Abstract
This study examined modulation of corticospinal excitability during both actual and imagined movements. Seven young healthy subjects performed actual (3% to 50% maximal voluntary contraction) and imagined index finger force production, and rest. Individual responses to focal transcranial magnetic stimulation (TMS) in four fingers (index, middle, ring, and little) were recorded for all three tested conditions. The force increments at the threshold of activation were derived from regression analysis, representing the TMS-induced response at the threshold activation of the corticospinal pathways. The increment in the index finger during motor imagery was larger than that at rest, but smaller than that at the threshold of activation. On the other hand, the increment in the uninstructed (middle, ring and little), slave fingers during motor imagery was larger than that at rest, but not different from that at the threshold of activation. These contrasting results suggest that the degree of imagery-induced enhancement in corticospinal excitability was significantly less than what could be predicted for threshold levels from regression analysis, but only for the index finger, and not the adjacent slave fingers. It is concluded that corticospinal excitability for the explicitly instructed index finger is specifically enhanced at subthreshold levels during motor imagery.
Keywords: motor imagery, transcranial magnetic stimulation (TMS), corticospinal excitability, finger
Introduction
Motor imagery describes a phenomenon of imagining an actual movement without executing it. As such, motor imagery corresponds to an active cognitive process during which the representation of a specific action is internally reproduced within working memory without any overt motor output, i.e., no discernible EMG activities (Decety and Grezes 1999). Accumulated evidence has demonstrated that motor imagery is subject to the same movement rules and constraints that physical movements follow, including Fitts’s laws (Decety and Jeannerod 1995), autonomic reactions (Decety et al. 1991), kinematic constraints (Sirigu et al. 1995), temporal properties (Decety et al. 1989; Sirigu et al. 1995), effects on motor performance (Yue and Cole 1992), the role in skill acquisition (Pascual-Leone et al. 1995) and in motor recovery after stroke (Page et al. 2001; Yoo et al. 2001; Stevens and Stoykov 2003; Malouin et al. 2004). These functional similarities between actual and imagined movements could be ascribed to, at least in part, shared common neural substrates along the neuroaxis, including primary motor cortex (M1) areas, as demonstrated by different brain mapping/imaging studies. Such techniques include functional magnetic resonance imaging (fMRI) (Porro et al. 1996; Roth et al. 1996; Lotze et al. 1999), positron emission tomography (PET) (Decety et al. 1994; Parsons et al. 1995; Stephan et al. 1995; Deiber et al. 1998), and electroencephalography (EEG) (Pfurtscheller and Neuper 1997; Pfurtscheller et al. 1999).
A recent series of studies (Jeannerod 1995; Yahagi et al. 1996; Decety 1996a; Kasai et al. 1997; Fadiga et al. 1999; Yahagi and Kasai 1999; Filippi et al. 2001; Facchini et al. 2002; Sparing et al. 2002; Patuzzo et al. 2003; Sohn et al. 2003; Stinear and Byblow 2003; Li et al. 2004) using transcranial magnetic stimulation (TMS) provide further evidence that motor imagery enhances corticospinal excitability. Enhanced corticospinal excitability is manifested by decreased motor threshold and facilitatory effects on motor-evoked potentials (MEPs) of the contralateral target muscles. The term “corticospinal excitability” refers to the excitability of all the structures/pathways involved in the generation of responses to TMS.
Due to the limitation of time resolution, brain imaging techniques may not be fine enough to detect dynamic changes of activation over periods less than 1 second in duration. The changes in the MEPs induced by focal TMS applications during motor imagery, on the other hand, do not provide information about origins of enhanced corticospinal excitability along the neuroaxis (Paus et al. 1997). Thus, it’s not clear whether or not an increase in the corticospinal excitability during motor imagery is simply a subsequence of an active cognitive process.
In general, there are two broadly different ways regarding the involvement of the motor system during motor imagery and its relation to those for actual movements of the same action. In the first, centers and motor pathways used for imagined movement could be separate from those used for actual movement, but they could be connected in programmable manner, i.e., non-specific involvement. In such an arrangement, imagery-associated facilitatory effects of corticospinal excitability could be similar to those non-specific facilitatory effects observed during other active cognitive processes, such as object/action observation (Fadiga et al. 1995; Baldissera et al. 2001; Grezes and Decety 2002) and speech listening (Fadiga et al. 2002). This non-specific involvement hypothesis may be plausible because of the existence of multiple motor representations in the motor cortex, especially for distal hand muscles. Stinear and Byblow’s findings support this hypothesis (Stinear and Byblow 2004). They found enhanced MEP amplitudes from both the instructed first dorsal interoseesus (FDI) and the uninstructed abductor pollicis brevis (APB) of the same side during motor imagery of phasic depression tasks with the index finger.
