35 | 14.9 (4.1) | 13.1 (3.8) | 16.1 (4.8) | 14.3 (4.4) | 35 | 15.0 (4.1) | 12.8 (4.4) | 16.1 (4.8) | 14.3 (5.0) | ||||
| I/L | 15 | 6.5 (1.8) | 6.3 (1.8) | 6.9 (2.1) | 8.2 (2.0) | I/L | 15 | 6.4 (1.8) | 6.8 (2.3) | 6.7 (2.1) | 9.1 (3.6) | ||
| 25 | 10.7 (2.9) | 8.4 (2.6) | 11.5 (3.3) | 11.9 (3.9) | 25 | 10.7 (2.9) | 8.6 (3.6) | 11.3 (3.3) | 11.5 (5.1) | ||||
| 35 | 15.0 (4.1) | 10.6 (3.1) | 16.1 (4.7) | 15.7 (5.2) | 35 | 15.0 (4.2) | 9.6 (2.6) | 16.0 (4.7) | 14.2 (5.5) | ||||
| L/I | 15 | 3.0 (1.0) | 3.8 (2.1) | 3.6 (1.4) | 3.9 (2.3) | L/I | 15 | 3.0 (1.0) | 3.8 (1.3) | 3.7 (1.4) | 4.1 (1.2) | ||
| 25 | 5.0 (1.7) | 5.9 (2.8) | 6.0 (2.3) | 5.8 (2.9) | 25 | 5.0 (1.6) | 5.3 (2.1) | 6.4 (2.7) | 5.5 (2.2) | ||||
| 35 | 7.0 (2.3) | 7.3 (3.0) | 8.9 (3.4) | 7.8 (3.2) | 35 | 7.0 (2.3) | 6.5 (4.2) | 8.9 (3.5) | 6.8 (4.7) | ||||
| NH/NH | I/I | 15 | 5.6 (1.7) | 5.9 (2.1) | 6.1 (2.1) | 6.3 (2.5) | NH/DH | I/I | 15 | 5.6 (1.7) | 6.9 (3.4) | 6.2 (2.0) | 7.3 (3.6) |
| 25 | 9.3 (2.7) | 9.4 (3.2) | 10.5 (3.8) | 10.7 (4.1) | 25 | 9.3 (2.7) | 9.7 (4.0) | 10.4 (3.5) | 10.6 (4.3) | ||||
| 35 | 12.9 (3.9) | 11.8 (3.9) | 14.5 (5.2) | 13.5 (5.2) | 35 | 12.9 (3.8) | 13.1 (5.8) | 14.6 (5.3) | 14.5 (6.6) | ||||
| I/L | 15 | 5.6 (1.7) | 6.2 (2.3) | 6.2 (2.1) | 7.7 (2.7) | I/L | 15 | 5.6 (1.6) | 6.9 (2.7) | 6.1 (2.1) | 9.1 (4.3) | ||
| 25 | 9.2 (2.7) | 7.8 (3.0) | 10.2 (3.3) | 10.1 (4.1) | 25 | 9.3 (2.8) | 8.6 (3.4) | 10.4 (3.6) | 12.0 (6.5) | ||||
| 35 | 12.9 (3.9) | 8.7 (3.5) | 14.3 (4.8) | 13.1 (6.7) | 35 | 12.9 (3.8) | 10.5 (3.8) | 14.3 (4.9) | 16.2 (10.2) | ||||
| L/I | 15 | 2.9 (0.9) | 4.3 (2.1) | 3.6 (1.2) | 4.5 (2.0) | L/I | 15 | 2.9 (0.9) | 4.7 (2.9) | 3.4 (1.1) | 4.9 (2.8) | ||
| 25 | 4.9 (1.8) | 6.0 (2.6) | 5.9 (2.1) | 6.6 (2.7) | 25 | 4.7 (1.6) | 6.7 (4.0) | 5.8 (2.0) | 7.2 (4.0) | ||||
| 35 | 6.6 (2.2) | 7.5 (3.8) | 7.9 (2.6) | 8.2 (3.9) | 35 | 6.6 (2.1) | 7.4 (4.8) | 7.8 (2.6) | 8.0 (4.9) | ||||
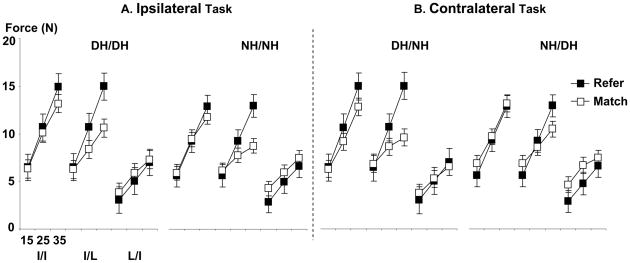
As illustrated in Fig 2, the index finger reference force was not different from the index finger matching force (i.e., I/I tasks) in each of four experimental conditions (DH/DH, NH/NH, DH/NH, and NH/DH), except for when the reference force was 35% MVC. For example, in the DH/DH condition, there was a main effect of FL (F(2,20)=143.71, p<0.001) and an interaction of PERIOD x FL (F(2,20)=4.64, p=0.022), but no main effect of PERIOD. The post-hoc test showed that the reference forces were underestimated at 35% MVC (14.9 N vs. 13.1 N, p<0.001), but not at 15% and 25% MVCs. Similar results were seen in the NH/NH, DH/NH, and NH/DH conditions.
