Abstract
G-protein coupled receptors (GPCRs) are targets of approximately 30 % of currently marketed drugs. Over the last few years, a number of GPCRs expressed in pancreatic β cells and activated by lipids have been discovered. GPR40 was shown to be activated by medium- to long-chain fatty acids (FAs). It has since been shown that GPR40 contributes to FA amplification of glucose-induced insulin secretion. Although some controversy still exists as to whether GPR40 agonists or antagonists should be designed as novel type 2 diabetes drugs, data obtained in our laboratory and others strongly suggest that GPR40 agonism might represent a valuable therapeutic approach. GPR119 is expressed in pancreatic β cells and enteroendocrine L-cells, and augments circulating insulin levels both via its direct insulinotropic action on β cells and via FA stimulation of glucagon-like peptide 1 (GLP-1) secretion. GPR120 is expressed in L-cells and was also shown to mediate FA-stimulated GLP-1 release. Finally, GPR41 and GPR43 are receptors for short-chain FAs and may indirectly regulate β-cell function via adipokine secretion. While the discovery of these various lipid receptors opens new and exciting avenues of research for drug development, a number of questions regarding their mechanisms of action and physiological roles remain to be answered.
Keywords: G-protein coupled receptors, Islet of Langerhans, Diabetes, Insulin secretion
Introduction
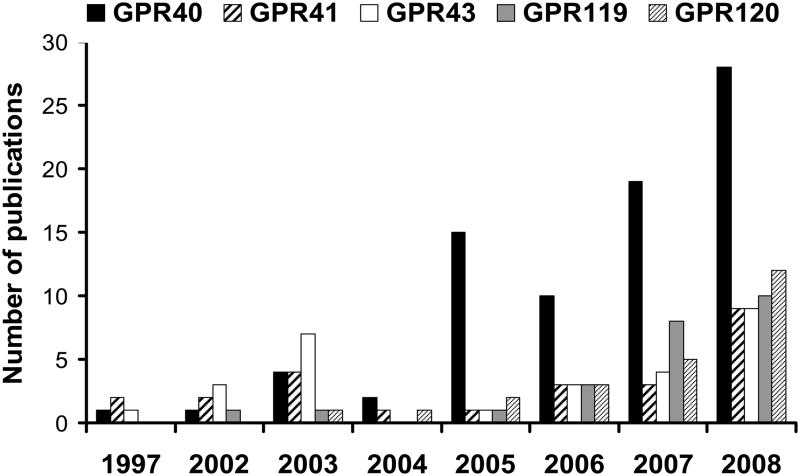
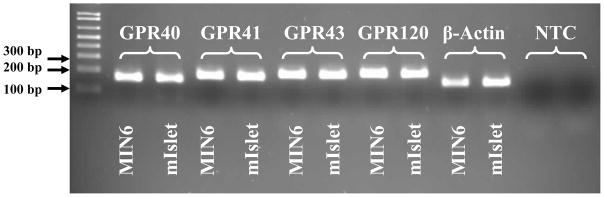
G-protein coupled receptors (GPCRs) constitute a superfamily of membrane proteins characterized by a common seven transmembrane helical bundle. GPCRs are activated by a variety of endogenous ligands such as hormones, neurotransmitters, peptides, proteins, steroids as wells as fatty acids (FAs) and other lipids. GPCR signaling is involved in a large number of physiological processes and, as a result, more than 30 % of currently marketed drugs target GPCRs [1]. This review focuses on the growing number of GPCRs that are expressed in the pancreatic β cell and activated by lipids, and their potential implication for the treatment of type 2 diabetes. This family of receptors includes the closely related GPR40, GPR41 and GPR43 as well as GPR119 and GPR120. The genes encoding GPR40 and its family members (GPR41 and GPR43) were isolated in 1997, during a search for novel human galanin receptor subtypes, as a group of tandemly encoded intronless genes clustered downstream of the CD22 gene on human chromosome locus 19q13.1 [2]. This family of receptors shares an overall sequence homology of 30–50 % [3] and a higher homology within their putative transmembrane domains [4]. Until 2003, the ligands for these GPCRs were unknown and hence they were called orphan receptors. Using ligand-fishing strategies the receptors were then deorphanized and GPR40, also referred to as free fatty acids 1 receptor (FFA1R), was shown to be activated by medium- to long-chain FAs [5–7]; and GPR41 (FFA3R) and GPR43 (FFA2R) to be activated by short-chain FAs [8, 9]. GPR119 and GPR120 were identified in 2003 in conjunction with five other GPCRs as new members of the rhodopsin (class A) GPCR superfamily by sequence analysis of the human genome database [10]. GPR119 and GPR120 bear little homology to the GPR40 family. At the time of identification both receptors were orphans; however, in 2005 GPR119 was shown to be activated by lysophosphatidylcholine (LPC) [11] and GPR120 by medium to long-chain unsaturated FAs [12]. Fatty acids provide an important energy source and also act as signaling molecules in various cellular processes. The deorphanization of this family of lipid receptors and their potential involvement in FA-induced insulin secretion sparked major interest from the diabetes research community, as reflected by the ever growing number of publications on these GPCRs over the last few years (Figure 1). Initially GPR41, GPR43 and GPR120 were not reported to be expressed in islets; however, we detected mRNA expression of all 3 receptors by RT-PCR in both isolated mouse islets and the insulin-secreting MIN6 cell line (Figure 2). In this article we will review the available literature on lipid receptors expressed in β cells, their mechanisms of action, their functional role, and discuss potential therapeutic implications in type 2 diabetes.
Figure 1.
Number of articles referenced in PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez) since 1997 on the different GPCRs discussed in this review, as of March 2009.
Figure 2.