To the contrary, Facchini et al. (Facchini et al. 2002) showed facilitatory effect on the MEP from APB and no such effect in FDI during motor imagery of thumb abduction on the same side. Another study by Li et al. (Li et al. 2004) extended this finding and showed that subjects were able to distinguish one- vs. four-finger imagined force production tasks. Given the existence of multiple finger representations that are highly interconnected in the motor cortex (Schieber and Hibbard 1993), the ability to imagine individual finger movements suggests that neural centers and motor pathways are specifically activated during motor imagery. These studies lead to an alternative hypothesis of specific involvement (cf. (Li et al. 2004; Li et al. 2005)), i.e., centers and motor pathways used for imagined movement could be essentially the same as those used for actual movement, but could simply be facilitated at subthreshold levels.
This study aimed to test the hypothesis of specific involvement during motor imagery by investigating modulation of corticospinal excitability for the distal hand muscles. TMS-induced force increments in four fingers at rest, during imagined and submaximal voluntary force production of the index finger were obtained. The force increments at the threshold of activation were derived from regression analysis. The increment in the target index finger during motor imagery was larger than that at rest, but smaller than that at the threshold of activation. On the other hand, the increment in the uninstructed (middle, ring and little) fingers during motor imagery was larger than that at rest, but not different from that at the threshold of activation. These strikingly contrasting results suggest that the corticospinal excitability for the index finger is specifically enhanced at the subthreshold levels among those highly interconnected centers/pathways for the distal hand muscles, favoring the specific-involvement hyposthesis.
Methods
Subjects
Seven young and healthy males (27.1 ± 4.3 years) participated in the experiments. All of them were right-handed according to their preferential use of the right hand during writing and eating. All subjects gave informed consent according to the procedures approved by the Office for Regulatory Compliance of the Pennsylvania State University. Most subjects participated in an earlier study (Li et al. 2004).
Setting
Subjects placed their two upper limbs symmetrical with respect to the body midline on the table. Their upper arms were placed at approximately 45° of abduction in the frontal plane and 45° of flexion in the sagittal plane, elbow joints at approximately 135° of flexion. Bipolar electromyographic (EMG) recordings from the flexor digitorum superficialis (FDS) of the right forearm were obtained from pairs of disposable surface electrodes placed over the muscle belly. The diameter of each electrode was 1 cm, the distance between the centres of two electrodes within a pair was 3 cm. The EMG signals were amplified, high pass filtered at 10 Hz and low pass filtered at 500 Hz.
The right hand and fingers were positioned and stabilized into a suspension device for force measurement using four unidirectional piezoelectric force sensors (208A03, PCB Piezotronics, Inc., Depew, NY). A hand fixation device (Fig 2) was located at the bottom of the frame and used to stabilise the palm of the hand and to ensure a constant hand configuration throughout the experiment, i.e., approximately 20° of wrist extension and approximately 20° of flexion at the metacarpophalangeal joints. The middle of the distal phalanxes was placed against the rubber-coated loops. Each loop was connected in parallel to a force sensor via wire cables, suspended by swivel attachments from slots in the top plate of the suspension device. The left forearm and hand rested on the testing table at the same height as the right forearm.
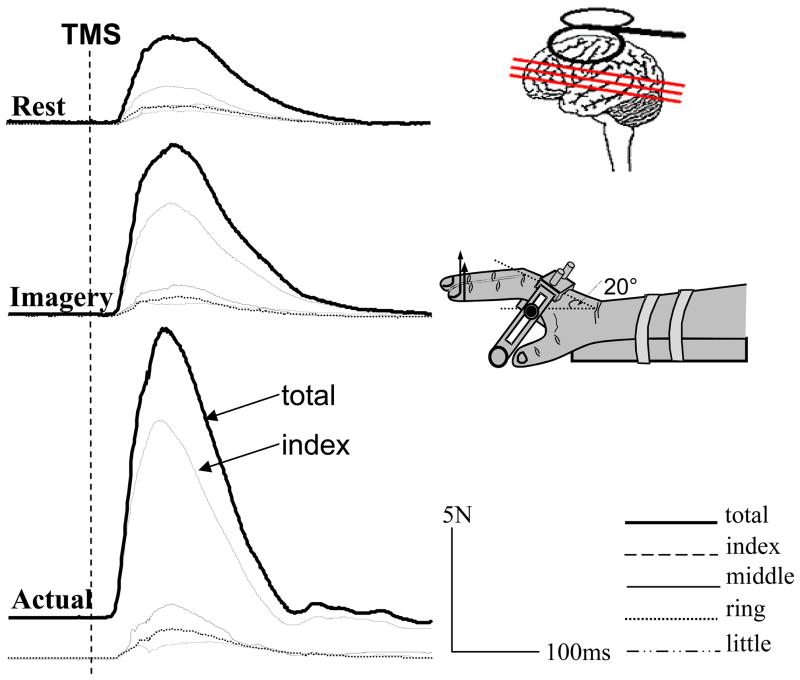
Figure 2.
