Abstract
Fraction of end tidal exhaled nitric oxide (FeNO) has been introduced as a non-invasive marker of airway inflammation in patients with asthma and may have value in monitoring disease activity in patients with sarcoidosis. This pilot study explored: 1) feasibility of the multiple flow rates maneuver to estimate alveolar (CAlvNO) and airway wall (JAWNO) NO in patients with sarcoidosis; and 2) utility of exhaled NO (FeNO, CAlvNO and JAWNO) measurements to detect and monitor treatment response in patients with active pulmonary sarcoidosis. Patients with sarcoidosis (n=42) and healthy non-smokers (n=20) underwent FeNO measurement at 7 flow-rates (50 to 400 ml/s). Using the Tsoukias and George (1998) model, CAlvNO and JAWNO were estimated. Both patients and healthy non-smokers were able to perform the multiple flow rates maneuver without discomfort, with first measurement success rate of 57% and 65%, respectively. No significant difference was found between patients with sarcoidosis and healthy non-smokers in exhaled NO. None were correlated with pulmonary function tests, except a significant negative correlation between CAlvNO and FVC% (p=0.001) and DLCO% (p=0.012). In 8 patients with active sarcoidosis, FeNO, CAlvNO or JAWNO were not different from those of patients with inactive sarcoidosis. Treatment of active sarcoidosis using oral prednisone and methotrexate did not show any consistent pattern of changes in CAlvNO or JAWNO. Due to a large inter-subject variability and difficulty controlling use of the inhaled corticosteroids, exhaled NO measurement did not appear to be a clinically useful method of monitoring disease progression in sarcoidosis.
Keywords: pulmonary sarcoidosis, exhaled nitric oxide, multiple flow- rates maneuver
INTRODUCTION
Sarcoidosis is a granulomatous inflammatory disorder that can affect multiple organs [1]. Pulmonary involvement occurs in more than 90% of cases [2]. Although relatively rare, sarcoidosis causes substantial patient burden of illness due to the unpredictable, insidious and, sometimes, irreversible progression of disease [3-5]. Pharmacological treatment, which mainly consists of corticosteroids and immunosuppressive agents, can decrease symptoms. However, spontaneous remission may occur and, therefore, the optimal approach for initiating and titrating treatment is still controversial [3]. The availability of a non-invasive technique that could reliably detect changes in airway inflammation could benefit disease management [2, 6].
Exhaled nitric oxide (NO) was first discovered in the exhaled breath of rabbits, guinea pigs, and humans by Gustaffson et al. in early 1990s [7]. This discovery was followed by intense interest in the potential use of exhaled NO as a noninvasive marker of inflammation and oxidative stress in the lungs [8]. Two techniques are currently used for measuring exhaled NO [9]. In offline measurements, expired air is collected into a reservoir and the concentration of NO is analyzed from this sample [9]. The more commonly used method involves analyzing the fraction of end tidal exhaled NO (FeNO) [9]. The concentration of FeNO is inversely related to flow-rate. Therefore, when patients exhale at lower flow rates, more NO is contributed from the airways relative to the overall concentration in the breath [10]. This characteristic pattern occurs because the slower flow rate allows more time for NO to enter from the airway and be exhaled.
