Abstract
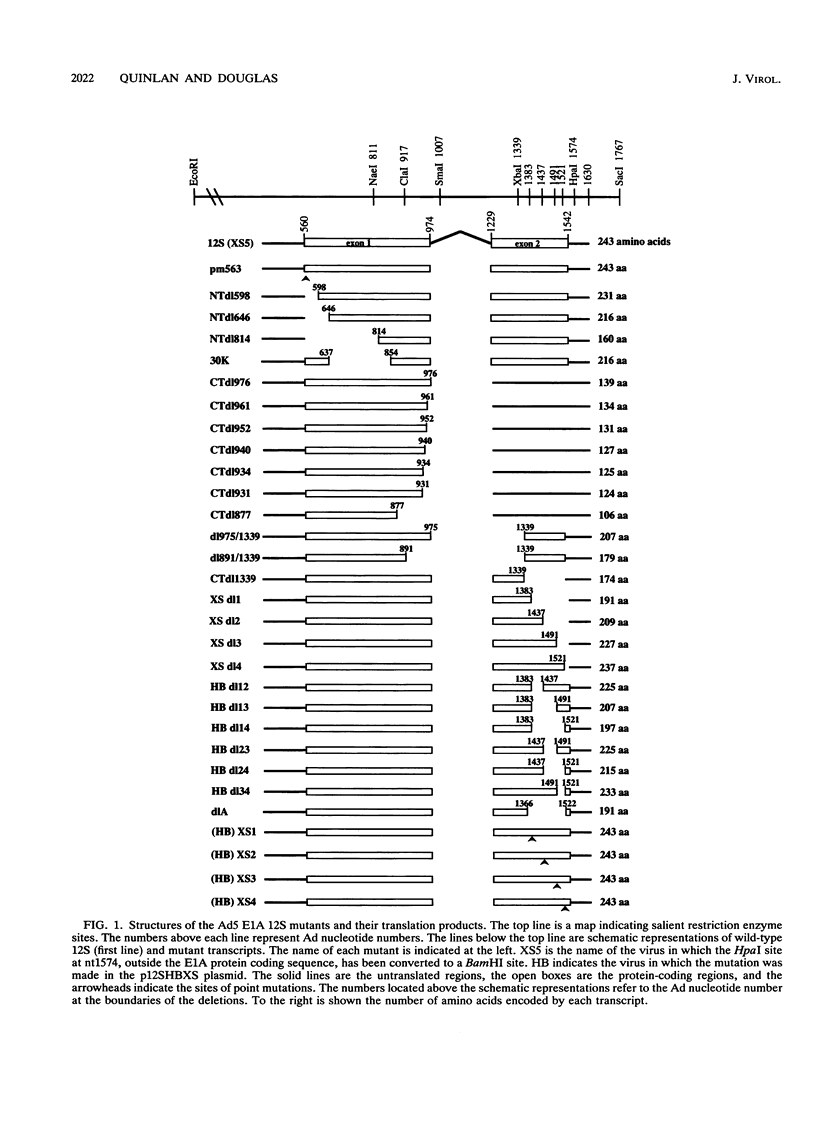
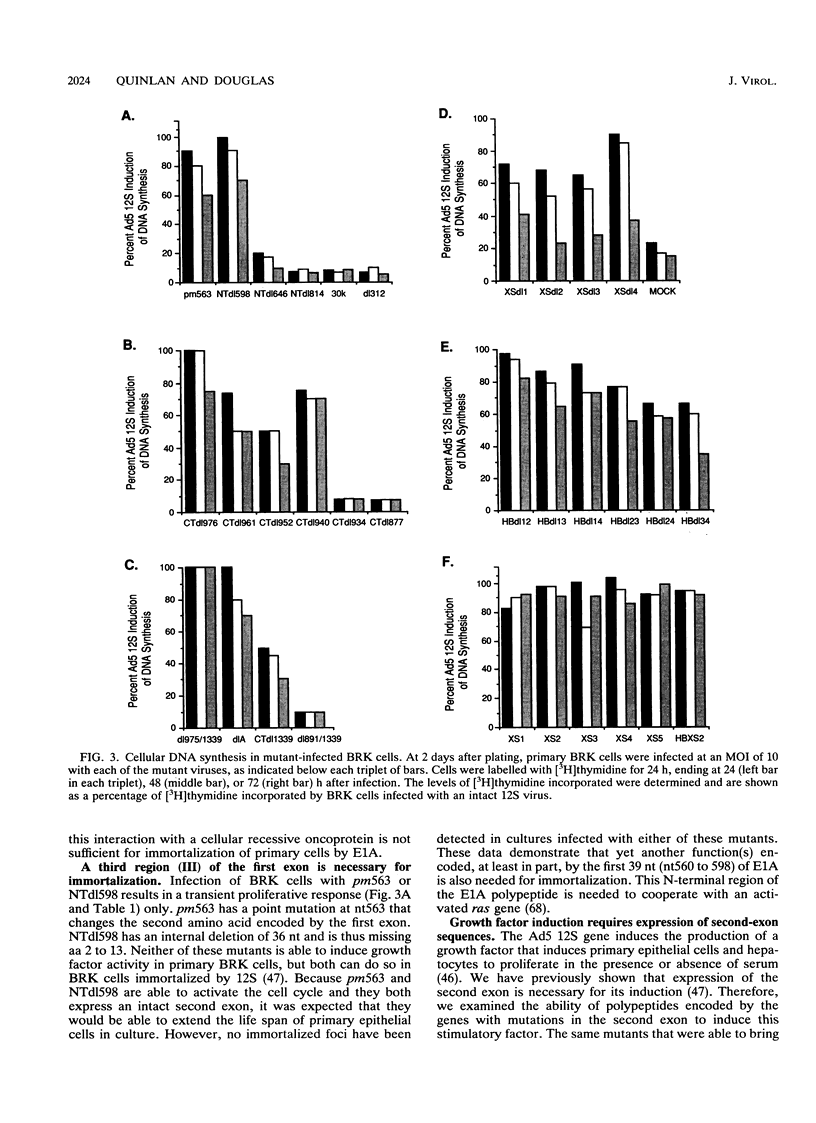
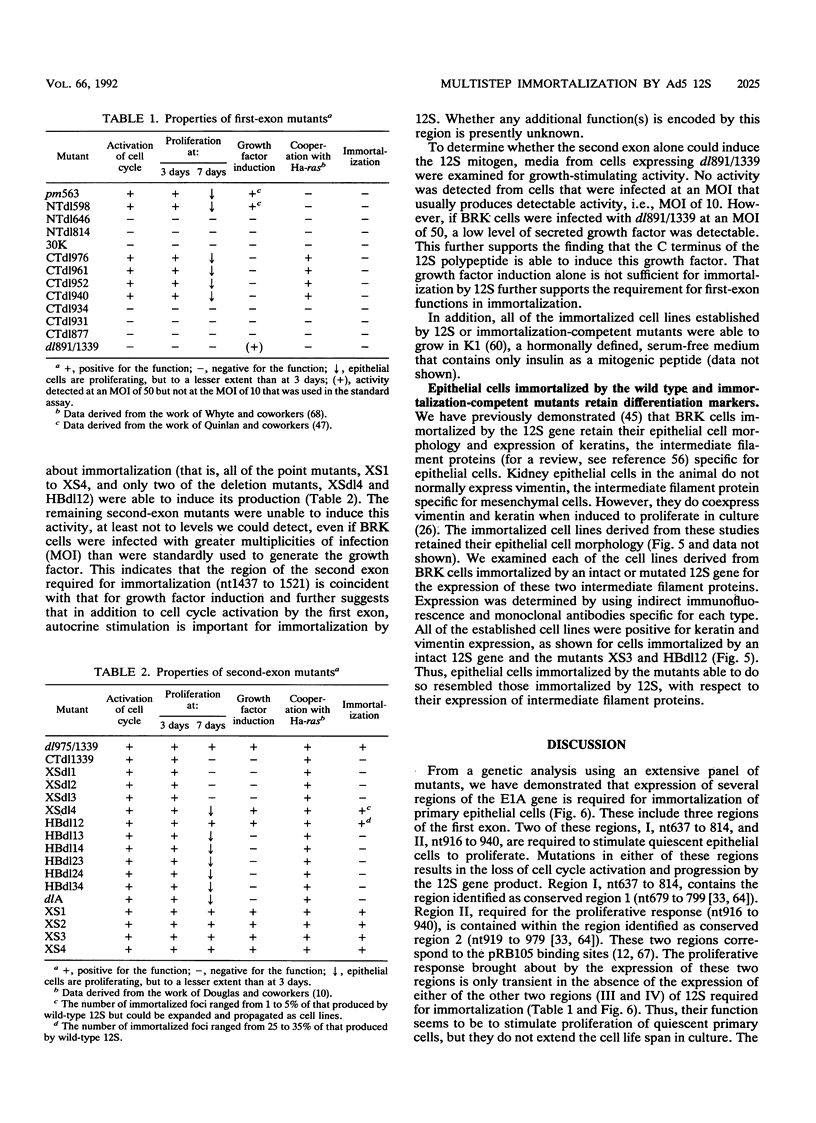
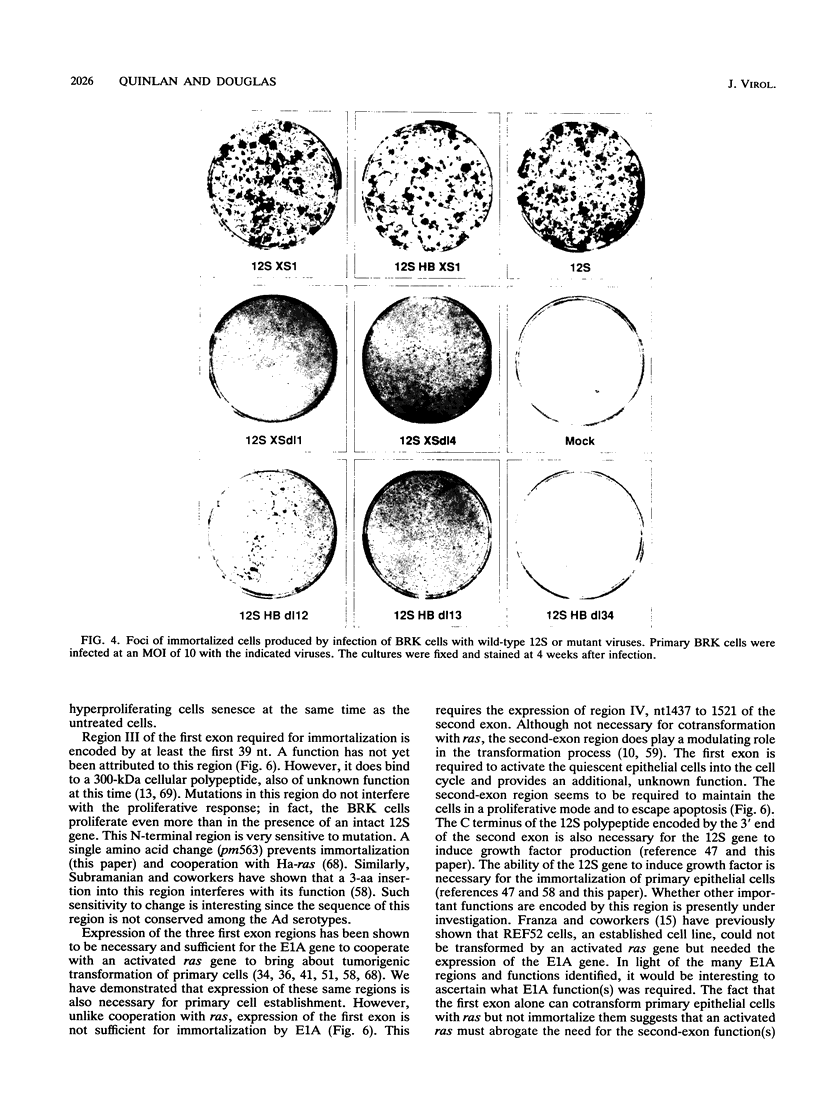
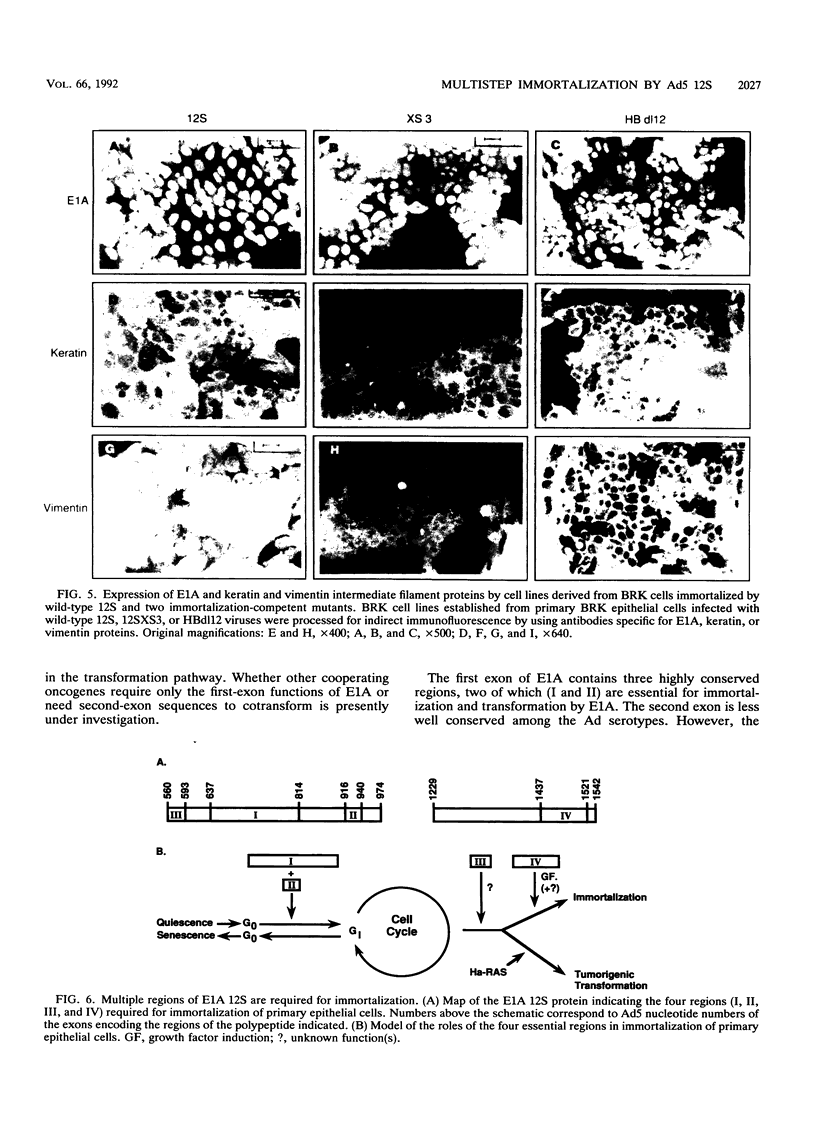
Immortalization of primary cells is a multistep process. The adenovirus E1A 12S gene product is a member of the class of oncoproteins that have the ability to establish primary cells as cell lines in culture. It is encoded by two exons. Extensive mutational analysis demonstrates that four regions of the E1A 12S gene, encoded by both exons, are necessary for immortalization of primary epithelial cells. Expression of two regions is necessary to activate quiescent cells into the cell cycle but is unable to extend the life span of these cells in culture and thus cannot immortalize them. These regions are encoded by the first exon. A third first-exon region, for which no function has yet been identified, is also required. These three regions are also required for 12S to cooperate with an activated ras gene to bring about tumorigenic transformation. The fourth region is required to maintain the cells in a proliferative mode, extend their life span in culture, and induce an autocrine growth factor. These functions are encoded by the second exon. The cells immortalized by wild-type 12S and immortalization-competent mutants retain their epithelial morphology and expression of keratin and vimentin intermediate filament proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks L., Barnett S. C., Crook T. HPV-16 E7 functions at the G1 to S phase transition in the cell cycle. Oncogene. 1990 Jun;5(6):833–837. [PubMed] [Google Scholar]