Representative trials of individual and total forces during imagined (imagery) and actual finger force production and at rest when focal TMS was delivered on the contralateral motor cortex from one subject. Dashed vertical line: TMS application; actual: 3% MVC of index finger force.
The method and procedures of application of TMS was the same as previously described (cf. (Danion et al. 2003; Li et al. 2004)). Briefly, a tight elastic cap was placed on the subject’s head. A grid of 1×1 cm was marked on the left side of the scalp, with its centre positioned 2 cm to the left of Cz. To optimally activate the corticospinal pathways trans-synaptically (Brasil-Neto et al. 1992), the intersection of the coils of a figure-of-8-shaped stimulation coil (mean diameter of each wing 45 mm, MagStim Corp., UK) was placed tangentially to the center of the grid with the handle pointing backward and laterally at a 45° angle away from the midline. In search of an optimal position for TMS applications, the stimulus intensity was set at 60% of the stimulator output. The optimal position was defined where the largest increment in the total force of all fingers was evoked in three consecutive trials by moving the coil over the scalp in steps of 1 cm. The optimal position was then marked with a pen.
Keeping the coil at the optimal location, the intensity of the stimulation was slowly decreased until the motor threshold (MT) was found. The MT was defined as the lowest stimulus capable of evoking at least 3 of 6 motor evoked potentials (MEPs) with the amplitude of at least 50 μV. The MT was recorded for motor imagery and rest conditions separately. The coil position and orientation was ensured with double-sided adhesive tape. The coil position was stabilized by the experimenter as in the previous study(Li et al. 2004).
A Gateway 450 MHz computer was used for data acquisition and processing. All signals were sampled at 1000 Hz by a 16-bit A/D board using LabVIEW software (National Instruments, TX).
Procedures
At the beginning of the experiment, subjects were asked to produce maximal voluntary contractions (MVCs) at the fingertips using the index finger only. The highest peak value from three trials was considered as MVC for the index finger. Three experimental conditions were investigated:
Rest: stimulations were randomly delivered within 1–3 seconds after a trial began.
Motor imagery (Imagery): Subjects were asked to imagine pressing the index finger down isometrically as hard as possible after a verbal command and to sustain this condition until a TMS stimulus was delivered (unexpectedly, within 3 s). Then the subject was instructed to relax. Subjects were allowed to practice for a few minutes prior to testing in order to keep the EMG silent during motor imagery. EMG silence was defined as the absence of any background activity at the sensitivity of 25 μV per division(Facchini et al. 2002; Li et al. 2004). The employed high resolution of EMG/force sensing systems was capable of detecting deviation of EMG and force signals from the background levels due to the slightest movement of individual fingers. Such trials, if happened, were discarded by the experimenter to ensure motor imagery tasks purely imagined. It was confirmed during data analysis that there was no significant difference in baseline EMG and force signals between rest and motor imagery.
Voluntary force production by the index finger only: Subjects were explicitly instructed to produce forces using the index finger to match target forces. The target levels were indicated by a computer-generated red line on the screen, corresponding to 3, 6, 10, 20, 30, 40, 50% MVC. Subjects were also explicitly instructed not to produce forces with other fingers, but these fingers are not allowed to lift up. Stimulations were randomly delivered within 1–3 seconds after the desired target was reached.
The order of three conditions was randomized. Five trials were recorded for each condition. For all trials, the same stimulation was delivered at the stimulation intensity of 150% of the resting MT (rMT), on average 60.6% of the stimulator output. The interval between two consecutive trials was approximately 20 seconds.
