Abstract
Substituted trityl radicals are important spin probes for functional electron paramagnetic resonance spectroscopy and imaging including oxygen and pH mapping in vivo. Here we report the synthetic procedure for large scale synthesis of deuterated Finland Trityl radical with superior EPR spectral properties and higher sensitivity towards oxygen concentrations in solution. Additionally Finland Trityl radicals substituted with linkers suitable for attaching peptide, or other synthetic precursors have been synthesized. The effect of deutero-substitution on EPR spectra of homologous derivatives has been evaluated. The compounds are potential candidates for targeted spin probes in EPR Imaging.
Triarylmethyl radicals, TAMs, and nitroxyl radicals, NRs, represent two main classes of soluble paramagnetic materials used for EPR spectroscopy and imaging applications. TAMs have advantages over NRs in extraordinary stability toward tissue redox processes, longer relaxation time and narrower line width making them particularly attractive for imaging applications1. However, undeveloped chemistry of the TAM radicals limits the number of available structures and their functional applications.
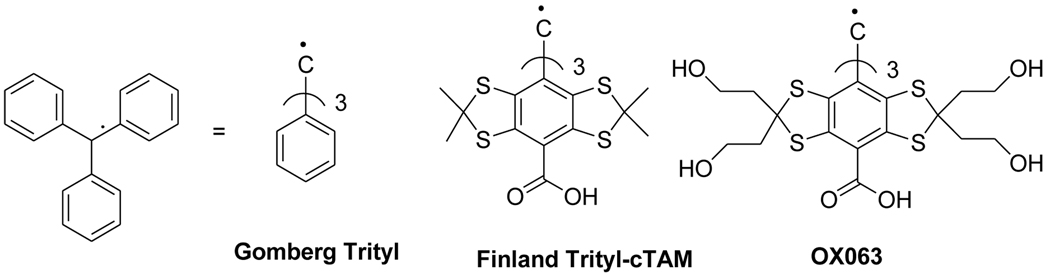
Triphenylmethyl radical was the first organic free radical synthesized by Gomberg more than hundred years ago2. Nevertheless, only recently the compounds with sterically protected trivalent carbon regained attention as the basic structural fragment for the synthesis of stable organic radicals. By the late 90s, Nycomed Innovation AB refined Gomberg’s original trityl radical in order to avoid hydrogen hyperfine coupling and enhance its stability and water solubility1, 3. A new family of trityl spin probes, tetrathiatriarylmethyls, bearing four sulfur atoms on the phenyl ring was developed. The most representative members are TAM derivatives containing carboxyl group, namely cTAM, deuterated cTAM and more hydrophilic Oxo63 derivative (see Scheme 1).
Scheme 1.
Representative structures of trityl radicals.
The EPR spectra of these TAM derivatives1, 4, 5 display a very narrow single line which is generally not broadened by interaction with proteins and other biological molecules, making them particularly attractive for imaging applications using EPR imaging, EPRI, and proton electron double resonance imaging, PEDRI (also known as Overhouser magnetic resonance imaging, OMRI)6, 7. In the latter case, the long relaxation time of TAMs makes them easily saturatable by radio frequency irradiation, and provides an advantage over NR for PEDRI applications by allowing enhancement of sensitivity and resolution with less heating of the sample1, 8, 9. Moreover, TAM radicals, due to their long relaxation time, are the superior probes for pulsed EPR/EPRI. Applications of TAM radicals include EPR oximetry1, 8, 9, recently reported sensitivity to the superoxide radical anion10, 11 and pH12, 13 and their use as hyperpolarizing agents in dynamic nuclear polarization (DNP)-enhanced NMR14 and MRI15.