- Bellett A. J., Jackson P., David E. T., Bennett E. J., Cronin B. Functions of the two adenovirus early E1A proteins and their conserved domains in cell cycle alteration, actin reorganization, and gene activation in rat cells. J Virol. 1989 Jan;63(1):303–310. doi: 10.1128/jvi.63.1.303-310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellett A. J., Li P., David E. T., Mackey E. J., Braithwaite A. W., Cutt J. R. Control functions of adenovirus transformation region E1A gene products in rat and human cells. Mol Cell Biol. 1985 Aug;5(8):1933–1939. doi: 10.1128/mcb.5.8.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Bols B. L., Naaktgeboren J. M., Simons J. W. Immortalization of Syrian hamster embryo cells is in itself a multistep event. Cancer Res. 1991 Feb 15;51(4):1177–1184. [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite A. W., Nelson C. C., Bellett A. J. E1a revisited: the case for multiple cooperative trans-activation domains. New Biol. 1991 Jan;3(1):18–26. [PubMed] [Google Scholar]
- Cerni C., Binétruy B., Schiller J. T., Lowy D. R., Meneguzzi G., Cuzin F. Successive steps in the process of immortalization identified by transfer of separate bovine papillomavirus genes into rat fibroblasts. Proc Natl Acad Sci U S A. 1989 May;86(9):3266–3270. doi: 10.1073/pnas.86.9.