Fig 2.
Absolute forces of the instructed fingers in the ipsilateral matching task (A) and the contralateral matching task (B). Refer and Match represents the reference and matching forces, respectively. DH and NH represent the dominant and non-dominant hands, respectively. The ipsilateral task includes DH/DH and NH/NH conditions and the contralateral task includes DH/NH and NH/DH conditions. I/I, I/L, and L/I tasks are included in each HAND condition (refer to Force-matching Tasks and Procedure). There are three force levels (15%, 25%, and 35% MVCs) in each experimental condition.
During I/L tasks, the index finger reference force was greater than the little finger matching force in the DH/DH, NH/NH, and DH/NH conditions, but the reference and matching forces were not different in the NH/DH condition. In the DH/DH condition, there was an main effect of PERIOD (F(1,10)=19.19, p=0.001), and an interaction of PERIOD x FL (F(2,20)=60.11, p<0.001). The post-hoc test showed that the index finger reference forces were greater than the little finger reference force at 25% (p<0.001) and 35% (p<0.001) MVCs, but not at 15% MVC. A similar result was found in the NH/NH and DH/NH condition. However, in the NH/DH condition, there was an interaction of PERIOD x FL (F(2,20)=18.07, p<0.001), but no main effect of PERIOD. The post-hoc test showed that the reference forces were underestimated at 35% MVC (p<0.001), but not at 15% and 25% MVCs.
During L/I tasks, the reference and matching forces were not different in all HAND conditions. There was no interaction in each HAND condition.
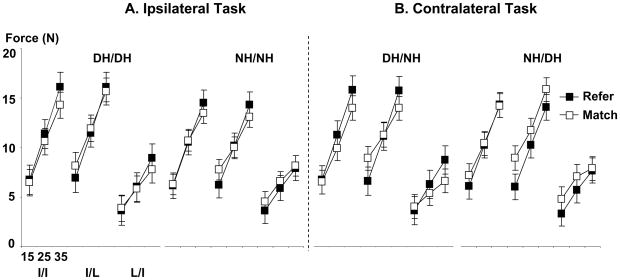
Interestingly, when the total finger forces during the reference and matching periods were compared, the reference force and the matching force were not different in all tasks and experimental conditions (Fig 3).
Fig 3.
Absolute forces of the total fingers in the ipsilateral matching task (A) and the contralateral matching task (B).