Data analysis
Data processing techniques were similar to those described earlier (Danion et al. 2003; Li et al. 2004). Changes in individual and combined finger forces were used as the main indices to evaluate the effects of TMS, while EMG signals were mainly used to monitor the background activity during motor imagery tasks and to quantify changes in the corticospinal excitability.
TMS-induced force increment for a finger (ΔFi, i= I, M, R, L) was defined as the difference between its force at the time of peak force response of all fingers and its background force (Fi, i=I, M, R, L). Fi was the mean force from – 100 ms to the moment of TMS application (t0 = 0 ms). This parameter was calculated for the target index finger only (Δ Ftarget), and for other uninstructed fingers ΔFother= ΣΔFi (i = M, R, and L), respectively. The latency of TMS induced force responses was defined as the time interval between the application of the stimulation and the time when the total force exceeded 2 standard deviations (SD) of its background value. The background force was the weight of fingers (offset to zeros) at the beginning of a trial.
Regression analyses were performed to estimate the relation between the background force (F, ranging from 3% to 50%MVC) and ΔF during voluntary force production. Thus, the regression equation reflects ΔF as a function of F for voluntary force production. When F equals to zero, a predicted ΔF(ΔFpredicted) can be calculated. ΔFpredicted was then used to compare with ΔF in other experimental conditions (motor imagery, rest) where the background force was zero. The potential mechanism for ΔFpredicted will be discussed later.
The EMG signal was rectified and low-pass filtered at 100 Hz using a second-order, zero-lag Butterworth filter. The baseline EMG (EMGbaseline) was defined as the mean rectified, filtered EMG calculated from – 100 ms to t0. The EMGbaseline size was expressed in arbitrary units (AU). The MEP latency was computed as the time that took the baseline EMG to increase by 2 SDs. Both the force and EMG indices were averaged across 5 trials for each condition.
Statistics
The descriptive statistics was used. Paired Student’s t-Tests were also used to compare motor imagery and rest conditions. Repeated-measures ANOVAs were used with factors CONDITION (3 levels, rest, imagery, and predicted), FORCE (7 levels, 3, 6, 10, 20, 30, 40, 50%MVC), and FINGER (3 levels, M, R, L). Whenever necessary, post-hoc Tukey’s honest significant difference tests were used to compare the various levels of the factors.
Results
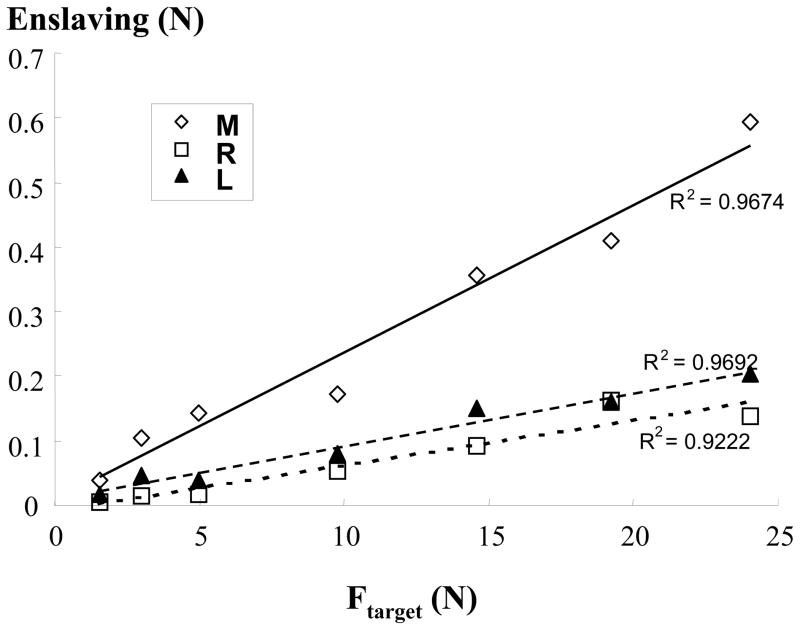
Subjects were able to match the desired target level of corresponding MVCs from 3% to 50% (R2=0.99, linear regression) with the index finger. The increase in the level of force production was paralleled by increased EMG activities recorded from the FDS (R2=0.97, linear regression). The uninstructed slave fingers also produced forces, i.e., enslaving. The enslaving increased with the force produced by the index finger in a linear fashion (Fig 1; R2, 0.97, 0.92, 0.97 for M, R, L, respectively). A 3×7 two-way ANOVA (FINGER × FORCE) showed a significant factor of FORCE (F[6,48] = 10.59, p<0.001). The analysis also showed a significant interaction FINGER × FORCE (F[12, 96] = 2.67, p=0.003). Turkey’s post-hoc analysis revealed that enslaving in the middle finger was significantly larger than in the ring and little fingers, particularly at a relatively high level of force production by the master finger.