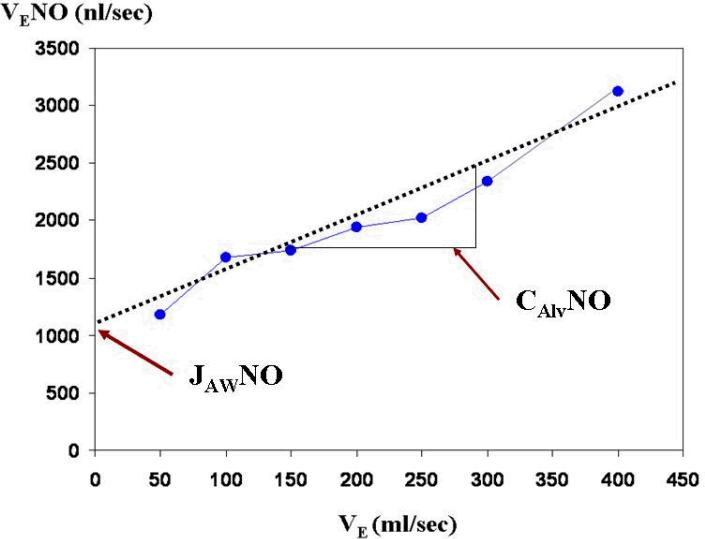
Using this flow-rate dependent characteristic, Tsoukias and George [10] developed a two-compartment model that can be used to calculate steady-state NO levels in alveoli (CAlvNO, ppb) and flux of NO from the airway wall (JAWNO, nl/sec). According to this model, the lung is comprised of two separate regions: a rigid and non-expansible airway compartment and an expansible alveolar compartment [10, 11]. Two parameters, CAlvNO, and JAWNO, define the contributions from each compartment. While alveolar NO (CAlvNO) moves through the conducting airways toward the mouth during exhalation, NO diffuses from airway wall (JAWNO). CAlvNO and JAWNO are estimated by measuring NO elimination rate (VLNO, nl/sec), which is a product of FeNO and measurement flow-rate. When FeNO is measured at two or more flow-rates (VE, ml/s), a linear relationship is displayed between VE and VLNO (Figure 1). CAlvNO and JAWNO can be estimated from the slope and intercept. This technique has the advantage of potentially providing more specific information about airway inflammation as contributions of the two compartments can be analyzed separately [9].
Figure 1.
Schematics of Tsoukias and George (1998) model to estimate the flow-independent NO parameters using a minimum of two constant exhalation flow rates
The utility of FeNO in detecting and monitoring airway inflammation has been extensively studied in patients with asthma [12]. The potential of using CAlvNO and JAWNO to differentiate whether the inflammation involves peripheral or conducting airways has been under investigation in patients with various lung diseases [13-17]. However, few studies have examined the utility of FeNO or CAlvNO and JAWNO as a monitoring method in patients with sarcoidosis[18].
The aim of this pilot study was to explore: 1) feasibility of the multiple flow rates maneuver to estimate alveolar (CAlvNO) and airway wall (JAWNO) NO in patients with sarcoidosis; and 2) utility of exhaled NO (FeNO, CAlvNO or JAWNO) to detect and monitor treatment response in patients with active pulmonary sarcoidosis. Healthy non-smokers were recruited as a control group.
MATERIALS AND METHODS
Site and sample
Patients with sarcoidosis (n=42) were recruited from the Dorothy P. & Richard P. Simmons Center for Interstitial Lung Disease, University of Pittsburgh Medical Center. Healthy non-smokers (n=20) were recruited by physician referral and advertisements. The study protocol was reviewed and approved by the institutional review board and informed consent was obtained from each participant prior to data collection.
For patients with sarcoidosis, the entry criteria were: 1) ≥ 18 years of age; 2) diagnosis of sarcoidosis, confirmed by histological evidence of noncaseating epithelioid cell granulomas in one or more tissues [19]; and 3) not being on oral corticosteroids or other immunosuppressive agents at the time of enrollment. We did not exclude patients with sarcoidosis who were on inhaled corticosteroids (ICS). Active sarcoidosis was diagnosed when the patient met three or more of following criteria over 6-12 weeks: 1) complaints of progressive respiratory symptoms, such as shortness of breath, cough, dyspnea on exertion; 2) exercise desaturation of 10% or greater in arterial oxygen saturation by pulse oximetry (SpO2) or the need to increase the flow rate of supplemental oxygen during exertion; 3) pulmonary function test results (FVC, DLCO) that indicated a deterioration of 10% or greater, or 4) evidence of worsening radiographical changes [2]. For cases of newly diagnosed sarcoidosis, disease activity was determined at the next follow-up visit, which typically occurred 6-12 weeks after the initial visit. For patients diagnosed with active sarcoidosis, data collection occurred twice within a 6-12 week interval (time 1 and time 2) if possible depending on scheduled appointments.
For healthy non-smokers (n=20), entry criteria were: 1) ≥ 18 years of age; 2) non-smoker or stopped smoking more than 6 months ago. Exclusion criteria were: 1) prior history of heart, lung, liver, kidney, endocrine, or neurological disorders, e.g., heart attack, COPD, cirrhosis, hepatitis, renal failure, diabetes, thyroid disease, stroke or seizure disorders (self-report); 2) symptoms of a respiratory tract infection < 1 month prior to the study; 3) use of medications seven days prior to data collection (self-report).