The extraordinary stability in vivo, very narrow single EPR line of about 100 mG or less, and oxygen-induced line broadening make TAMs the most efficient soluble oxygen probes. Oxygen-induced broadening of the TAMs in water is about (300–500) mG/mM of oxygen1, 13 similar to that for the NRs. On the other hand, the concentration broadening of the TAMs is about 10–30 mG/mM1 which is one order of magnitude less than that for the NRs16. These properties make TAM radicals superior oximetric probes for in vivo EPR, EPRI and PEDRI applications 1, 8.
The synthetic chemistry of TAM probes, while it is still in its infancy, is becoming a fast developing area of research. First reports on the synthesis of TAMs useful for EPRI and PEDRI have appeared in the patent literature3. The published procedures have been difficult to duplicate, and the large scale synthesis of these compounds has proven to be very challenging. Recently creative efforts have been done for the synthesis of these complex molecules4, 5, 17. We have published a large scale synthesis of the Finland trityl, cTAM, based on a few modifications of the original literature4. Fluorinated TAMs18 possessing a high affinity to fluorous media were designed for assessment of tumor oxygenation using biocompatible perfluorocarbon emulsions. Recently we developed TAM structures with dual function pH and oxygen sensitivity 12, 13, and probes with enhanced sensitivity to oxygen due to doublet spectral pattern19. Dendritic20 and ester21 derivatives of Finland trityl were also reported as potentially valuable probes with enhanced stability and ability for intracellular delivery.
The isotopic substitution of the 36 methyl protons in the three aryl groups of TAMs for deuterons result in further significant decrease of their EPR linewidth which is important for the applications of TAMs as functional probes1. However, until now the synthesis of several deuterated TAMs were published only in patented literature3. Herein, we report the improved procedure for the synthesis of deuterated Finland trityl (cTAM*) and several new deuterated cTAM derivatives with the linkers attached to the carboxylic groups allowing for further functional modification. The effect of the isotopic substitution on the EPR spectra is described.
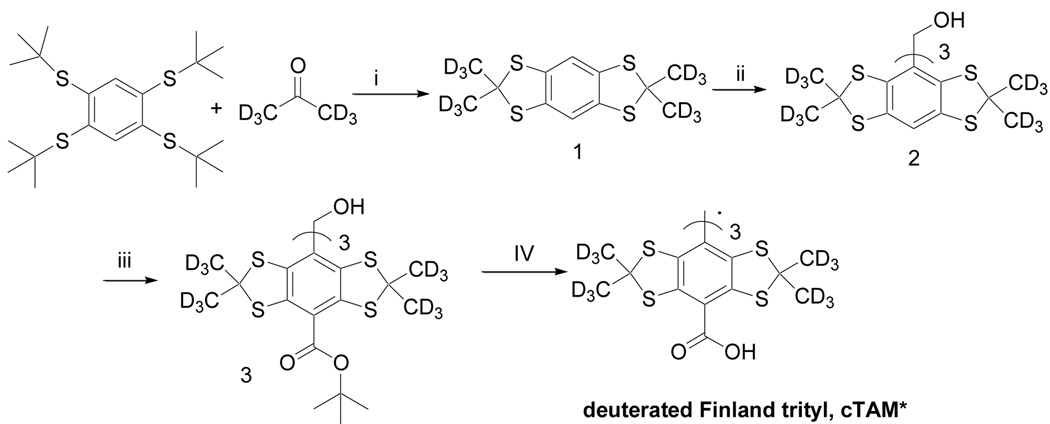
To synthesize cTAM* a slightly modified procedure previously developed in our group for large scale synthesis of cTAM radical was applied4 (see Scheme 2).
Scheme 2.
Synthesis of deuterated Finland trityl radical, cTAM*: (i) HBF4, toluene, d6-acetone 70 %; (ii) n-BuLi, Et2O; CH3COCl, 70 %; (iii) n-BuLi, TMEDA, DiBoc, 44 %; (IV) CF3COOH; 95%.
The exchange of proton with deuterons observed during the first step was less than a few percent. The degree of deuteration of the product 2 in all preparations exceeded 95 % according to 1H NMR data and was even larger (> 97 %) at larger scale synthesis.