Relative Instructed Finger Force
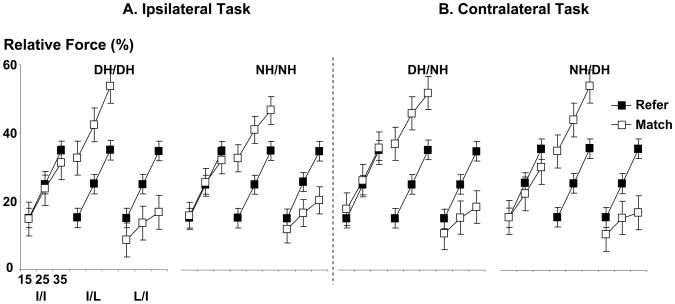
When relative reference and matching forces of the instructed fingers (i.e., with regard to corresponding MVCs) were compared, a different pattern of results was observed (compare Fig 2 and 4). Similarly, two-way ANOVAs were performed to compare relative reference and matching forces in each experimental condition, with factors of PERIOD and FL.
Fig 4.
Relative forces of the instructed fingers in the ipsilateral matching task (A) and the contralateral matching task (B).
During I/I tasks, the relative reference and matching forces of the index finger were not different in ipsilateral matching conditions (DH/DH and NH/NH). In the contralateral matching conditions, each HAND condition (i.e., DH/NH and NH/DH) showed no main effect of PERIOD; however, an interaction of PERIOD x FL (F(2,20)=6.13, p=0.008) was found in the NH/DH condition, but not in the DH/NH condition. The post-hoc test showed that the relative reference force of the non-dominant index finger was greater than of the dominant index finger at 35% MVC (p=0.001), but not at 15% and 25% MVCs.
During I/L tasks, the relative reference forces of the index finger were smaller than the relative matching forces of the little finger in all HAND conditions. There was a main effect of PERIOD (F(1,10)>36.99, p<0.001), but not an interaction. To the contrary, during L/I tasks, the relative reference force of the little finger were greater than the relative matching force of the index finger in all HAND conditions. There was a main effect of PERIOD ((F(1,10)>107.73, p<0.001), and an interaction of PERIOD x FL ((F(2,20)>84.93, p<0.001).
Constant Errors
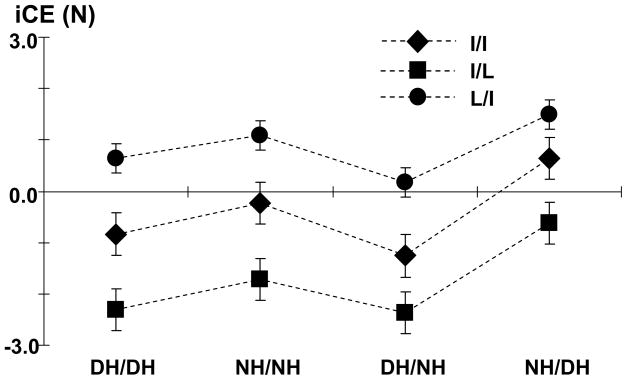
Constant errors in absolute finger forces of the instructed fingers (iCE) were used to compare matching performance across different experimental conditions (Fig 5). Two-way ANOVAs were performed separately to examine the effect of handedness on matching performance in the ipsilateral conditions and the difference in matching performance between ispilateral and contralateral conditions. Factors were HAND and FINGER.
Fig 5.
Constant errors of instructed finger forces (iCE) averaged across three force levels.
In ipsilateral conditions, no handedness effect on matching errors was found. Matching errors (iCE) of the ipsilateral matching tasks in the dominant hand (DH/DH) were not different from those in the non-dominant hand (NH/NH). Matching errors, however, were different among finger force matching tasks, showing a main effect of FINGER (F(2,20)=12.50, p<0.001). In both DH/DH and NH/NH conditions, there was an overestimation in L/I tasks (iCE = 0.9 N) and an underestimation in I/L tasks (iCE = −2.0 N). In I/I tasks, the reference and matching forces were closely approximated.
Handedness did impact matching performance in contralateral tasks when compared to ispilateral tasks, however (Fig 5). When finger forces in the dominant hand were instructed to be matched by the contralateral fingers in the non-dominant hand (DH/NH), iCEs were the same as those in ispilateral tasks (DH/DH). Similarly, iCEs were not different when finger forces of the non-dominant hand were to be matched by fingers in the same hand or in the contralateral hand (Fig 5, NH/NH vs. NH/DH). In other words, matching errors were the same when finger forces of both dominant and non-dominant hands were instructed to be matched by fingers in the same (ipsilateral) or in the other hand (contralateral).