Data collection
Measurement of Dyspnea
Patients with active sarcoidosis completed the University of California San Diego Shortness of Breath Questionnaire (UCSD-SOBQ). The UCSD-SOBQ asked subjects to rate the severity of shortness of breath on a 6-point scale (0=not at all to 5=maximal or unable to do because of breathlessness) while performing 21 activities of daily living associated with varying levels of exertion [20]. Three additional questions asked about daily life limitations due to shortness of breath, fear of over exertion and fear of shortness of breath. If subjects did not routinely perform the activity, they were asked to estimate the shortness of breath anticipated. The score was obtained by summing responses on the 24 items to form a total score (range 0-120) [20]. Reliability and validity of UCSD-SOBQ have been well established [20].
Measurement of FeNO
FeNO was measured using a chemiluminescence analyzer (model LR1800; Logan Research, Rochester, UK) which has sensitivity to NO from 1 to 5000 parts per billion (ppb) by volume and a resolution of 0.3 ppb adapted for on-line recording of exhaled NO concentration. The measurement technique was consistent with guidelines in the product manual and standards published by the American Thoracic Society/European Respiratory Society in 2005 [9]. Subjects were sitting in a chair during the entire measurement. When the machine gave a signal to exhale with a green light, subjects were asked to take a full inspiration from room air and then exhale into the machine while putting their lips around the mouthpiece which was connected to the analyzer. Subjects were asked to make a full exhalation as long as possible.
Prior to each measurement, a constant flow rate was programmed in the machine to provide a different level of resistance when subjects exhaled. To determine if subjects maintained a constant flow-rate during exhalation, two procedures were used: 1) continuous monitoring of expiratory flow-rate by graphic display on the monitor screen, and 2) feedback noise (clicking sound) from the machine if the flow-rate exceeded the programmed limit. When this occurred, the subject was asked to exhale slowly and not fight the resistance. If a subject exhaled slower than desired as indicated by the graphical change on the screen, faster exhalation was encouraged.
Estimation of CAlvNO, and JAWNO
In this study, we used the model by Tsoukias and George [10] to estimate CAlvNO, and JAWNO. For each subject, FeNO values were obtained at a total of seven flow rates (50, 100, 150, 200, 250, 300, 400 mL/s). A random order generator was used in order to select ordering of the seven separate flow-rates [21]. Between each measurement, subjects rested for five minutes.
Statistical analyses
Data were analyzed using the SPSS 16.0 (SPSS, Inc.; Chicago, IL, USA). Descriptive statistics (mean, median, standard deviation, percentage, and 95% confidence intervals of the mean) were used to analyze all demographic and clinical variables, and exhaled NO (FeNO at each flow rate, CAlvNO, and JAWNO). A two-sided p-value < 0.05 was considered statistically significant. Because of large within-group variability and differences in sample sizes, Mann-Whitney U test, Spearman's rank correlation, and Wilcoxon signed rank test were used.
This study was supported by the National Institute of Health/ National Institute of Nursing Research, U.S. Public Health Service (F31-NR009457) and the Leslie Hoffman Endowed Acute Care Research Award, University of Pittsburgh. Preparation of this manuscript was supported by the National Institute of Health/ National Institute of Nursing Research, U.S. Public Health Service (T32 NR008857)
RESULTS
Sample characteristics
The study sample consisted of a total of 42 patients with sarcoidosis and 20 healthy non-smokers recruited from May 2006 to November 2007. All patients had pulmonary involvement of sarcoidosis that was confirmed by transbrochial lung biopsy or mediastinoscopy. Eight patients were diagnosed with active sarcoidosis. The majority were prescribed inhaled corticosteroids (ICS) at enrollment (87.5% active sarcoidosis; 64.7 % inactive sarcoidosis). Six patients with active sarcoidosis participated in at least one or more follow-up measurements at various intervals (3 to 23 weeks) after starting treatment with oral corticosteroids and methotrexate. Detailed sample characteristics are summarized in Table 1.