GPR41, GPR43 and GPR120 are expressed in isolated mouse islets and cultured MIN6 cells. Total RNA was extracted from isolated C57BL/6 mouse islets and MIN6 cells and reverse transcribed. cDNA was DNAse-treated and subjected to RT-PCR analysis for the indicated mRNAs. The resulting PCR products were resolved by electrophoresis on a 2% agarose gel. mIslet: Mouse islets; NTC: no template control.
GPR40
Fatty acids are known to have complex and divergent effects on β-cell function. In contrast to their acute, stimulatory effects on glucose-stimulated insulin secretion (GSIS), prolonged exposure to elevated levels of FAs impairs β-cell function, including insulin secretion, gene expression and survival (reviewed in [13]). Until recently, the prevailing model postulated that the effects of FAs on β cells were mediated by their intracellular metabolism and the generation of lipid-derived signals such as cytoplasmic long-chain CoA (LC-CoA), which in turn activate downstream signals including stimulation of protein kinase C isoforms, acylation of proteins or direct activation of insulin exocytosis [14, 15]. This model has been challenged by accumulating evidence suggesting that FAs induce a variety of physiological responses by activating GPR40. GPR40 is highly expressed in pancreatic β cells and insulin-secreting cell lines [5–7] including human islets [16] and also in the ileum [6], monocytes [6], pancreatic alpha-cells [17], enteroendocrine cells [18], breast cancer cells [19], osteoclasts [20] and some area of the human brain [5]. Since GPR40 has been proposed to be involved in the regulation of FA-potentiation of GSIS [6], it has received considerable attention as a potential therapeutic target for type-2 diabetes [21–26]. However, the potential contribution of GPR40 to the chronic, deleterious effects of FAs on β-cell function remains controversial. Therefore, it is still debatable whether an agonist or an antagonist should be developed as a therapeutic agent [23]. In the next section we will discuss first the role of GPR40 in insulin secretion and, second, its contribution to the long-term maintenance of glucose homeostasis. We will then attempt to speculate on the therapeutic implications of these findings.
Role of GPR40 in insulin secretion
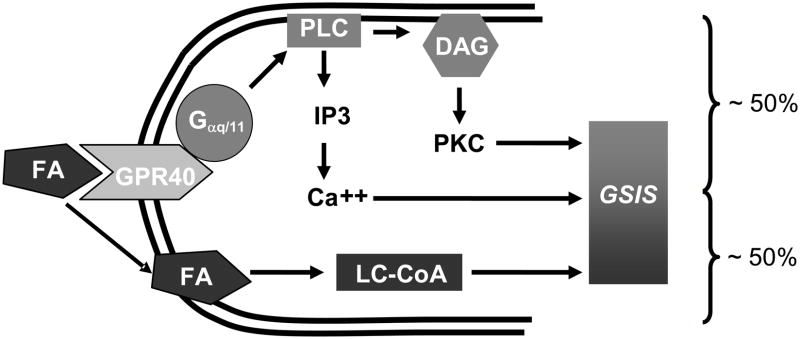
Accumulating evidence suggests that GPR40 is implicated in FA-potentiation of GSIS. Thus, down regulation of GPR40 by small interfering RNA [6, 27, 28], antisense oligonucleotides [29], pharmacological antagonists [21] or gene deletion in the mouse [30, 31] decreases the ability of FAs to potentiate GSIS. Conversely, transgenic overexpression of GPR40 [32] or administration of GPR40 agonists [33, 34] augments insulin secretion. A role for GPR40-mediated signaling in FA-potentiation of GSIS is in apparent contradiction with the well-established notion that the insulinotropic effect of FAs requires their intracellular metabolism [15, 35]. Our hypothesis is that both mechanisms of action, namely receptor-mediated signaling and intracellular metabolism, are implicated in FA-potentiation of GSIS (Figure 3). In support of this hypothesis, insulin secretion in response to FAs in vivo is reduced by approximately 50 % in GPR40 knock-out (KO) mice [30]. Recent studies in our laboratory led us to conclude that these mechanisms are independent from one another, i.e. that acute stimulation of GPR40 by FAs does not alter intracellular fuel metabolism in islets (unpublished observations). Therefore, we postulate that GPR40 is responsible for approximately half of the insulin secretory response to FAs, the other half being accounted for independently by intracellular metabolism of FAs and the generation of lipid-derived signals.
Figure 3.
Schematic representation of the mechanisms of FA-potentiation of GSIS in β cells illustrating the concept that both intracellular metabolism of FAs and GPR40-mediated signals are necessary.
The role of GPR40 in FA-potentiation of insulin release has important implications for the regulation of insulin secretion in vivo, because FAs are always present in the circulation and thereby contribute to the integrated response of the β cell to the many factors that amplify the effects of glucose. Accordingly, we have recently shown that GPR40 KO mice have defective insulin secretion in hyperglycemic clamp studies (unpublished observations). GPR40 KO mice also had fasting hyperglycemia and hypoinsulinemia, consistent with the concept put forth by McGarry and colleagues [36] that FAs are essential for insulin secretion after a period of fast.
Overall, the available evidence indicates that GPR40 is implicated in FA-potentiation of GSIS and, therefore, in the regulation of insulin secretion in vivo. Therefore, at least in an acute setting, GPR40 agonists are expected to enhance insulin secretion in a glucose-dependent manner, similar to the mode of action of GLP-1 agonists currently on the market.