Figure 1.
Enslaving during voluntary force production. Enslaving, accompanied force production in the uninstructed fingers, increases with the target finger force (Ftarget) in a linear fashion. M: middle; R: ring; L: little fingers; R2: coefficient of regression.
A burst of EMG activity (MEPs) and ensuing increments in finger forces were observed following a single TMS stimulation. The motor threshold (MT) was lower during motor imagery (36.4%) than at rest (40.4%) (paired t-test, p=0.003). The EMG latency for FDS was, on average, 17.9ms, ranging from 14.9ms to 18.5ms. No difference was found between rest and imagery conditions. The force response started at a latency of 27.6ms, ranging from 25.7ms to 33ms. The present study focused on the magnitude of force responses in individual fingers across different conditions, i.e., rest, imagined and voluntary force production.
A single TMS stimulation generated a larger magnitude of force response (ΔF) during motor imagery than at rest (Fig 2). At rest, averaged across conditions and all subjects, ΔFtarget was 0.68 N, and ΔFother was 1.25 N, while ΔFtarget was 1.82 N, and ΔFother was 1.96 N during motor imagery. Paired Student’s t-tests showed that the difference between ΔFtarget was on the boundary of significance (p=0.051). In contrast, the difference between ΔFother was statistically significant (p=0.012).
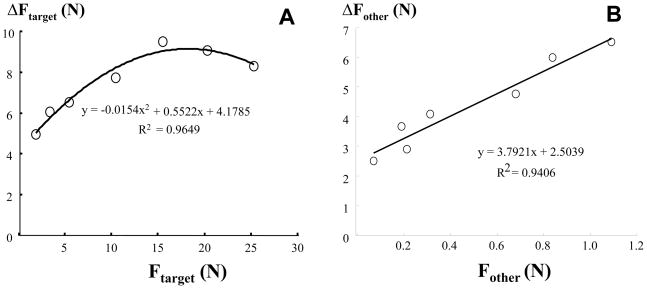
During voluntary force production, TMS-induced force increment (ΔF) increased with the background force for both index and slave fingers. For the index finger, a second-order regression analysis was performed for each subject, according to a previous study(Danion et al. 2003). ΔFtarget showed an inverted-U dependence on the background force of the index finger (R2=0.97, Fig 3A), with a peak increment around 35%MVC. One-way ANOVA showed a main effect of FORCE on ΔFtarget (F[6,48]=13.73, p<0.001). The combined TMS-induced force increment (ΔFother) showed a linear dependence on the combined background force of these fingers (R2=0.94, Fig 3B). One-way ANOVA revealed a main effect of FORCE (F[6,48]=3.48, p=0.007). Both second-order regression (R2: 0.64 – 0.98) and linear regression (R2: 0.60 – 0.93) showed a good fit to the data in all subjects.
Figure 3.
TMS-induce force increment (ΔF) as a function of background force (F) in the target finger (A) and other uninstructed slave fingers (B). I: index; M: middle; R: ring; L: little fingers. Regression equations and R2 are shown.
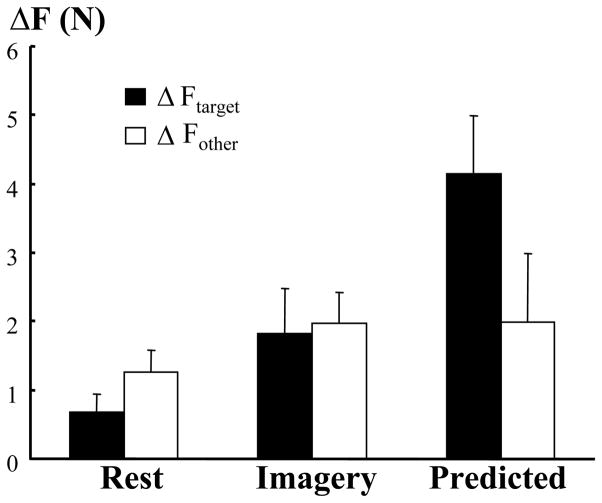
In an attempt to estimate the TMS-induced force increment when voluntary force production of a finger was zero, a predicted value (ΔFpredicted) was obtained from regression equations for the index and slave fingers separately. When compared to ΔF during motor imagery and at rest, ΔFpredicted showed a unique characteristic (Fig 4). On average, ΔFpredicted for the index finger (4.14 N) was significantly larger than ΔFtarget during motor imagery (1.82 N) or at rest (0.68 N), according to one-way ANOVA (CONDITION) (F[2,20]=7.77, p=0.004). In contrast, ΔFpredicted for the slave fingers (M, R, L) (1.99 N) was not different from ΔFother during motor imagery (1.96 N) or ΔFother at rest (1.25 N).