In contrast, when the fingers in the dominant hand were instructed to match reference finger forces in the contralateral hand, greater iCEs were observed as compared to ipsilateral tasks (F[1,10]=6.29, p=0.031) (Fig 5, DH/DH vs. NH/DH). However, no difference in matching errors was found when fingers in the non-dominant hand were instructed to reference finger forces in the same hand or in the contralateral hand (Fig 5, NH/NH vs. DH/NH).
In contralateral tasks, iCE was greater in NH/DH (0.5 N) than in DH/NH (−1.1 N). The ANOVA showed main effects of HAND (F(1,10)=8.00, p=0.018) and FINGER (F(2,20)=5.37, p=0.014). There was no interaction.
Enslaving effect
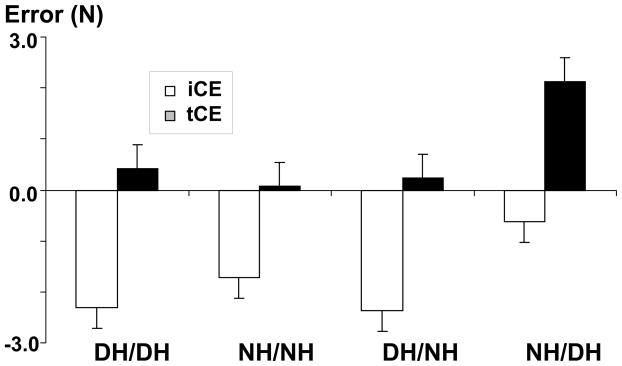
To examine the effect of uninstructed finger forces (enslaving forces) on matching errors, two-way ANOVAs were performed to compare matching errors with (tCE) or without (iCE) enslaving forces, with factors of HAND and ERROR. Similar to our previous studies (Li and Leonard 2006; Park et al. 2007), we focused on enslaving effects in I/L tasks (Fig. 6).
Fig 6.
Constant errors of instructed finger forces (iCE) and of the total finger forces (tCE) in I/L tasks of different HAND conditions
Enslaving forces significantly minimized matching errors in I/L tasks in all experimental conditions except for NH/DH (Fig 6A). There were main effects of ERROR (F(1,10)=30.66, p=0.001) and HAND (F(3,30)=3.05, p=0.044). No interaction was found. Post-hoc tests showed that enslaving forces minimized matching errors from −2.1 N (iCE) to 0.2 N (tCE) in DH/DH, NH/NH, and DH/NH conditions (p<0.001). In NH/DH, enslaving forces amplified errors (iCE = −0.6 N to tCE=2.1 N) (p=0.001).
Discussions
The present study aimed to compare finger force perception within the hand and in the contralateral hand and to examine the handedness effect on finger force perception. During the experiment, subjects were instructed to produce reference forces by an instructed finger (the index or little finger) and estimate the reference forces by the same or different fingers within the hand (i.e., ipsilateral tasks), or the fingers of the other hand (i.e., contralateral tasks). Our main findings were: 1) similarity in finger force matching performance between ipsilateral and contralateral matching tasks; and 2) the effect of handedness on finger force matching performance in contralateral tasks.
Absolute finger force matching in ipsilateral and contralateral tasks
Contralateral finger force matching tasks showed a similar pattern of matching performance as compared to ipsilateral tasks. Similarities were in both ipsilateral and contralateral tasks that 1) the absolute reference and matching forces were matched closely in homo-finger (I/I) tasks (Fig 2); 2) a weaker little finger underestimated the index finger reference force (I/L tasks) (Fig 2), even with a higher level of relative force (effort) (Fig 4), while the total forces in the reference and matching periods were not different (Fig 3); 3) matching errors of the instructed fingers were not different between ipsilateral and contralateral tasks (Fig 5); 4) enslaving forces helped minimize matching errors of total force except for the task when the index finger reference force of the non-dominant hand was matched by the little finger of the dominant hand (I/L in NH/DH). These results of ipsilateral and contralateral finger force matching were in general agreement with our previous studies using ipsilateral matching paradigms (Li and Leonard 2006; Park et al. 2007). As such, the present experiment confirmed and expanded the previous findings and supported the idea that absolute finger forces were perceived in both ipsilateral and contralateral tasks.