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A., de Villiers J., Kamen R. Immortalization of rat embryo fibroblasts by mutant polyomavirus large T antigens deficient in DNA binding. Mol Cell Biol. 1986 Dec;6(12):4344–4352. doi: 10.1128/mcb.6.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. L., Gopalakrishnan S., Quinlan M. P. Modulation of transformation of primary epithelial cells by the second exon of the Ad5 E1A12S gene. Oncogene. 1991 Nov;6(11):2093–2103. [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Egan C., Bayley S. T., Branton P. E. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene. 1989 Mar;4(3):383–388. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard C., Galiana E., Rouget P. Establishment of 'normal' nervous cell lines after transfer of polyoma virus and adenovirus early genes into murine brain cells. EMBO J. 1986 Dec 1;5(12):3157–3162. doi: 10.1002/j.1460-2075.1986.tb04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Grossman S. R., Mora R., Laimins L. A. Intracellular localization and DNA-binding properties of human papillomavirus type 18 E6 protein expressed with a baculovirus vector. J Virol. 1989 Jan;63(1):366–374. doi: 10.1128/jvi.63.1.366-374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger P. B., Chen Q., Dong L. Q., Stevens J. L. Alterations in intermediate filament proteins in rat kidney proximal tubule epithelial cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1316–1322. doi: 10.1016/s0006-291x(88)81018-0. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz D. R., Chinnadurai G. Evidence that a second tumor antigen coded by adenovirus early gene region E1a is required for efficient cell transformation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):163–167. doi: 10.1073/pnas.82.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Ferguson B., Rosenberg M., Baserga R. Induction of cellular DNA synthesis by purified adenovirus E1A proteins. Virology. 1986 Jul 15;152(1):1–10. doi: 10.1016/0042-6822(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy M. N., Chinnadurai G. Relationship between the transforming and transcriptional regulatory functions of adenovirus 2 E1a oncogene. Virology. 1987 Jul;159(1):31–38. doi: 10.1016/0042-6822(87)90344-8. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi J. J., Prives C. Binding of p53 and p105-RB is not sufficient for oncogenic transformation by a hybrid polyomavirus-simian virus 40 large T antigen. J Virol. 1990 Nov;64(11):5250–5259. doi: 10.1128/jvi.64.11.5250-5259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Grodzicker T. Adenovirus E1A 12S protein induces DNA synthesis and proliferation in primary epithelial cells in both the presence and absence of serum. J Virol. 1987 Mar;61(3):673–682. doi: 10.1128/jvi.61.3.673-682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Sullivan N., Grodzicker T. Growth factor(s) produced during infection with an adenovirus variant stimulates proliferation of nonestablished epithelial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3283–3287. doi: 10.1073/pnas.84.10.