Figure 4.
TMS-induced force increment in the target (ΔFtarget) and other (ΔFpredicted) slave fingers. Imagery-induced increment was significantly less the predicted magnitude for the target finger, not for the other slave fingers. Standard errors are shown. Imagery: motor imagery of index finger force production.
Discussion
Most findings were consistent with previous reports, including 1) enslaving increased with the force of target finger during voluntary force production (Slobounov et al. 2002; Danion et al. 2003); 2) The TMS-induced force increment showed an inverted U-shaped dependence on the background force (from 3% to 50%) during voluntary force production by the index finger(Danion et al. 2003). Danion et al.(Danion et al. 2003) reported the same pattern with the maximal increment for the index finger at its 35% MVC. A linear relation between the increment and the background force for the slave fingers could be viewed as the rising phase on the inverted U relation, due to forces of these fingers at low levels. 3) As compared to rest, motor imagery decreased the motor threshold for cortical stimulation, increased force increment in both target and slave fingers (Li et al. 2004).
Before further discussion on the novel findings of this study, one needs to accept the main assumption that the predicted force increment (ΔFpredicted) reflects mainly force increment to TMS when the cortiocospinal structures/pathways responsible for force production of a finger are fully depolarized to the threshold of activation, but not discharging. Thus, no smallest EMG or force signals could be discerned. The assumption is based on the following facts. First, there exists a period of rising excitability of the motor system from rest to the threshold of activation (EMG onset). For example, the excitability of the corticospinal pathways increases gradually from about 100 ms prior to the EMG onset during simple reaction time movements. This gradual rise in the corticospinal excitability is concomitantly evidenced by parallel enhancements in MEPs of the target muscle (Pascual-Leone et al. 1992; Chen and Hallett 1999). Secondly, enhanced excitability during this period occurs along the neuroaxis, including the spinal level (Eichenberger and Ruegg 1984). Thirdly, motor imagery enhances the corticospinal excitability, including the spinal levels(Li et al. 2004). Given that TMS activates the corticospinal pathways trans-synaptically (Brasil-Neto et al. 1992), inducing responses along the neuroaxis (Paus et al. 1997), it is conceivable that, when the corticospinal pathways/structures are depolarized to the threshold of activation, the TMS-induced response could be estimated from regression equations derived from voluntary contraction in which motorneurons are already suprathreshold. Mathematically, the value of this response (ΔFpredict) is estimated by the intercept from the equations in Figure 3, representing the TMS-induced response at the threshold activation of the corticospinal pathways. ΔFpredict provides a useful reference for objective assessment of the corticospinal excitability during motor imagery. The limitation of the assumption is that the predicted value may not be a reflection of the true response at the threshold of activation. Such response, unfortunately, is unlikely to be examined.
Using this novel approach, the increment in the instructed index finger (ΔFtarget) during motor imagery was found to be larger than that at rest, but smaller than that at the threshold of activation. These results could be interpreted as the corticospinal excitability for the instructed finger during motor imagery was between rest and the threshold of activation, i.e., subthreshold enhancement.
The increment in the uninstructed (middle, ring and little), slave fingers (ΔFother) during motor imagery was significantly larger than that at rest. The increment in slave fingers could be ascribed to the complex and unique organization of the human hand in M1. Each finger has multiple representations in M1 that are highly interconnected (Schieber and Hibbard 1993). A single-finger movement could lead to activation of representations distributed throughout the M1 hand area (Porter and Lemon 1993; Schieber 1999). Due to the diverging projections from finger representations, activation of one finger representation could project to adjacent fingers (Schieber and Hibbard 1993; Schieber 2001), namely, enslaving effect (Danion et al. 2000; Li et al. 2000; Li et al. 2001; Latash et al. 2002). The results of significant enhancement in the force increment in both instructed and non-instructed fingers (cf (Stinear and Byblow 2004)) could suggest that motor imagery enhanced the corticospinal excitability non-specifically for both instructed and uninstructed fingers at the subthreshold levels, thus favouring the non-specific involvement hypothesis.