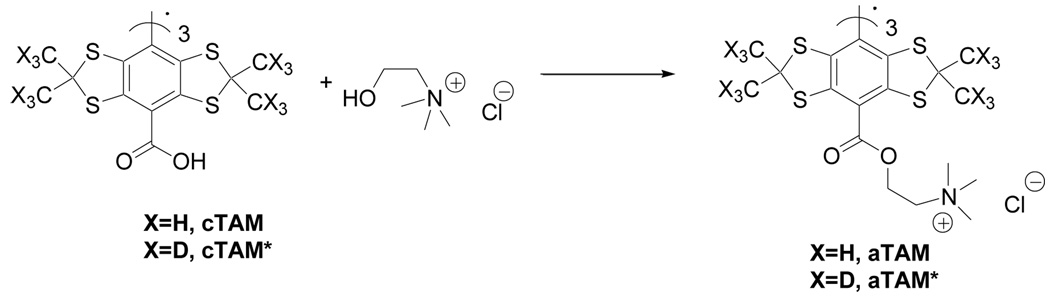
An introduction of new groups and/or linkers in the TAM structure allows for adjustment of their functional properties, such as solubility, stability and sensitivity to oxygen and pH13, 17, 18, 20. The aTAM derivative with positively charged ammonium group and its deuterated analog, aTAM*, were synthesized as shown in the Scheme 3. The carboxylic moiety of cTAM was activated by standard peptode coupling conditions using O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, HBTU, N,N-dimethylaminopyridine, DMAP, and triethylamine, TEA, followed by the addition of choline chloride.
Scheme 3.
HBTU, DMAP, TEA, DMF, 65 %.
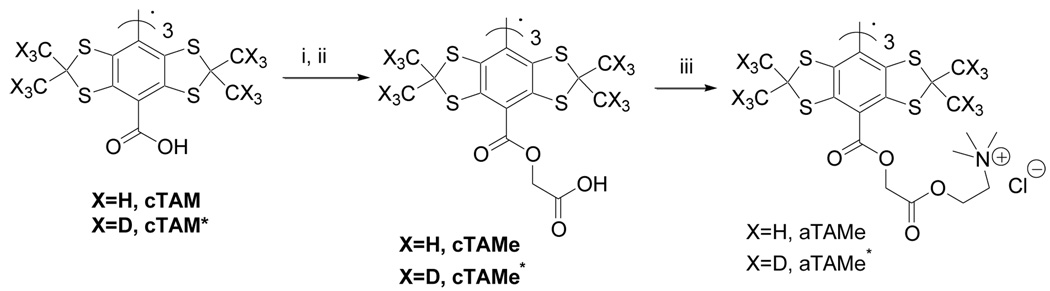
An additional series of TAMe derivatives based on glycolic acid ester, followed by addition of choline chloride was synthesized as shown in the Scheme 4. The compound cTAMe and its deuterated analog cTAMe* with carboxylic end group, and aTAMe and aTAMe* with ammonium end group were obtained. The structures and EPR spectral parameters are summarized in Table 1 and detailed procedures and EPR spectra are available in the supporting information.
Scheme 4.
(i) HBTU, DMAP, TEA, DMF glycolic acid, tert-butylester; (ii) Trifluoroacetic acid 98 % over two steps; (iii) HBTU, DMAP, TEA, DMF, choline chloride; 60 %.
Table 1.
EPR spectral parameters of the cTAM, aTAM, aTAMe, cTAMe and their deuterated analogs obtained from EPR measurements in anoxic solutions.
| TAM | Δpp, ±5 mG (resolved line) |
aH (CH2), ±2 mG |
Δppt, ±5 mG (unresolved multiplet) |
|---|---|---|---|
| cTAM | 95 | - | - |
| cTAM* | 65 | - | - |
| aTAM | 92 | 110 | - |
| aTAM* | 60 | 110 | - |
| cTAMe | 100 | 60 | 220 |
| cTAMe* | 70 | 60 | ≈190* |
| aTAMe | 100 | 70 | 230 |
| aTAMe* | 60 | 70 | - |
the spectral line was only partially resolved
The EPR peak-to-peak linewidth, Δpp, of cTAM* was found to be 65 mG in anoxic solution in agreement with the previously reported data1. This 1.5 fold line narrowing compared with 95 mG linewidth of cTAM corresponds to more than two fold increase in spectral intensity, and provides significant advantage for EPR/EPRI applications.