Our finding of absolute finger force perception in contralateral finger force matching tasks seems to be contradictory to the result of previous weight estimation studies using contralateral matching paradigms (Kilbreath and Gandevia 1991; 1992). In which errors of perceived heaviness of a weight lifted by a finger increased when the magnitude of concurrently lifted weight by an adjacent finger within the same hand increased. For example, the index finger lifted 200 g while the ring finger of the same hand concurrently lifted 300 g (Kilbreath and Gandevia 1991). This procedure possibly altered the normal force sharing pattern between the index and ring finger during voluntary force production by these two fingers, as such the activation level for the index finger was likely to be increased due to increased level of activation for the adjacent ring finger. A heavier weight was estimated subsequently by the contralateral index finger. As a result, errors in perceived heaviness of the index finger weight increased. This result, however, could be alternatively interpreted that the altered activation for the index finger is accurately estimated by the contralateral index finger force. In this regard, the result of this weight estimation study is consistent with our study.
Our finding of absolute finger force matching is contradictory to earlier reports of relative force matching involving other muscle groups. Relative force matching has been reported in contralateral force matching paradigms involving other homologous [triceps (Carson et al. 2002); triceps and biceps (Cafarelli and Bigland-Ritchie 1979; Jones and Hunter 1982; Jones and Hunter 1983)] and non-homologous [finger flexors and elbow flexors (Jones 2003; Jones and Piateski 2006)] muscles. The contrasting results may be related to different methodologies. Subjects were instructed to maintain the reference force during the matching period in some studies, e.g., Carson et al. (Carson et al. 2002). This might have biased subjects to match the effort (the relative force) (cf.(Li et al. 2002)). In contrast, reference and matching forces were produced sequentially after a brief resting period in our contralateral matching paradigm. The methodological explanation needs to be further explored, however. On the other hand, this disparity is likely to be resulted from difference in anatomical design and control between distal hand muscles and proximal muscles. The great density of mechanoreceptors in the hand (Johansson and Vallbo 1983) as well as the greater specificity in afferent innervation of motoneurons for hand muscles (Illert and Kummel 1999) make the fingers innately have greater peripheral feedback than the proximal muscles. Furthermore, accumulated data have demonstrated that afferent inputs from one hand ascend to both ipsilateral and contralateral somatosensory cortices (Wegner et al. 2000; Simoes et al. 2002; Nihashi et al. 2005; Blatow et al. 2007). As such, tactile stimuli applied to fingertips in one hand are reported to be identified and localized in the contralateral corresponding fingertips (Braun et al. 2005). This phenomenon of bilateral projections of unilateral hand afferent inputs could also account for a previous report that patients with surgical disconnection of the cerebral hemispheres are able to estimate accurately heaviness of the weight lifted by the contralateral hand (Gandevia 1978).
Persistent observation of relative force matching in contralateral matching tasks involving other muscles and the result of absolute finger force matching may reflect the uniqueness of finger force perception and control and its relation to hand function. Fingers are unique functionally as compared to other muscle group(s). Each finger (element) is part of the hand (a system) and individual fingers are interdependent within the hand (Schieber and Santello 2004). Perception of absolute forces of individual fingers (Li 2006) allow them to be integrated into a meaningful multi-finger synergy to achieve many daily activities, e.g., to provide pen stabilization during hand-writing (Latash et al. 2003) and to grasp (Johansson et al. 1999; Pataky et al. 2004a; Pataky et al. 2004b). In case of individual finger impairment, e.g., after fatigue (Kruger et al. 2007), other fingers are able to compensate for one finger’s impairment to preserve performance of the hand. Similarly, such synergy has been demonstrated to be present during two-hand multi-finger tasks (Gorniak et al. 2007). Furthermore, as in one-hand multi-finger tasks (Li et al. 1998a), un-instructed finger forces (enslaving) help minimize a trunk rotational moment during two-hand multi-finger pressing tasks (Li et al. 2000a; Li et al. 2001). In the present study, enslaving forces also minimize matching errors in ipsilateral tasks and most contralateral tasks. The ability to perceive absolute finger forces within the hand and in the contralateral hand could permit skilled and coordinated two-hand movements, ranging from tieing a necktie to operating microsurgery. In contrast, the CNS seems to adopt a different strategy for coordinated movements when involving other muscles in two limbs (cf. (Carson et al. 2002; Li and Leonard 2006)). Selection of appropriate strategies for different functional tasks suggests that the ability of the CNS to choose a strategy which imparts most success and efficiency.