Table 1.
Sample Characteristics
| Sarcoidosis (n=42) | Healthy non-smokers (n=20) | ||
|---|---|---|---|
| Active (n=8) | Inactive (n=34) | ||
| Gender (Female/Male) | 2/6 | 13/21 | 10/10 |
| Age (years) | 46.0 ± 12.2 | 51.7 ± 10.7 | 45.4 ± 10.3 |
| Race | |||
| White Caucasian | 3 (37.5 %) | 22 (64.7 %) | 18 (90 %) |
| African American | 5 (62.5 %) | 12 (35.3 %) | 0 |
| Asian | 0 | 0 | 2 (10 %) |
| Smoking history | |||
| Current | 1 (12.5 %) | 3 (8.8 %) | 0 |
| Never | 6 (75.0 %) | 20 (58.8 %) | 20 (100 %) |
| Ex-smoker | 1 (12.5 %) | 11 (32.4 %) | 0 |
| Duration of diagnosis (months) | 48.6 ± 73.7 | 92.2 ± 107.3 | |
| ICS use | 7 (87.5 %) | 22 (64.7 %) | |
| UCSD-SOBQ | 38.17 ± 29.77 | 30.9 ± 23.48 | |
| Asthma or Allergy symptoms | 3 (50 %) | 15 (44.1 %) | |
| Pulmonary function tests (% predicted) | |||
| FEV1 | 68.6 ± 24.0 | 76.4 ± 14.5 | |
| FVC | 74.4 ± 19.5 | 75.0 ± 12.2 | |
| DLCO | 74.4 ± 30.4 | 69.8 ± 24.2 | |
| Radiographic Stage | |||
| Stage 0 | 0 | 6 (17.6 %) | |
| Stage 1 | 3 (37.5 %) | 8 (23.5 %) | |
| Stage 2 | 5 (62.5 %) | 14 (41.2 %) | |
| Stage 3 | 0 | 3 (8.8 %) | |
| Stage 4 | 0 | 3 (8.8 %) | |
| Other system involvement | |||
| None | 6 (75.0 %) | 28 (82.4 %) | |
| Gastrointestinal | 1 (12.5 %) | 0 | |
| Skin | 0 | 4 (11.8 %) | |
| Neuro | 1 (12.5 %) | 1 (2.9 %) | |
| Bone | 0 | 1 (2.9 %) | |
| Serum ACE (U/min.l) | 59.3 ± 28.3 | 57.3 ± 26.8 | |
ICS = Inhaled Corticosteroids; UCSD-SOBQ=University of SanDiego Shortness of Breath Questionnaire; FEV1 = Forced Expiratory Volume at one second; FVC = Forced Vital Capacity; DLCO = diffusion capacity of the lung for carbon monoxide; ACE = Angiotensin-Converting Enzyme
Data are missing for: Pulmonary function tests 3 subjects; Length of Diagnosis 4 subjects; UCSD-SOBQ 11 subjects; Serum ACE 24 subjects
All participants were able to perform the multiple flow rates maneuver and none reported discomfort. The majority of patients with sarcoidosis (55%) and healthy non-smokers (65%) were able to complete FeNO measurement at seven flow rates in their first attempt. There was no significant difference between groups in first trial success, χ2 (1) = .58, p=NS. In less than 15% of cases, participants had to repeat the expiratory efforts twice or more for at least one flow rate to obtain successful measurement, mostly at the lowest (50 ml/sec) or highest (400 ml/sec) flow rate. In patients with sarcoidosis, two or more measurements were required in 5 patients (11.9 %) at a flow rate of 50 ml/sec and 6 patients (14.3 %) at a flow rate of 400 ml/sec. In healthy non-smokers, no additional measurement was required at a flow rate of 50 ml/sec. However two or more measurements were required in 3 participants (15 %) at a flow rate of 400 ml/sec.
When obtaining these measurements, a learning effect was observed. When we examined the number of attempts at the first assigned flow rate, additional measurements were required in 12 patients with sarcoidosis (28.6 %) and 3 healthy non-smokers (15%).