Role of GPR40 in the long-term maintenance of glucose homeostasis
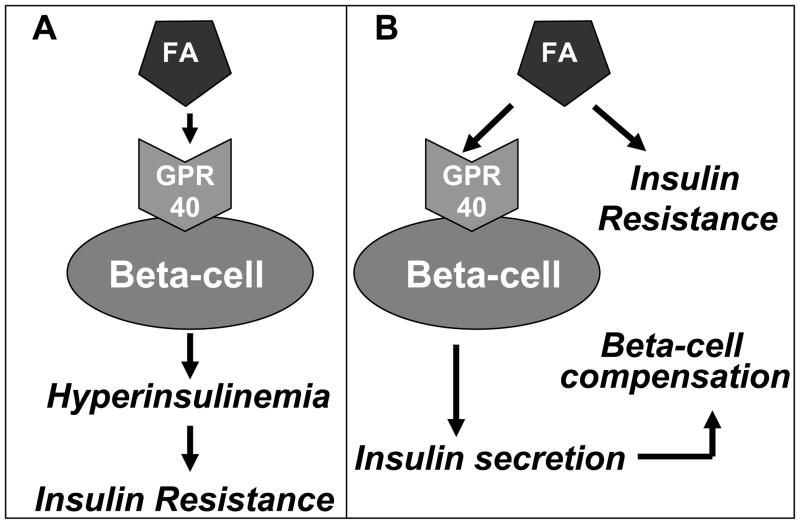
Because FAs become inhibitory to pancreatic β-cell function when present at elevated concentrations for prolonged periods of time, it is conceivable that chronic agonism of the receptor might be detrimental. Such possibility was supported by a study from Steneberg et al. [31] who reported that lack of GPR40 protected mice from high-fat diet (HFD)-induced insulin resistance. In the same study, transgenic overexpression of GPR40 under the pancreas and duodenum homeobox 1 (PDX-1) promoter led to impaired insulin secretion and diabetes. Based on these results, the authors concluded that excessive activation of GPR40, either by HFD or by overexpression of the receptor, is detrimental to β-cell function [31] and therefore the development of a GPR40 antagonist may be an attractive approach for the treatment of diabetes. Similar findings were obtained by Brownlie et al. [37] using a different KO strain. Steneberg et al. [31] proposed that the GPR40-mediated increase in insulin secretion in response to HFD causes insulin resistance which is prevented by GPR40 deletion (Figure 4A), in line with the notion that therapeutic strategies aimed at enhancing insulin secretion are in fact counterproductive because they favor insulin resistance [38].
Figure 4.
Schematic representation of two alternative hypotheses regarding the mechanisms of lipid-induced insulin resistance and β-cell compensation. A: According to Stenberg et al. [31] the GPR40 mediated increase in insulin secretion in response to HFD causes insulin resistance. B: In contrast, we propose that the lipid-induced, GPR40-mediated increase in insulin secretion contributes to β-cell compensation for insulin resistance.
Several observations, however, appear to contradict the aforementioned conclusions. First, in our hands islets from GPR40 KO mice are not protected from FA inhibition of insulin secretion after prolonged exposure [30]. Second, culture of islets in the presence of a specific GPR40 agonist does not impair insulin secretion [33]. Third, two independent studies have found that GPR40 KO mice were not protected from HFD-induced insulin resistance [39, 40]. If anything, in our study the mice became hyperglycemic earlier than their wild-type littermates, and GPR40 became rate limiting for GSIS after HFD [39]. Fourth, transgenic overexpression of the human GPR40 gene under the control of the mouse insulin II promoter (hGPR40-Tg) did not induce lipotoxicity and, to the contrary, these mice were less susceptible to HFD-induced hyperglycemia [32]. Fifth, GSIS was improved by administration of a GPR40 agonist in rats fed a high-fructose diet [34]. Lastly, islets from hGPR40-Tg mice had increased GSIS, and transgenic overexpression of GPR40 in the diabetic KK background resulted in improved insulin secretion and glucose tolerance [32]. Overall, these studies provide support to the concept that FA-induced hyperinsulinemia represents a mechanism by which the β cell attempts to compensate for insulin resistance, and that this ability is compromised by GPR40 deletion (Figure 4B). By inference, chronic activation of GPR40 may be beneficial to the maintenance of glucose homeostasis.
The major discrepancies between the studies that reported a negative role of GPR40 [31, 37] and those that reported a positive role [30, 32–34, 39, 40] are difficult to explain. Regarding the KO mice, differences in genetic background must be taken into consideration because insulin secretion after HFD is markedly influenced by the strain [41], although it is difficult to imagine that this variable alone would explain the dramatically different results. Resolving this controversy will likely require confirmation of the impact of GPR40 deletion specifically in pancreatic β cells, ideally in an inducible manner, in different mouse strains. Regarding the transgenic mice, three possible reasons could explain the differences in phenotypes between the study of Steneberg et al. [31] and that of Nagasumi [32]. First, the levels of overexpression of the receptor were 20–100 fold in the study of Steneberg et al. [31] vs. 10 fold in the study by Nagasumi et al. [32]). Second, the PDX-1 promoter used by Steneberg et al. [31] also drives expression in non-β cells, whereas expression of the mouse insulin II promoter is β-cell specific. Finally, transgenic expression during embryonic development under the PDX-1 promoter might have affected islet morphogenesis. Indeed, the transgenic line generated by Steneberg et al. [31] showed disorganized islet architecture and decreased insulin content [31], whereas Nagasumi et al. [32] did not observe changes in islets morphology or β-cell mass.
Overall, despite these important discrepancies and although additional investigation is required, our conclusion is that the evidence in favor of developing GPR40 agonists for the treatment of type 2 diabetes outweighs that supporting the development of antagonists. Such contention is further supported by the observation that loss-of-function mutations of the GPR40 gene in humans are associated with altered insulin secretion [42].
Role of GPR40 in other tissues
GPR40 is expressed in high levels in the pancreatic β cell [5–7] and was also shown to be expressed, albeit to a lesser extent, in other tissues. At the outset of this section it is important to mention the important caveat that, to our knowledge, no specific rodent GPR40 antibody is commercially available. Therefore, most of the studies discussed below rely on RT-PCR measurements of GPR40 mRNA expression. Nevertheless, GPR40 mRNA has been detected in non-β cells yet its function in these tissues is largely unknown. Clearly, the role of GPR40, if any, in non-β cells needs to be understood in details if it is to be targeted for drug discovery.