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P. The Ad5 12S growth factor induces F9 cell proliferation and differentiation. Oncogene. 1989 Aug;4(8):1051–1055. [PubMed] [Google Scholar]
- Quinlan M. P., Whyte P., Grodzicker T. Growth factor induction by the adenovirus type 5 E1A 12S protein is required for immortalization of primary epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3191–3203. doi: 10.1128/mcb.8.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. A., Pusztai R., Green M. Chemical synthesis of human papillomavirus type 16 E7 oncoprotein: autonomous protein domains for induction of cellular DNA synthesis and for trans activation. J Virol. 1990 Dec;64(12):6121–6129. doi: 10.1128/jvi.64.12.6121-6129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H., Yamashita T. Induction of DNA synthesis by adenoviruses in contact-inhibited hamster cells. Virology. 1968 Nov;36(3):422–433. doi: 10.1016/0042-6822(68)90167-0. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Eng C. Y., Berk A. J. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985 Mar;53(3):742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987 Jul;6(7):2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Nasr R. J., Chinnadurai G. An N-terminal region of adenovirus E1a essential for cell transformation and induction of an epithelial cell growth factor. Oncogene. 1988 Feb;2(2):105–112. [PubMed] [Google Scholar]
- Subramanian T., La Regina M., Chinnadurai G. Enhanced ras oncogene mediated cell transformation and tumorigenesis by adenovirus 2 mutants lacking the C-terminal region of E1a protein. Oncogene. 1989 Apr;4(4):415–420. [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfendahl P. J., Linder S., Kreivi J. P., Nordqvist K., Sevensson C., Hultberg H., Akusjärvi G. A novel adenovirus-2 E1A mRNA encoding a protein with transcription activation properties. EMBO J. 1987 Jul;6(7):2037–2044. doi: 10.1002/j.1460-2075.1987.tb02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Sato H., Furuno A., Yoshiike K. Mutational analysis of human papillomavirus type 16 E7 functions. J Virol. 1990 Jan;64(1):207–214. doi: 10.1128/jvi.64.1.207-214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J. L., Byrd P. J., Grand R. J., Gallimore P. H. Isolation and characterization of four adenovirus type 12-transformed human embryo kidney cell lines. Mol Cell Biol. 1984 Jan;4(1):110–116. doi: 10.1128/mcb.4.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., Dijkema R. Comparison of nucleotide sequences of the early E1a regions for subgroups A, B and C of human adenoviruses. Gene. 1980 Dec;12(1-2):63–76. doi: 10.1016/0378-1119(80)90016-5. [DOI] [PubMed] [Google Scholar]
- van den Elsen P. J., Houweling A., van der Eb A. J. Morphological transformation of human adenoviruses is determined to a large extent by gene products of region E1a. Virology. 1983 Nov;131(1):242–246. doi: 10.1016/0042-6822(83)90549-4. [DOI] [PubMed] [Google Scholar]