Handedness effect on finger force perception in contralateral tasks
In contrast to no difference in matching performance between the dominant and non-dominant ipsilateral tasks, handedness influenced matching performance in contralateral tasks. Specifically, the index finger in the dominant hand produced greater matching errors when estimating the contralateral reference forces than the ipsilateral reference forces (NH/DH vs. DH/DH), while no such difference was observed when the index finger in the non-dominant hand produced matching forces (DH/NH vs. NH/NH) (Fig 5). Moreover, in contralateral I/L tasks (Fig 6), enslaving forces increased matching errors of the total force when the dominant little finger produced matching forces, while errors were minimized when the non-dominant little finger produced the matching force. Taken together, these results suggest that the dominant hand tends to overestimate the forces of the non-dominant hand, and the non-dominant hand tends to match the forces of the dominant hand in contralateral tasks. Our results of handedness effect on force matching are in general agreement with previous studies (Gordon et al. 1994; Henningsen et al. 1995; Shergill et al. 2003). For example, when a brief constant force is applied to the left index fingertip and the perceived force is estimated by the right index finger, the right index finger consistently overestimates the left index finger force (Shergill et al. 2003). When acting in alternative turns, force escalation is subsequently observed. Since approximately 90% of humans use the right hand as their dominant hand, the result could be interpreted that the dominant index finger overestimates the force of the non-dominant index finger.
The effect of handedness on matching performance of contralateral matching tasks may be related to asymmetries in the somatosensory system. The primary somatosensory cortex (SI) is activated by contralateral sensory stimuli. SI activations are symmetrical at the same level of peripheral stimuli. The secondary somatosensory cortex (SII), which receives ipsilateral and contralateral afferent inputs, is however preferentially activated in the dominant hemisphere (Wegner et al. 2000; Simoes et al. 2002). Conceivably, at the same level, the non-dominant index finger force triggers a higher SII activation than the dominant index finger force. In the subsequent matching force production period of I/I tasks, the dominant index finger produces predictably greater matching errors in the NH/DH than DH/DH condition. Alternatively, the tendency of the non-dominant hand to match the dominant hand could make it suitable for controlling movement trajectories and final position, even in case errors may be introduced, during bimanual coordinated movement. On the other hand, the tendency of the dominant hand to overestimate finger forces of the non-dominant hand may indicate that the CNS simply favors the use of a command efferent signal in bimanual tasks. Thus, our findings support the dynamic dominance model of handedness (Sainburg 2002).
In conclusion, we provided evidence that pattern of matching performance were similar between ipsilateral and contralateral finger force matching. Further, handedness played an important role in contralteral force matching. Matching errors were greater when fingers in the dominant hand estimated the contralateral than ipsilateral reference finger forces. These results support the notion that the absolute magnitude of finger forces is perceived and reproduced. The results are likely related to the unique anatomical and physiological design and the function of the hand.
Acknowledgments
This study was supported in part by an NIH grant (1R15NS053442-01A1).