The first demonstration of extra-pancreatic function of GPR40 comes from a study in the MCF7 breast cancer cell line [19] which showed that knock-down of GPR40 by RNA interference reduced, while GPR40 overexpression increased, the ability of oleate to stimulate breast cancer cell proliferation. The unsaturated FAs oleate and linoleate, but not the saturated FAs palmitate or stearate, induced an increase in cellular Ca++ concentration in these cells and mediated proliferation by activating GPR40 [19, 43]. Pertussis toxin treatment partially blocked the effect of oleic acid [43]. The mechanism by which GPR40 may be implicated in the control of cell growth and survival has been proposed to involve activation of the extracellular-regulated kinases (ERK) and phosphatidylinositol 3 (PI3)-kinase/protein kinase B signaling pathways [19, 43]. Whether or not GPR40 contributes to tumorigenesis in humans is unknown. If this were the case, it would clearly impede the safety of GPR40 agonists.
Of particular relevance to glucose homeostasis, GPR40 was shown to be expressed in glucagon-producing cells and primary mouse α-cells [17], in which FAs are known to stimulate glucagon secretion [44, 45]. Flodgren et al. [17] showed that In-R1-G9 hamster glucagonoma cells respond dose-dependently to linoleic acid stimulation by elevated phosphatidyl inositol hydrolysis and glucagon release and that the cells become increasingly responsive to FA stimulation upon overexpression of GPR40. Also, Lan et al. [40] have recently observed that GPR40 KO mice secrete significantly less glucagon than WT mice in response to elevated plasma FA levels. Therefore, evidence suggests that GPR40 might be implicated in FA-stimulation of glucagon secretion.
Edfalk et al. [18] have recently shown that GPR40 is expressed in endocrine cells of the gastrointestinal tract, including cells expressing the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), and that GPR40 mediates FA-stimulated incretin secretion. Furthermore, the authors have shown that GLP-1 and GIP secretion is reduced in GPR40 KO mice [18]. These findings suggest that GPR40 mediates FA stimulated insulin secretion from the β cell not only directly but also indirectly via regulation of incretin secretion [18].
GPR40 expression has been detected in the brain, at least in primates (including humans) [5], but its role in this tissue is unknown. In the mouse, we were not able to detect GPR40 mRNA in whole brain or hypothalamic extracts, but found significant levels of GPR40 expression in isolated brain microvessels (unpublished observations in collaboration with S. Chemtob and A. Kooli). Previously, Kermorvant-Duchemin et al. [46] had shown that during the sub-acute phase following hypoxia-ischemia, generation of nitric oxide induces the formation of trans-arachidonic acid (TAA) from arachidonic acid. In collaboration with S. Chemtob and A. Kooli, we have shown that TAA can activate GPR40, and that the brain infarct size following a hypoxic-ischemic insult is significantly reduced in GPR40 KO mice (unpublished results). The patho-physiological implications of these observations in humans have yet to be confirmed.
Finally, a recent study found expression of GPR40 as well as GPR41, GPR43 and GPR120, in osteoclastic cells [20] and suggested that activation of GPR40 and GPR120 might mediate in part the antiosteoclastogenic effects of FAs.
In conclusion, GPR40 mRNA has been detected in several tissues besides the β cell, but the functional impact of these observations has, in most cases, not been fully investigated. This clearly needs to be further examined in the context of the development of GPR40 compounds as new drug targets. On the one hand, a contribution of GPR40 to incretin-potentiation of insulin secretion (via its mediation of FA-induced incretin release) might provide an additional mechanism by which stimulation of the receptor enhances insulin secretion. On the other hand, confirmation of the implication of GPR40 in breast cancer formation or in the pathogenesis of brain microvascular degeneration after hypoxia-ischemia would raise concerns regarding undesirable side-effects of GPR40 agonists.
Mechanisms of action and regulation
The mechanisms of action of GPR40 are incompletely understood. The lack of sensitivity of GPR40 signaling to pertussis toxin pretreatment suggested that it couples to the G-protein subunit Gαq/11 [6] and in turn activates phospholipase-C-mediated hydrolysis of phosphatidylinositol 4,5-biphosphate into diacylglycerol and inositol triphosphate (IP3), which activates protein kinase C and mobilization of Ca++ from endoplasmic reticulum stores [23], respectively, similar to the mechanisms underlying cholinergic stimulation of insulin release [47] (Figure 3). Furthermore, pharmacological inhibition of Gαq/11 [28, 30] or phospholipase C [28] blocks FA-potentiation of GSIS in vitro. GPR40 also activates L-type Ca++ channels [28, 48]. Finally, Feng et al. [49] reported that oleic acid inhibits delayed rectifying voltage-gated K+ channels through GPR40, thereby amplifying insulin secretion. Overall, the amount of direct information on the mechanism of action and intracellular signaling pathways activated by GPR40 remains relatively limited, considering the interest shown in this receptor as a potential drug target.
The regulation of the GPR40 gene is also essentially unknown. Bartoov-Shifman et al. [50] elucidated the structure of the 5′-upstream region of GPR40 and showed that β-cell specific expression is conferred by the conserved region HR2, a potent β-cell specific transcriptional enhancer. Systematic mutational analysis of the HR2 region identified sites that bind the transcription factors signal transducer and activator of transcription, PDX-1 and BETA2 [50]. PDX-1 and BETA2 are glucose-responsive transcription factors essential to the regulation of insulin gene expression. Since PDX-1 activity is stimulated by glucose and inhibited by palmitate in islets [51], it is conceivable that GPR40 may also be regulated by these nutrients. Indeed, we observed that glucose stimulates the expression of GPR40 mRNA in isolated mouse and rat islets as well as in insulin secreting MIN6 cells, and that this stimulation by glucose was inhibited in the presence of palmitate (unpublished observations). Furthermore, we found that GPR40 expression was also enhanced in a dose-dependent manner by exogenous insulin in MIN6 cells and that the effects of both insulin and glucose were lost when insulin signaling was pharmacologically blocked with the PI3 kinase inhibitor Ly 294002 and in β-cells lacking insulin receptors (unpublished observations). Understanding the mode of regulation of GPR40 will provide valuable information regarding its role in physiological and pathophysiological states.