End-tidal exhaled NO (FeNO) at 7 flow rates
FeNO levels showed a similar pattern for both patients with sarcoidosis and healthy non-smokers, i.e., the highest level at the lowest flow rate (50 ml/sec) and lowest level at the highest flow rate (400 ml/sec). Intersubject variability was large in all three groups for all seven flow rates. Mean FeNO at each flow rate tended to be lower in patients with active sarcoidosis compared with those with active sarcoidosis or healthy non-smokers. However, there were no significant differences among groups in FeNO for all seven flow rates (Table 2).
Table 2.
Exhaled NO (FeNO at 7 flow rates, CAlvNO and JAWNO)
| Sarcoidosis (n=42) | |||
|---|---|---|---|
| Active (n=8) | Inactive (n=34) | Healthy non-smokers (n=20) | |
| FeNO at 50ml/sec (ppb) | |||
| Mean ± SD | 20.5±13.0 | 24.8 ± 14.7 | 37.0± 16.3 |
| Median | 15.1 | 20.5 | 29.2 |
| 95% CI | 9.6-31.4 | 19.7-30.0 | 24.4 – 39.6 |
| FeNO at 100ml/sec (ppb) | |||
| Mean ± SD | 13.0±6.7 | 15.4 ± 9.7 | 18.0 ± 9.6 |
| Median | 11.45 | 12.3 | 15.9 |
| 95% CI | 7.3-18.6 | 12.0-18.8 | 13.5 – 22.5 |
| FeNO at 150ml/sec (ppb) | |||
| Mean ± SD | 10.1±4.3 | 12.2 ± 7.1 | 13.1 ± 7.9 |
| Median | 9.6 | 10.8 | 11.4 |
| 95% CI | 6.4-13.7 | 9.7-14.7 | 9.5 – 16.8 |
| FeNO at 200ml/sec (ppb) | |||
| Mean ± SD | 8.3±4.3 | 11.4 ± 7.3 | 11.9 ± 7.1 |
| Median | 8.6 | 9.4 | 10.7 |
| 95% CI | 4.8-11.9 | 8.8-13.9 | 8.7 – 15.2 |
| FeNO at 250ml/sec (ppb) | |||
| Mean ± SD | 8.1±3.4 | 9.4 ± 5.5 | 10.8 ± 6.6 |
| Median | 8.1 | 7.8 | 9.4 |
| 95% CI | 5.3-10.9 | 7.5-11.3 | 7.7 – 13.8 |
| FeNO at 300ml/sec (ppb) | |||
| Mean ± SD | 7.4±3.6 | 9.1 ± 5.2 | 9.4 ± 5.5 |
| Median | 7.1 | 8.1 | 7.4 |
| 95% CI | 4.4-10.3 | 7.3-10.9 | 6.8 – 12.0 |
| FeNO at 400ml/sec (ppb) | |||
| Mean ± SD | 5.7±3.2 | 8.1 ± 4.8 | 7.9± 4.3 |
| Median | 4.4 | 7.6 | 6.9 |
| 95% CI | 3.00-8.3 | 6.4-9.8 | 5.9 – 9.9 |
| CAlvNO (ppb) | |||
| Mean ± SD | 3.8±3.3 | 5.7 ± 4.2 | 4.7±3.0 |
| Median | 2.9 | 4.8 | 3.9 |
| 95% CI | 1.0-6.6 | 4.2-7.2 | 3.3-6.1 |
| JAWNO (nl/sec) | |||
| Mean ± SD | 927.4±729.7 | 990.0 ± 723.2 | 1368.7±759.3 |
| Median | 693.3 | 872.5 | 1193.4 |
| 95% CI | 317.4-1537.4 | 737.7-1242.4 | 1013.4-1724.1 |
FeNO = Fraction of end tidal nitric oxide
Estimated airway NO flux (JAWNO) and alveolar NO concentration (CAlvNO)
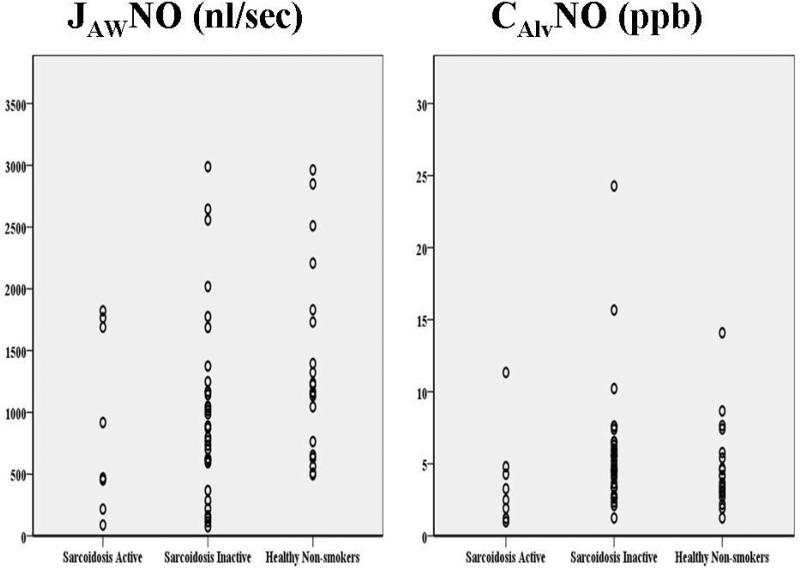
Intersubject variability was large and no statistical significance was found for JAWNO or CAlvNO among three groups (Figure 2). There was a tendency for a higher mean JAWNO and CAlvNO in healthy non-smokers compared to patients with active and inactive sarcoidosis.