References
- Blatow M, Nennig E, Durst A, Sartor K, Stippich C. fMRI reflects functional connectivity of human somatosensory cortex. NeuroImage. 2007;37:927. doi: 10.1016/j.neuroimage.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Braun C, Hess H, Burkhardt M, Wuhle A, Preissl H. The right hand knows what the left hand is feeling. Exp Brain Res. 2005;162:366–373. doi: 10.1007/s00221-004-2187-4. [DOI] [PubMed] [Google Scholar]
- Cafarelli E, Bigland-Ritchie B. Sensation of static force in muscles of different length. Exp Neurol. 1979;65:511. doi: 10.1016/0014-4886(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S, Shahbazpour N. Central and peripheral mediation of human force sensation following eccentric or concentric contractions. J Physiol. 2002;539:913–925. doi: 10.1113/jphysiol.2001.013385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion F, Latash ML, Li S. Finger interactions studied with transcranial magnetic stimulation during multi-finger force production tasks. Clin Neurophysiol. 2003;114:1445–1455. doi: 10.1016/s1388-2457(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of fatigue on multifinger coordination in force production tasks in humans. J Physiol. 2000;523(Pt 2):523–532. doi: 10.1111/j.1469-7793.2000.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. The sensation of heaviness after surgical disconnection of the cerebral hemispheres in man. Brain. 1978;101:295–305. doi: 10.1093/brain/101.2.295. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Roles for perceived voluntary motor commands in motor control. Trends in Neurosciences. 1987;10:81–85. [Google Scholar]
- Gandevia SC, McCloskey DI. Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anaesthesia in man. J Physiol. 1977a;272:673–689. doi: 10.1113/jphysiol.1977.sp012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Effects of related sensory inputs on motor performances in man studied through changes in perceived heaviness. J Physiol. 1977b;272:653–672. doi: 10.1113/jphysiol.1977.sp012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, Iwasaki N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia. 1994;32:555–568. doi: 10.1016/0028-3932(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Gorniak SL, Zatsiorsky VM, Latash ML. Hierarchies of synergies: an example of two-hand, multi-finger tasks. Exp Brain Res. 2007;179:167–180. doi: 10.1007/s00221-006-0777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen H, Ende-Henningsen B, Gordon AM. Asymmetric control of bilateral isometric finger forces. Exp Brain Res. 1995;105:304–311. doi: 10.1007/BF00240966. [DOI] [PubMed] [Google Scholar]
- Illert M, Kummel H. Reflex pathways from large muscle spindle afferents and recurrent axon collaterals to motoneurones of wrist and digit muscles: a comparison in cats, monkeys and humans. Exp Brain Res. 1999;128:13–19. doi: 10.1007/s002210050812. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Backlin JL, Burstedt MK. Control of grasp stability during pronation and supination movements. Exp Brain Res. 1999;128:20–30. doi: 10.1007/s002210050813. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensory coding in the glabrous skin of the human hand. Trends in Neurosciences. 1983:27–32. [Google Scholar]
- Jones LA. Perceptual constancy and the perceived magnitude of muscle forces. Exp Brain Res. 2003;151:197–203. doi: 10.1007/s00221-003-1434-4. [DOI] [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Force sensation in isometric contractions: a relative force effect. Brain Res. 1982;244:186–189. doi: 10.1016/0006-8993(82)90919-2. [DOI] [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Effect of fatigue on force sensation. Exp Neurol. 1983;81:640–650. doi: 10.1016/0014-4886(83)90332-1. [DOI] [PubMed] [Google Scholar]
- Jones LA, Piateski E. Contribution of tactile feedback from the hand to the perception of force. Exp Brain Res. 2006;168:298–302. doi: 10.1007/s00221-005-0259-8. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Independent digit control: failure to partition perceived heaviness of weights lifted by digits of the human hand. J Physiol (Lond) 1991;442:585–599. doi: 10.1113/jphysiol.1991.sp018810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Independent control of the digits: changes in perceived heaviness over a wide range of force. Exp Brain Res. 1992;91:539–542. doi: 10.1007/BF00227850. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gorman RB, Raymond J, Gandevia SC. Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J Physiol. 2002;543:289–296. doi: 10.1113/jphysiol.2002.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Refshauge K, Gandevia SC. Differential control of the digits of the human hand: evidence from digital anaesthesia and weight matching. Exp Brain Res. 1997;117:507–511. doi: 10.1007/s002210050247. [DOI] [PubMed] [Google Scholar]