GPR119
Tissue distribution
GPR119 is another member of the class A rhodospin like GPCR which exhibits little homology with but shares similar expression pattern as GPR40. It is expressed in the pancreas and gastrointestinal tract in rodents and humans, as well as several areas of the brain in rodents but not in humans [11]. Although Sakamoto et al. [52] initially reported, using immunohistochemical analysis, that GPR119 was expressed in pancreatic polypeptide (PP) cells but not in β or α cells, subsequent studies have documented β cell expression of GPR119 [11, 52–54]. Importantly, Chu et al. presented convincing evidence that GPR119 is functionally active in pancreatic β cells [53] and in enteroendocrine L-cells [55].
Activation and mechanism of action
The FA derivatives LPC [11] and oleoylethanolamide (OEA) [54] have been initially described as endogenous ligands for GPR119. LPC is a lysophospholipid known to have insulinotropic action on pancreatic β cells through cAMP production [11]. Soga et al. [11] showed that activation of GPR119 by LPC leads to glucose-dependent insulin secretion through cAMP production in the perfused pancreas as well as in the NIT-1 cell line. GPR119 is coupled to the G protein α-subunit Gαs [11], causing activation of adenylate cyclase and an increase in cAMP levels (Figure 5). Deletion of GPR119 by small interfering RNA in NIT-1 cells or inhibiton of adenylate cyclase partially reduced LPC-induced insulin secretion [11]. In contrast, Ning et al. [56] showed that LPC stimulated insulin secretion in the rat RINm5f insulinoma cell line that does not express GPR119 endogenously and that cells transfected with human GPR119 had an increased response to LPC, suggesting that LPC may also act through GPR119-independent mechanisms. Furthermore, Lan et al. [57] recently showed in GPR119 KO mice that the effects of LPC and OEA on insulin secretion were GPR119-independent. Overall, the nature of the endogenous ligands to GPR119, and whether this GPCR plays a physiological role in directly regulating insulin secretion by the pancreatic β cell, remains largely unknown.
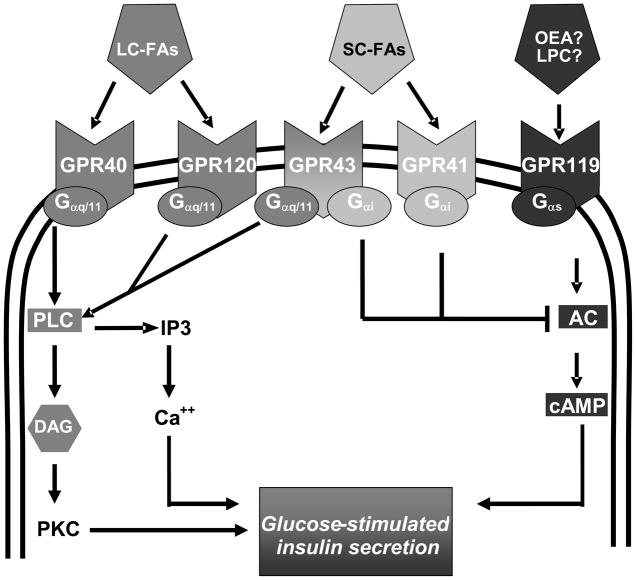
Figure 5.
Potential islet GPCRs and their downstream signaling pathways. Both GPR40 and GPR120 are activated by long-chain FAs (LC-FA) and couple to Gαq/11. GPR41 and GPR43 are activated by short-chain FAs (SC-FA). GPR41 couples to Gαi, whereas GPR43 couples to both Gαq/11 and Gαi. GPR119 is activated by the long-chain FA derivatives oleoylethanolamide (OEA) and lysophophatidylcholine (LPC) and couples to Gαs. Activation of the Gαq/11 pathway leads to stimulation of phospholipase C, generation of inositol-3-phosphate (IP3) and diacylglycerol (DAG), and mobilization of Ca++ from endoplasmic reticulum stores. This will contribute to the release of insulin granules either directly or partly through subsequent activation of protein kinase c (PKC). Activation of Gs stimulates adenylate cyclase (AC) and increases cAMP levels leading to an increase in insulin secretion. On the other hand, activation of Gαi proteins causes inhibition of AC and a decrease in cAMP levels.
In addition, recent evidence suggests that GPR119 indirectly stimulates insulin secretion by mediating FA-induced GLP-1 release from the L-cell via OEA [55, 58]. The recent study by Lauffer et al. [58] convincingly demonstrated that OEA stimulates GLP-1 secretion via activation of GPR119 and protein kinase A. Importantly, in that study enteral, but not intravenous, administration of OEA increased circulating levels of GLP-1 and insulin, suggesting that the insulinotropic action of OEA via activation of GPR119 in vivo is mediated through GLP-1 secretion from enteroendocrine cells rather than directly from the β cell [58].