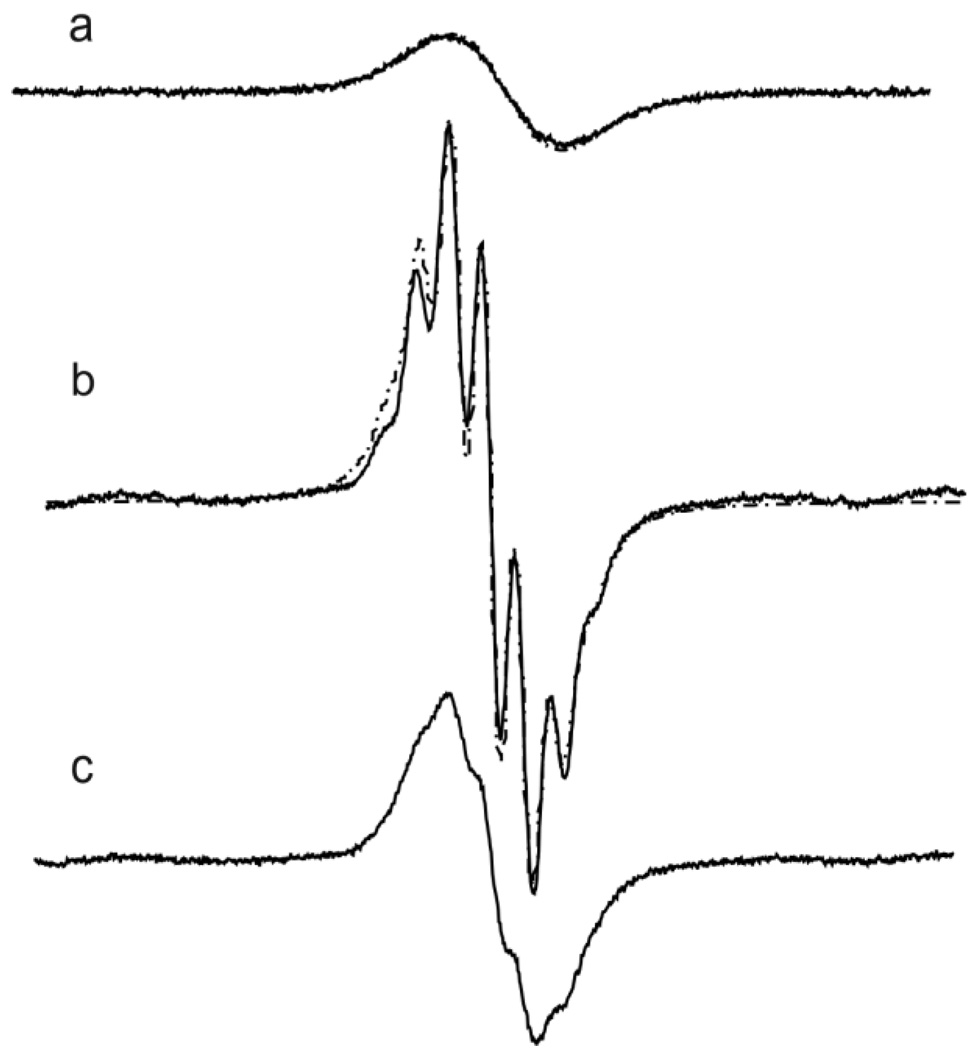
Similar narrowing effect of deutero-substitution on the EPR linewidth was observed for aTAM, cTAMe, and aTAMe (see Table 1 for the linewidth of the resolved line). However, additional hyperfine splitting (septet with peak intensity ratio 1:6:15:20:15:6:1) appears from 6 protons of methylene group, aH, adjacent to aryl caboxylate. Interestingly, the aH hyperfine splitting is almost twice larger for aTAM (110 mG) with positively charged ammonium group compared with cTAMe (60 mG) with carboxyl group in agreement with expected higher electron attracting inductive effect of the ammonium function. An increase of the linker length between aryl and ammonium groups in the compound aTAMe resulted in decrease of aH hyperfine splitting from 110 mG to 70 mG. Note that the hyperfine structure from methylene protons was not resolved for the non-deuterated cTAMe and aTAMe derivatives with aH values being significantly lower than the linewidth of the individual spectral components (see Table 1). For these compounds aH values were first determined from the corresponding deuterated radicals and then used for calculation of aH and Δpp values of non-deuterated compounds from their spectral simulation (see Fig.1 for the aTAMe). The origin of multiplet spectral structure was further confirmed by the overnight incubation of aTAMe* in CF3COOD/D2O solution, which resulted in partial substitution of the methylene protons for deuterons and corresponding disappearance of the multiplet structure (see Fig. 1c for the EPR spectrum of the aTAMe**).
Figure 1.
X-band EPR spectra of 1 mM aTAMe (a), aTAMe* (b) and aTAMe** (c) measured in anoxic DMSO solutions radicals at room temperature. The spectra are shown with the same receiver gain. Spectral parameters were as follows: microwave power, 0.63 mW; modulation amplitude, 0.01 G; sweep width, 2G; number of points, 1024. The simulated EPR spectra (a) and (b) are shown by dotted lines.