- Kruger ES, Hoopes JA, Cordial RJ, Li S. Error compensation during finger force production after one-and four-finger voluntarily fatiguing exercise. Exp Brain Res. 2007;181:461–468. doi: 10.1007/s00221-007-0942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Danion F, Scholz JF, Zatsiorsky VM, Schoner G. Approaches to analysis of handwriting as a task of coordinating a redundant motor system. Hum Mov Sci. 2003;22:153–171. doi: 10.1016/s0167-9457(02)00157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one-and two-hand force production at distal and proximal phalanges. Brain Res. 2002;924:198–208. doi: 10.1016/s0006-8993(01)03234-6. [DOI] [PubMed] [Google Scholar]
- Li S. Perception of individual finger forces during multi-finger force production tasks. Neurosci Lett. 2006;409:239–243. doi: 10.1016/j.neulet.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Characteristics of finger force production during one and two-hand tasks. Hum Mov Sci. 2000;19:897–924. [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Bilateral deficit and symmetry in finger force production during two-hand multifinger tasks. Exp Brain Res. 2001;141:530–540. doi: 10.1007/s002210100893. [DOI] [PubMed] [Google Scholar]
- Li S, Danion F, Zatsiorsky VM, Latash ML. Coupling phenomena during asynchronous submaximal two-hand, multi-finger force production tasks in humans. Neurosci Lett. 2002;331:75–78. doi: 10.1016/s0304-3940(02)00869-8. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Zatsiorsky VM. Finger interaction during multi-finger tasks involving finger addition and removal. Exp Brain Res. 2003;150:230–236. doi: 10.1007/s00221-003-1449-x. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Zatsiorsky VM. Effects of motor imagery on finger force responses to transcranial magnetic stimulation. Cogn Brain Res. 2004;20:273–280. doi: 10.1016/j.cogbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Li S, Leonard CT. The effect of enslaving on perception of finger forces. Exp Brain Res. 2006;172:301–309. doi: 10.1007/s00221-005-0332-3. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Newell KM, Zatsiorsky VM. Motor redundancy during maximal voluntary contraction in four-finger tasks. Exp Brain Res. 1998a;122:71–78. doi: 10.1007/s002210050492. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Exp Brain Res. 1998b;119:276–286. doi: 10.1007/s002210050343. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin Biomech (Bristol, Avon) 2000;15:203–211. doi: 10.1016/s0268-0033(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Nihashi T, Naganawa S, Sato C, Kawai H, Nakamura T, Fukatsu H, Ishigaki T, Aoki I. Contralateral and ipsilateral responses in primary somatosensory cortex following electrical median nerve stimulation--an fMRI study. Clin Neurophysiol. 2005;116:842. doi: 10.1016/j.clinph.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Park WH, Leonard CT, Li S. Perception of finger forces within the hand after index finger fatiguing exercise. Exp Brain Res. 2007;182:169–177. doi: 10.1007/s00221-007-0978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataky TC, Latash ML, Zatsiorsky VM. Prehension synergies during nonvertical grasping, I: experimental observations. Biol Cybern. 2004a;91:148–158. doi: 10.1007/s00422-004-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataky TC, Latash ML, Zatsiorsky VM. Prehension synergies during nonvertical grasping, II: Modeling and optimization. Biol Cybern. 2004b;91:231–242. doi: 10.1007/s00422-004-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- Simoes C, Alary F, Forss N, Hari R. Left-Hemisphere-Dominant SII Activation after Bilateral Median Nerve Stimulation. NeuroImage. 2002;15:686. doi: 10.1006/nimg.2001.1007. [DOI] [PubMed] [Google Scholar]
- Wegner K, Forss N, Salenius S. Characteristics of the human contra-versus ipsilateral SII cortex. Clin Neurophysiol. 2000;111:894. doi: 10.1016/s1388-2457(99)00319-3. [DOI] [PubMed] [Google Scholar]