Therapeutic implications
Despite the remaining uncertainties regarding its endogenous ligands and physiological role, the therapeutic potential of GPR119 has been clearly established in preclinical studies. Chu et al. [53] first reported that the selective GPR119 agonist AR231453 stimulated cAMP accumulation and glucose-dependent insulin release in vitro and improved GSIS and oral glucose tolerance in both normal and diabetic mice. The effect of AR231453 was blocked in cells lacking GPR119 as well as in GPR119 KO mice [53]. They further showed that the effect of AR231453 on insulin secretion was reduced by almost 50 % when glucose was administered intraperitoneally [53], suggesting that its action may also involve modulation of incretin signaling, in accordance with subsequent experiments using OEA ([58]; see above). Furthermore, inhibition of the GLP-1 receptor by exendin (9–39) reduced the ability of AR231453 to improve glucose tolerance in mice [55]. These authors also showed that activation of GPR119 led to an increase in GIP secretion in vivo, a surprising finding considering that GPR119 is not expressed in GIP-producing K cells [55]. Nevertheless, there is strong evidence to suggest that pharmacological activation of GPR119 can increase circulating insulin levels both directly by activating insulin secretion from the β cell and also indirectly by activating the release of the incretin hormones GLP-1 and GIP.
Finally, GPR119 was also proposed to mediate the hypophagic effect of its endogenous ligand OEA [54]. Overton et al. [54] described two small molecule GPR119 synthetic agonists, PSN632408 and PSN375963, and showed that oral administration of PSN632408 as well as OEA lead to an increase in cAMP accumulation and markedly suppressed food intake, reduced weight gain, and decreased white adipose tissue deposition upon sub-chronic HFD in rat. These findings were not confirmed in subsequent studies [53, 57], and the potential for GPR119 as a therapeutic target to regulate food intake remains to be established.
Other GPCRs
GPR120
GPR120 has only 10 % amino acid identity to the GPR40 family [59] but shares the same ligand as GPR40, i.e. medium to long-chain FAs [12]. It is abundantly expressed in the intestinal tract in the mouse and human as well as in the mouse intestinal endocrine STC-1 cell line [12]. GPR120 is coupled to pertussis toxin-insensitive Gαq/11 proteins and activation of the receptor by medium- to long-chain FAs reportedly increases intracellular Ca++ levels and activates the ERK and PI3-kinase pathways, leading to GLP-1 secretion [12, 60, 61]. In addition, GPR120 was shown to mediate the protective effects of linoleate against serum deprivation-induced apoptosis in STC-1 cells [60], reminiscent of the effect of GPR40 mediating anti-apoptotic effect of oleate in insulin secreting cells [62]. GPR120 is also highly expressed in adipocytes where it is suggested to function as a regulator of adipocyte development and differentiation [63] as well as in type II taste cells of the circumvallate and in the fungiform papillae where it is suggested to function as a sensor for dietary fat [64]. Hirasawa et al. [12] reported lack of GPR120 expression in MIN6 cells; in contrast, we find GPR120 mRNA expression in MIN6 cells as well as in isolated mouse islets (Figure 2). Although it needs to be further substantiated, this finding raise the possibility that in addition to its indirect involvement in the regulation of islet function through GLP-1 secretion, GPR120 may also play a role in the regulation of insulin secretion directly from the pancreatic β cell.
GPR41 and GPR43
GPR41 and GPR43 are activated by short-chain FAs such as acetate (C2), propionate (C3), butyrate (C4) and pentanoate (C5) with distinct rank order potencies [8, 9, 65]. C3-C5 FAs are more potent for GPR41 whereas C2-C3 FAs are more potent for GPR43 [66]. Short-chain FAs are the major anions in the large intestine and are produced by bacterial fermentation of dietary fibers. Although GPR41 and GPR43 share the same endogenous agonists, they display key differences in their tissue distribution pattern and G-protein coupling specificities. GPR41 has a more widespread expression pattern than GPR43 with the highest level of GPR41 detected in adipose tissues [9, 65, 67] where it has been implicated in short-chain FA-stimulated leptin production [67]. This observation however is not supported by that of Hong et al. [68] who have shown that GPR43, not GPR41, is expressed in adipocytes and plays a role in adipogenesis. High levels of GPR41 were also reported in the pancreas, spleen, bone marrow and lymph nodes [9, 65].
GPR43 was originally cloned from leukocytes [69] and its highest levels are detected in immune cells such as neutrophils, monocytes, peripheral blood mononuclear cells, polymorphonuclear cells as well as in spleen, bone marrow and B-lymphocytes [8, 9, 65, 69]. The tissue distribution of GPR43 suggests a potential role in immune cell activation and differentiation [9, 65, 69]. GPR43 is also expressed in peptide-YY (PYY)-containing enteroendocrine and 5-hydroxytryptamine-containing mucosal mast cells in the rat intestine and in human colon [70] where short-chain FAs stimulate the release of PYY [71] and serotonin [72]. PYY is an enteroendocrine cell derived hormone that normally inhibits gut motility, increases intestinal transit rate and reduces harvest of energy from diet. Furthermore, GPR43 is expressed in adipocytes [73] and was suggested to play a role in the regulation of lipid metabolism.
Upon ligand stimulation both GPR41 and GPR43 couple to IP3 generation, elevated intracellular Ca++ release through activation of phospholipase C, ERK½ activation and inhibition of cAMP accumulation [9]. However the two receptors are coupled to different G-proteins. GPR41 couples exclusively to the pertussis toxin-sensitive Gαi/o family and GPR43 couples to both Gαi/o and pertussis toxin-insensitive Gαq/11 ([9, 65]; Figure 5).