The EPR spectra of the cTAM and its synthesized derivatives show similar oxygen-induced line-broadening about 70 mG/20 % [O2]. Among the deuterated cTAM derivatives the compounds cTAM*, cTAMe* and aTAMe** demonstrate single EPR line in anoxic solution with the narrowest line for cTAM*. The cTAM* radical, therefore, has an advantage in oxygen sensitivity and simplicity of the EPR spectrum. The multiplet character of the EPR spectra of aTAM* and aTAMe* derivatives make them less attractive for EPR oximetric applications. Nevertheless, they have their own advantages compared with Finland trityl derivatives containing carboxylic groups. First, as it was previously shown4 cTAM has tendency to aggregation associated with protonation of its carboxyl groups and facilitated in environments with low pH and low polarity, e.g. in the presence of biomembranes. On the other hand, aTAM derivatives show pH-independent solubility in aqueous solution in mM range of concentration. Apparently, the presence of positively charged ammonium group in the structure of aTAM derivatives prevents their aggregation and, therefore, makes them potentially less toxic. Second, in general the EPR oximetric probes with multiplet spectral pattern possess higher sensitivity to oxygen at low oxygen tension compared with single-line EPR probes due to the opportunity to follow changes in peak intensity ratio of partially resolved components rather than EPR linewidth19, 22. Recently we described the cTAM derivative with enhanced sensitivity to oxygen down to 1 mmHg due to its partially overlapped doublet EPR spectrum19. Therefore, aTAM* and aTAMe* derivatives may have an advantage when accurate measurements of low oxygen concentrations are required, e.g. to locate area of hypoxia (≤ 15 mmHg 23) or even to detect a threshold of true anoxia (≤ 1.5 mmHg).
In summary, we describe the synthesis of several new TAM radicals. The deuterated Finland trityl, cTAM*, was synthesized according to a modified protocol. The synthesis is very efficient in multigram scale. cTAMe structures, which can be used for further derivatization such as in designing peptide-TAM bioconjugates have been also synthesized. The EPR characterization of newly synthesized compounds should help in predicting the EPR hyperfine pattern of other derivatized TAMs.