Figure 2.
JAWNO and CAlvNO estimated in 42 patients with sarcoidosis (8 active; 34 inactive) and 20 healthy non-smokers. Intersubject variability was large. Neither JAWNO nor CAlvNO showed significant difference among groups.
Correlation with other clinical parameters and clinical outcomes
Pulmonary function data were available in 39 patients with sarcoidosis. There were significant negative correlations between CAlvNO and FVC% (r=−0.52, p=0.001) and DLCO% (r=−0.40, p=0.012). With this exception, no significant correlations were seen. None of the additional clinical parameters examined, i.e., UCSD-SOBQ scores, serum angiotensin-converting enzyme (ACE) levels, or radiographic stage, showed a significant correlation with FeNO, JAWNO or CAlvNO.
Of 34 patients with inactive sarcoidosis, 29 returned for follow-up after 24 ± 14 (mean±SD) weeks. None showed radiographic changes or required treatment medication at follow-up. With the exception of one patient who developed skin involvement, none developed a new organ system involvement. Given the limited change, it was not feasible to test for a possible relationship between NO data and clinical outcomes.
Use of exhaled NO in diagnosing active sarcoidosis and monitoring response to treatment
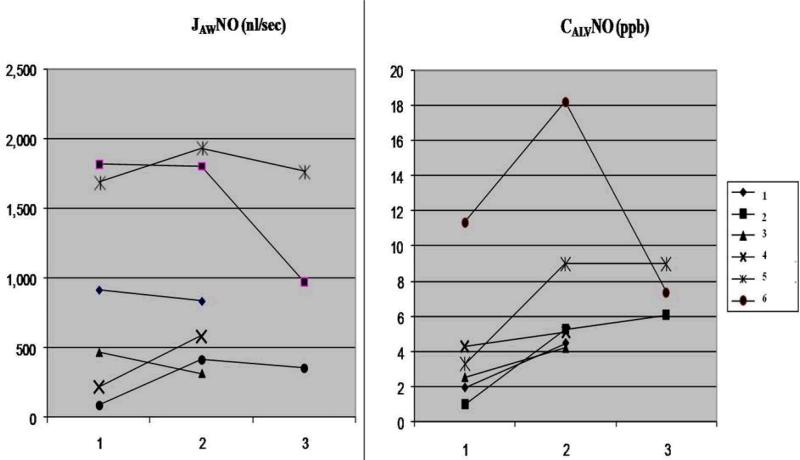
The sample included 8 patients with active sarcoidosis who had not yet received treatment. No significant difference was found in FeNO at seven flow rates, CAlvNO or JAWNO, compared with patients with inactive sarcoidosis. Six patients completed follow-up measurement at various intervals (3 to 23 weeks) while on treatment with various doses of oral prednisone and methotrexate. Clinical characteristics of these 6 patients with active sarcoidosis were summarized in Table 3. There was no consistent trend in the change in FeNO at each flow rate, JAWNO or CAlvNO (Table 4 and Figure 3). The trend of change in exhaled NO was not correlated with changes in UCSD-SOBQ scores. Clinical outcome of these 6 patients was monitored for up to 57 weeks after the last exhaled NO measurement. Two continued to have active sarcoidosis that required an increase in treatment medications. None developed a new organ system involvement. Due to the small sample size and variability in the pattern of changes in exhaled NO, it was not possible to explore a possible relationship between changes in exhaled NO and clinical outcome.