Although there was no previous report on the expression of GPR41 and GPR43 in pancreatic β cells, we detected mRNA expression of both receptors in mouse pancreatic islets as well as in insulin-producing MIN6 cells by RT-PCR (Figure 2). Recent patent applications by Arena Pharmaceuticals [74, 75] also reported expression of these receptors in pancreatic islets and showed that GPR41 mRNA is increased in db/db mice [74] and that GPR43 mRNA levels are enhanced in both db/db and ob/ob mouse islets [75]. Furthermore, Ximenes et al. [76] showed that propionate inhibits GSIS in perifused rat islets. Although the authors did not discuss the potential role of short-chain FA receptors, propionate is the most potent and efficacious ligand for GPR41 and also activates GPR43, suggesting that the effect of propionate on GSIS may be mediated through activation of either of these receptors in islets. As discussed earlier, activation of GPR41 and GPR43 is coupled with several intracellular signals. Some of these signals such as the increased generation of IP3, increase in intracellular Ca++ as well as activation of the ERK½ pathway are known to contribute to stimulation of GSIS in islets. On the other hand, inhibition of cAMP accumulation is known to inhibit GSIS. However, the exact physiological role of GPR41 and GPR43 is unknown and clearly needs to be studied in more details. Whether activation or inhibition of these receptors can offer therapeutic benefits in disease states such as diabetes remains to be examined. To date, to our knowledge no synthetic agonist or antagonist for GPR41 or GPR43 has been reported.
Conclusion
Islet GPCRs activated by lipids have received considerable interest in the last few years. The available evidence indicates that GPR40 mediates FA-potentiation of insulin secretion and is implicated in the regulation of insulin secretion in vivo. Data from our laboratory and others indicate that GPR40 does not mediate FA inhibition of insulin secretion upon long-term exposure, and that it appears necessary for β-cell compensation for insulin resistance. In addition, our recent, unpublished observations suggest that GPR40 expression is metabolically regulated, although the physiological consequences of such regulation remain to be determined. Finally, GPR40 is expressed and functionally active in non-β cells, but its physiological role is unclear. Although GPR40 has received the most interest, other lipid-receptors have recently been implicated in the regulation of β-cell function as well. GPR119 is involved in the regulation of glucose homeostasis both directly by stimulating insulin secretion from the β cell, and indirectly via FA-mediated incretin secretion from enteroendocrine cells. GPR120 also plays a role in incretin secretion and is expressed in β cells, although a direct role for this receptor in insulin secretion remains to be demonstrated. Finally, GPR41/43 might modulate β-cell function via adipokine release. They are both expressed in β cells, but their physiological relevance is unknown. Overall, we believe that the available evidence favours the development of agonist compounds to the islet lipid receptors GPR40, GPR119, and GPR120 as potential therapeutic agents. In this context, it is conceivable that a «network pharmacology» approach aimed at simultaneously targeting several receptors might be beneficial [77]. However, it is clear from the review of the available literature that several key questions regarding the physiological role and mechanisms of action of these receptors must be answered before any of them can successfully progress down the path of drug development.
Acknowledgments
Work performed in the authors’ laboratory was supported by the US National Institutes of Health (R21-DK070598 to VP) and the Canadian Institutes of Health Research (MOP 177381 to VP). TA is supported by a post-doctoral fellowship from the Canadian Diabetes Association. VP holds the Canada Research Chair in Diabetes and Pancreatic Beta-cell Function.
References
- 1.Howard AD, McAllister G, Feighner SD, et al. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 2.Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239:543–547. doi: 10.1006/bbrc.1997.7513. [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA and cell biology. 2005;24:54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- 4.Surgand JS, Rodrigo J, Kellenberger E, Rognan D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins. 2006;62:509–538. doi: 10.1002/prot.20768. [DOI] [PubMed] [Google Scholar]
- 5.Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 6.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 7.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003;301:406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 9.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- 11.Soga T, Ohishi T, Matsui T, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 13.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51 (Suppl 3):S405–413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- 15.Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53:1007–1019. doi: 10.2337/diabetes.53.4.1007. [DOI] [PubMed] [Google Scholar]
- 16.Tomita T, Masuzaki H, Noguchi M, et al. GPR40 gene expression in human pancreas and insulinoma. Biochem Biophys Res Commun. 2005;338:1788–1790. doi: 10.1016/j.bbrc.2005.10.161. [DOI] [PubMed] [Google Scholar]
- 17.Flodgren E, Olde B, Meidute-Abaraviciene S, Winzell MS, Ahren B, Salehi A. GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem Biophys Res Commun. 2007;354:240–245. doi: 10.1016/j.bbrc.2006.12.193. [DOI] [PubMed] [Google Scholar]
- 18.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem. 2005;280:13285–13291. doi: 10.1074/jbc.M410922200. [DOI] [PubMed] [Google Scholar]
- 20.Cornish J, MacGibbon A, Lin JM, et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008;149:5688–5695. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- 21.Briscoe CP, Peat AJ, McKeown SC, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrido DM, Corbett DF, Dwornik KA, et al. Synthesis and activity of small molecule GPR40 agonists. Bioorg Med Chem Lett. 2006;16:1840–1845. doi: 10.1016/j.bmcl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Gromada J. The free fatty acid receptor GPR40 generates excitement in pancreatic beta-cells. Endocrinology. 2006;147:672–673. doi: 10.1210/en.2005-1388. [DOI] [PubMed] [Google Scholar]
- 24.McKeown SC, Corbett DF, Goetz AS, et al. Solid phase synthesis and SAR of small molecule agonists for the GPR40 receptor. Bioorg Med Chem Lett. 2007;17:1584–1589. doi: 10.1016/j.bmcl.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 25.Song F, Lu S, Gunnet J, et al. Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J Med Chem. 2007;50:2807–2817. doi: 10.1021/jm070130j. [DOI] [PubMed] [Google Scholar]
- 26.Stoddart LA, Brown AJ, Milligan G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol Pharmacol. 2007;71:994–1005. doi: 10.1124/mol.106.031534. [DOI] [PubMed] [Google Scholar]