Supplementary Material
Acknowledgements
Authors thank Drs. Andrey Bobko, Olga Efimova and Denis Komarov for the help in spectra acquisition and analysis. This work was partially supported by the NIH grants R21 HL091423, R01 HL38324, EB0890 and EB4900.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ardenkjaer-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K. J Magn Reson. 1998;133(1):1–12. doi: 10.1006/jmre.1998.1438. [DOI] [PubMed] [Google Scholar]
- 2.Gomberg M. J. Am. Chem. Soc. 1900;22:757–771. [Google Scholar]
- 3.Anderson S, Golman K, Rise F, Wikstro¨m H, Wistrand L-G. Free radicals. US Patent. 1996;5:530. 140.
- 4.Dhimitruka I, Velayutham M, Bobko AA, Khramtsov VV, Villamena FA, Hadad CM, Zweier JL. Bioorg Med Chem Lett. 2007;17(24):6801–6805. doi: 10.1016/j.bmcl.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy TJ, Iwama T, Halpern HJ, Rawal VH. J Org Chem. 2002;67(14):4635–4639. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]
- 6.Lurie DJ, Bussell DM, Bell LH, Mallard JR. J Magn Reson. 1988;76:366–370. [Google Scholar]
- 7.Lurie DJ, Hutchison JMS, Bell LH, Nicholson I, Bussell DM, Mallard JR. J Magn Reson. 1989;84:431–437. [Google Scholar]
- 8.Golman K, Petersson JS, Ardenkjaer-Larsen JH, Leunbach I, Wistrand LG, Ehnholm G, Liu KJ. J Magn Reson Imaging. 2000;12:929–938. doi: 10.1002/1522-2586(200012)12:6<929::aid-jmri17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer-Larsen JH, Subramanian S, Mitchell JB. Proc Natl Acad Sci U S A. 2002;99(4):2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Arch Biochem Biophys. 2004;424(1):81–88. doi: 10.1016/j.abb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Kutala VK, Villamena FA, Ilangovan G, Maspoch D, Roques N, Veciana J, Rovira C, Kuppusamy P. J Phys Chem B. 2008;112(1):158–167. doi: 10.1021/jp076656x. [DOI] [PubMed] [Google Scholar]
- 12.Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. J Am Chem Soc. 2007;129(23):7240–7241. doi: 10.1021/ja071515u. [DOI] [PubMed] [Google Scholar]
- 13.Dhimitruka I, Bobko AA, Hadad CM, Zweier JL, Khramtsov VV. J Am Chem Soc. 2008;130(32):10780–10787. doi: 10.1021/ja803083z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Nature. 2008;453(7197):940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 16.Halpern HJ, Peric M, Yu C, Bales BL. J Magn Reson, Ser. A. 1993;103:13–22. [Google Scholar]
- 17.Liu Y, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J Org Chem. 2008;73(4):1490–1497. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]
- 18.Driesschaert B, Charlier N, Gallez B, Marchand-Brynaert J. Bioorg Med Chem Lett. 2008;18(15):4291–4293. doi: 10.1016/j.bmcl.2008.06.100. [DOI] [PubMed] [Google Scholar]
- 19.Bobko AA, Dhimitruka I, Eubank TD, Marsh CB, Zweier JL, Khramtsov VV. Free Radic Biol Med. 2009;47(5):654–658. doi: 10.1016/j.freeradbiomed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Villamena FA, Zweier JL. Chem Commun (Camb) 2008;(36):4336–4338. doi: 10.1039/b807406b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Villamena FA, Sun J, Wang TY, Zweier JL. Free Radic Biol Med. 2009;46(7):876–883. doi: 10.1016/j.freeradbiomed.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Proc Natl Acad Sci U S A. 1994;91(26):13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.vanFaassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro D, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier JL, Gladwin MT. Medicinal Research Reviews. 2009 doi: 10.1002/med.20151. in press (#0198-6325) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.