Table 3.
Parameters of clinical activity at initial measurement in 6 patients with active sarcoidosis.
| No. | Serum ACE >55 U/min.l | Fever | Other symptoms | BAL CD4/CD8 > 2.5 | Hypercalcemia | Uveitis | Erythema Nodosum | Polyarthralgia | Lymphadenopathy | X-ray progression | Worsening of respiratory function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | − | + | Not available | − | − | − | − | − | + | − |
| 2 | − | − | + | Not available | − | − | − | − | − | − | + |
| 3 | + | − | + | Not available | − | − | − | − | − | + | + |
| 4 | Not available | − | + | Not available | Not available | − | − | − | − | + | + |
| 5 | + | − | + | Not available | − | − | − | − | − | + | − |
| 6 | + | − | + | Not available | − | − | − | − | − | + | + |
Note. +, Presence; −, Absence
Table 4.
Changes in exhaled NO (FeNO at 50 ml/sec, CAlvNO, JAWNO) during treatment in 6 patients with active sarcoidosis.
| No. | Age Gender |
Duration of diagnosis (months) | ICS use | UCSD-SOBQ | Baseline pulmonary function tests (% predicted) |
Asthma or Allergy Symptoms | Follow-up interval (weeks) | Direction of Change in Exhaled NO at 1st and 2nd Visit |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 | FVC | DLCO | FeNO at 50ml/sec (ppb) | CAlvNO (ppb) | JAWNO (nl/sec) | |||||||
| 1 | 37 years Female |
17 | Yes | 42* | 93 | 98 | 64 | No | 23 | ↑ | ↑ | ↑ |
| 2 | Male 57 years |
22 | No | 3, 0+ | 106 | 97 | 118 | No | 3,14 | ↑, ↓ | ↑,↑ | ↓,↓ |
| 3 | 31 years Female |
6 | Yes | 88, 58 | 42 | 42 | 48 | Yes | 6 | ↓ | ↑ | ↓ |
| 4 | 63 years Female |
20 | Yes | 37, 28 | 58 | 65 | 39 | Yes | 7 | ↑ | ↑ | ↑ |
| 5 | 51 years Male |
36 | Yes | 13, 24, 18 | 90 | 83 | 109 | No | 6,8 | ↑, ↓ | ↑, - | ↑, ↓ |
| 6 | 47 years Female |
228 | Yes | 46, 50, 32 | 58 | 67 | 63 | Yes | 6,8 | ↑, ↓ | ↑, ↓ | ↑, ↓ |
Scores available for 2nd visit only *
Scores available for 2nd and 3rd visit only+
Increase in FeNO at 50ml/sec, CAlvNO, or JAWNO: ↑
Decrease in FeNO at 50ml/sec, CAlvNO, or JAWNO: ↓
No change in FeNO at 50ml/sec, CAlvNO, or JAWNO: -
Figure 3.
Change in JAWNO and CAlvNO in 6 patients with active sarcoidosis at baseline (1) and 2nd (2) and 3rd (3) follow-up visit after being on treatment. Three patients were available for 3rd follow-up measurement.
DISCUSSION
Sarcoidosis is a chronic multi-system inflammatory disease of unknown etiology, characterized by an increase in activity of macrophages and activated T-cells [22]. The granulomatous process is known to be driven by interactions between alveolar macrophages, CD4 T helper cells, and a Th1-cytokines [1]. Activation of inflammatory cells, such as alveolar macrophages, stimulate activities of inducible nitric oxide synthase (iNOS) that may possibly increase production of NO[23]. An increase in the expression of iNOS has been reported in inflammatory, but not fibrotic, lesions in sarcoidosis[24]. We explored whether differentiating production of NO in the conducting airways and peripheral part of lungs by estimating JAWNO and CAlvNO might provide valuable information in monitoring disease progression and response to treatment. To our knowledge, this is the first study that explored feasibility of the multiple flow rates maneuver to measure exhaled NO and attempted longitudinal follow-up measurements for patients with active pulmonary sarcoidosis.