This hypothesis, however, was not supported by other findings. Although imagined index finger movements resulted in enhanced excitability of the corcitospinal pathways for the index finger and slave fingers, the degree of enhancement was significantly less than what could be predicted for threshold levels from regression analysis, but only for the target index finger, and not the adjacent slave fingers. Note that force increments in the uninstructed slave fingers, a phenomenon of enslaving, result from diverging out from enhance corticospinal excitability of the instructed fingers. Li et al (Li et al. 2004) compared the combined force increment in the middle, ring and little fingers (ΔFMRL) as slave fingers during motor imagery of the index finger and instructed fingers during motor imagery of four fingers. They reported that ΔFMRL was significantly larger during four-finger tasks when acting as target fingers. ΔFMRL (ΔFother in the present study) was not significant differently from the predicted value when the corticospinal excitability of the instructed index finger resides at the threshold level. The contrasting results that imagery-induced enhancement was significantly less than the predicted value for the index, but not for other slave fingers, therefore, argue for movement-specific modulation of the corticospinal excitability for the target finger and less specific for the slave fingers. These results are in agreement with earlier findings (Facchini et al. 2002; Li et al. 2004) that subjects were able to specifically modulate the excitability for the instructed distal hand muscle during motor imagery.
To conclude, the present study adopts a new approach to examine modulation of corticospnal excitability during motor imagery. The obtained results support the newly-proposed hypothesis that motor imagery induces movement-specific subthreshold enhancement of the motor system.
Acknowledgments
The author thanks Professor Mark Latash for his comments on an earlier version of the manuscript. This study was in part supported by an NIH grant (1R15NS053442-01A1).
References
- Baldissera F, Cavallari P, Craighero L, Fadiga L. Modulation of spinal excitability during observation of hand actions in humans. Eur J Neurosci. 2001;13:190–194. doi: 10.1046/j.0953-816x.2000.01368.x. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Danion F, Latash M, Li S. Finger interactions studied with transcranial magnetic stimulation during multi-finger force production tasks. Clinical Neurophysiology. 2003;114:1445–1455. doi: 10.1016/s1388-2457(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of fatigue on multifinger co-ordination in force production tasks in humans. J Physiol. 2000;523:523–532. doi: 10.1111/j.1469-7793.2000.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996a;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J. Neural mechanisms subserving the perception of human actions. Trends in Cogn Sci. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M. Mentally simulated movements in virtual reality: does Fitts’s law hold in motor imagery? Behav Brain Res. 1995;72:127–134. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M, Germain M, Pastene J. Vegetative response during imagined movement is proportional to mental effort. Behav Brain Res. 1991;42:1–5. doi: 10.1016/s0166-4328(05)80033-6. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M, Prablanc C. The timing of mentally represented actions. Behav Brain Res. 1989;34:35–42. doi: 10.1016/s0166-4328(89)80088-9. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F. Mapping motor representations with positron emission tomography. Nature. 1994;371:600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage. 1998;7:73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- Eichenberger A, Ruegg DG. Relation between the specific H reflex facilitation preceding a voluntary movement and movement parameters in man. J Physiol. 1984;347:545–559. doi: 10.1113/jphysiol.1984.sp015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand. 2002;105:146–151. doi: 10.1034/j.1600-0404.2002.1o004.x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci. 2002;15:399–402. doi: 10.1046/j.0953-816x.2001.01874.x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Filippi MM, Oliveri M, Pasqualetti P, Cicinelli P, Traversa R, Vernieri F, et al. Effects of motor imagery on motor cortical output topography in Parkinson’s disease. Neurology. 2001;57:55–61. doi: 10.1212/wnl.57.1.55. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33:1419–1432. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawai S, Kawanishi M, Yahagi S. Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res. 1997;744:147–150. doi: 10.1016/s0006-8993(96)01101-8. [DOI] [PubMed] [Google Scholar]
- Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one- and two-hand force production at distal and proximal phalanges. Brain Res. 2002;924:198–208. doi: 10.1016/s0006-8993(01)03234-6. [DOI] [PubMed] [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Characteristics of finger force production during one and two-hand tasks. Hum Mov Sci. 2000;19:897–924. [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Bilateral deficit and symmetry in finger force production during two- hand multifinger tasks. Exp Brain Res. 2001;141:530–540. doi: 10.1007/s002210100893. [DOI] [PubMed] [Google Scholar]
- Li S, Kamper DG, Stevens JA, Rymer WZ. The effect of motor imagery on spinal segmental excitability. J Neurosci. 2004;24:9674–9680. doi: 10.1523/JNEUROSCI.2781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Latash ML, Zatsiorsky VM. Effects of motor imagery on finger force responses to transcranial magnetic stimulation. Cog Brain Res. 2004;20:273–280. doi: 10.1016/j.cogbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Li S, Stevens JA, Kamper DG, Rymer WZ. The movement-specific effect of motor imagery on the premotor time. Motor Control. 2005;9:119–128. doi: 10.1123/mcj.9.2.119. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Malouin F, Belleville S, Richards CL, Desrosiers J, Doyon J. Working memory and mental practice outcomes after stroke. Arch Phys Med Rehabil. 2004;85:177–183. doi: 10.1016/s0003-9993(03)00771-8. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Sisto S, Johnston MV. A randomized efficacy and feasibility study of imagery in acute stroke. Clin Rehabil. 2001;15:233–240. doi: 10.1191/026921501672063235. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115:1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia. 2003;41:1272–1278. doi: 10.1016/s0028-3932(02)00293-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett. 1997;239:65–68. doi: 10.1016/s0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Ramoser H, Muller-Gerking J. Visually guided motor imagery activates sensorimotor areas in humans. Neurosci Lett. 1999;269:153–156. doi: 10.1016/s0304-3940(99)00452-8. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford: Clarendon; 1993. [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, et al. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–1284. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Somatotopic gradients in the distributed organization of the human primary motor cortex hand area: evidence from small infarcts. Exp Brain Res. 1999;128:139–148. doi: 10.1007/s002210050829. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on Somatotopic Organization in the Primary Motor Cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. 1993;261:489–492. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y, Pierrot-Deseilligny C. Congruent unilateral impairments for real and imagined hand movements. Neuroreport. 1995;6:997–1001. doi: 10.1097/00001756-199505090-00012. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Johnston J, Chiang H, Ray W. The role of sub-maximal force production in the enslaving phenomenon. Brain Res. 2002;954:212–219. doi: 10.1016/s0006-8993(02)03288-2. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Dang N, Hallett M. Suppression of corticospinal excitability during negative motor imagery. J Neurophysiol. 2003;90:2303–2309. doi: 10.1152/jn.00206.2003. [DOI] [PubMed] [Google Scholar]
- Sparing R, Mottaghy FM, Ganis G, Thompson WL, Topper R, Kosslyn SM, Pascual-Leone A. Visual cortex excitability increases during visual mental imagery--a TMS study in healthy human subjects. Brain Res. 2002;938:92–97. doi: 10.1016/s0006-8993(02)02478-2. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil. 2003;84:1090–1092. doi: 10.1016/s0003-9993(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Motor imagery of phasic thumb abduction temporally and spatially modulates corticospinal excitability. Clin Neurophysiol. 2003;114:909–914. doi: 10.1016/s1388-2457(02)00373-5. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp Brain Res. 2004;157:351–358. doi: 10.1007/s00221-004-1851-z. [DOI] [PubMed] [Google Scholar]
- Yahagi S, Kasai T. Motor evoked potentials induced by motor imagery reveal a functional asymmetry of cortical motor control in left- and right-handed human subjects. Neurosci Lett. 1999;276:185–188. doi: 10.1016/s0304-3940(99)00823-x. [DOI] [PubMed] [Google Scholar]
- Yahagi S, Shimura K, Kasai T. An increase in cortical excitability with no change in spinal excitability during motor imagery. Percept Mot Skills. 1996;83:288–290. doi: 10.2466/pms.1996.83.1.288. [DOI] [PubMed] [Google Scholar]
- Yoo E, Park E, Chung B. Mental practice effect on line-tracing accuracy in persons with hemiparetic stroke: a preliminary study. Arch Phys Med Rehabil. 2001;82:1213–1218. doi: 10.1053/apmr.2001.25095. [DOI] [PubMed] [Google Scholar]
- Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol. 1992;67:1114–1123. doi: 10.1152/jn.1992.67.5.1114. [DOI] [PubMed] [Google Scholar]