- 27.Schnell S, Schaefer M, Schofl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263:173–180. doi: 10.1016/j.mce.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Biophys Res Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Salehi A, Flodgren E, Nilsson NE, et al. Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res. 2005;322:207–215. doi: 10.1007/s00441-005-0017-z. [DOI] [PubMed] [Google Scholar]
- 30.Latour MG, Alquier T, Oseid E, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Nagasumi K, Esaki R, Iwachidow K, et al. Overexpression of GPR40 in Pancreatic {beta}-Cells Augments Glucose Stimulated Insulin Secretion and Improves Glucose Tolerance in Normal and Diabetic Mice. Diabetes. 2009 doi: 10.2337/db08-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan CP, Feng Y, Zhou YP, et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes. 2008;57:2211–2219. doi: 10.2337/db08-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doshi LS, Brahma MK, Sayyed SG, et al. Acute administration of GPR40 receptor agonist potentiates glucose-stimulated insulin secretion in vivo in the rat. Metabolism. 2009;58:333–343. doi: 10.1016/j.metabol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992;267:5802–5810. [PubMed] [Google Scholar]
- 36.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownlie R, Mayers RM, Pierce JA, Marley AE, Smith DM. The long-chain fatty acid receptor, GPR40, and glucolipotoxicity: investigations using GPR40-knockout mice. Biochem Soc Trans. 2008;36:950–954. doi: 10.1042/BST0360950. [DOI] [PubMed] [Google Scholar]
- 38.Aston-Mourney K, Proietto J, Morahan G, Andrikopoulos S. Too much of a good thing: why it is bad to stimulate the beta cell to secrete insulin. Diabetologia. 2008;51:540–545. doi: 10.1007/s00125-008-0930-2. [DOI] [PubMed] [Google Scholar]
- 39.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008;57:2432–2437. doi: 10.2337/db08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan H, Hoos LM, Liu L, et al. Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes. 2008;57:2999–3006. doi: 10.2337/db08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrikopoulos S, Massa CM, Aston-Mourney K, et al. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J Endocrinol. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- 42.Vettor R, Granzotto M, De Stefani D, et al. Loss-of-function mutation of the GPR40 gene associates with abnormal stimulated insulin secretion by acting on intracellular calcium mobilization. J Clin Endocrinol Metab. 2008;93:3541–3550. doi: 10.1210/jc.2007-2680. [DOI] [PubMed] [Google Scholar]
- 43.Yonezawa T, Katoh K, Obara Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun. 2004;314:805–809. doi: 10.1016/j.bbrc.2003.12.175. [DOI] [PubMed] [Google Scholar]
- 44.Bollheimer LC, Landauer HC, Troll S, et al. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism. 2004;53:1443–1448. doi: 10.1016/j.metabol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Olofsson CS, Salehi A, Gopel SO, Holm C, Rorsman P. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes. 2004;53:2836–2843. doi: 10.2337/diabetes.53.11.2836. [DOI] [PubMed] [Google Scholar]
- 46.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat Med. 2005;11:1339–1345. doi: 10.1038/nm1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 48.Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab. 2005;289:E670–677. doi: 10.1152/ajpendo.00035.2005. [DOI] [PubMed] [Google Scholar]
- 49.Feng DD, Luo Z, Roh SG, et al. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology. 2006;147:674–682. doi: 10.1210/en.2005-0225. [DOI] [PubMed] [Google Scholar]
- 50.Bartoov-Shifman R, Ridner G, Bahar K, Rubins N, Walker MD. Regulation of the gene encoding GPR40, a fatty acid receptor expressed selectively in pancreatic beta cells. J Biol Chem. 2007;282:23561–23571. doi: 10.1074/jbc.M702115200. [DOI] [PubMed] [Google Scholar]
- 51.Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280:32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakamoto Y, Inoue H, Kawakami S, et al. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem Biophys Res Commun. 2006;351:474–480. doi: 10.1016/j.bbrc.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 53.Chu ZL, Jones RM, He H, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- 54.Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Chu ZL, Carroll C, Alfonso J, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149:2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 56.Ning Y, O’Neill K, Lan H, et al. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol. 2008;155:1056–1065. doi: 10.1038/bjp.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lan H, Vassileva G, Corona A, et al. GPR119 Is Required For Physiological Regulation of Glucagon-Like Peptide-1 Secretion but Not for Metabolic Homeostasis. J Endocrinol. 2009 doi: 10.1677/JOE-08-0453. [DOI] [PubMed] [Google Scholar]
- 58.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009 doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 60.Katsuma S, Hatae N, Yano T, et al. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280:19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515–522. doi: 10.1007/s00210-007-0250-y. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Xu M, Zhang S, et al. The role of G protein-coupled receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. J Mol Endocrinol. 2007;38:651–661. doi: 10.1677/JME-06-0048. [DOI] [PubMed] [Google Scholar]
- 63.Gotoh C, Hong YH, Iga T, et al. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007;354:591–597. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura S, Mizushige T, Yoneda T, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- 65.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 66.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34:770–773. doi: 10.1042/BST0340770. [DOI] [PubMed] [Google Scholar]
- 67.Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 69.Senga T, Iwamoto S, Yoshida T, et al. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101:1185–1187. doi: 10.1182/blood-2002-06-1881. [DOI] [PubMed] [Google Scholar]
- 70.Karaki S, Tazoe H, Hayashi H, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 71.Cherbut C, Ferrier L, Roze C, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415–1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 72.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 73.Ge H, Li X, Weiszmann J, et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 74.Leonard J, Chu ZL, Bruce MA, Boatman PD. WO 2006/052566 A2 Pat PCT/US2005/039551. 2006
- 75.Leonard J, Hakak Y. WO 2006/036688 A2 Pat PCT/US2005/033795. 2006
- 76.Ximenes H, Hirata A, Rocha M, Curi R, Carpinelli A. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochemistry and Function. 2006;25:173–178. doi: 10.1002/cbf.1297. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]