Findings of this study demonstrated that patients with active and inactive sarcoidosis could easily accomplish the seven flow rates maneuver required to measure JAWNO and CAlvNO. However, values did not differ significantly from healthy non-smokers for any of the seven flow rates. Neither JAWNO nor CAlvNO showed any significant difference between groups. In patients with sarcoidosis, CAlvNO showed a significant negative correlation with FVC% and DLCO%. In patients with active sarcoidosis, exhaled NO (FeNO at all seven flow rates, JAWNO and CAlvNO) values were not different from patients with inactive disease.
Consistent with this finding, prior studies have reported that FeNO measurement is easy to perform with minimum practice [9] by patients with chronic lung disease (e.g., asthma, COPD) [25]. The utility of FeNO as a non-invasive monitoring tool has been most extensively studied in patients with asthma [26], leading to FDA approval of this technique as an option for disease management [27]. Initial studies suggested the potential utility of FeNO in screening, diagnosis, and management of asthma. Findings indicated that FeNO levels in patients with asthma were higher than those of healthy controls[28, 29] and had good correlations with other markers of airway inflammation, e.g., eosinophils [30]; and steroid responsiveness[31]. Several randomized controlled trials concluded that FeNO measurement was effective in asthma control [32-34]. However, recent studies reported no added benefit from FeNO measurement in asthma control [35, 36] and the potential to prescribe higher dose of ICS [27]. Therefore the utility of FeNO measurement in patients with asthma remains unclear [28-31].
In patients with sarcoidosis, FeNO was not significantly different from healthy non-smokers consistent with prior studies [37-39]. While consistent, we believe this finding may, in part, have been influenced by sample characteristics. Approximately 70% of patients with sarcoidosis in the present study were prescribed ICS, whereas none of the patients in prior studies were prescribed oral or ICS [37-40]. The potential of ICS to reduce FeNO levels is well established [31, 41]. Therefore, it is unclear whether this finding resulted from the disease process or use of ICS. No literature was identified that examined the change in FeNO in response to the use of ICS in patients with sarcoidosis.
Compared with FeNO levels reported in four prior studies (range of mean 6.7- 9.8 ppb; [37-40]), mean FeNO in our study was much higher for both patients and healthy non-smokers. We therefore explored the potential that an extraneous factor, e.g., ambient NO or room temperature, caused this difference by examining FeNO and ambient NO levels at the time of measurement. No significant correlation was found (Spearman's rho = −0.16, p=0.93). It was also possible other factors that can elevate FeNO values influenced this change, e.g., air quality or temperature outside of lab. This potentially confounding influence was not controlled in this study. Also, we did not exclude current smokers (1 in active sarcoidosis and 3 in inactive sarcoidosis). Because cigarette smoking is known to decrease FeNO levels [38], we conducted a post hoc exploratory analysis after excluding these 4 smokers. However, the results did not differ from the original analysis.
In patients with sarcoidosis, whereas FeNO and JAWNO did not show any significant correlations, CAlvNO showed a significant negative correlation with FVC% and DLCO%. This finding may have been caused by worsening of lung function and decreased diffusion capacity that altered the alveolar concentration of NO. A similar correlation between CAlvNO and FVC% and DLCO% has been reported in studies that recruited patients with mixed types of interstitial lung disease [14, 15].
For patients with active sarcoidosis who completed follow-up, in all 6 cases, the trend of change in exhaled NO (FeNO, CAlvNO and JAWNO) did not show any consistent pattern as a result of treatment with prednisone and methotrexate. Our finding is not consistent with the report by Moodley et al. (1999) that showed a significant decrease in FeNO after 6 weeks of treatment with oral prednisone (40 mg) in 8 newly diagnosed patients with sarcoidosis. The potential effect of ICS, heterogenous patient characteristics (e.g. baseline UCSD-SOBQ scores), various treatment regimens and follow-up intervals might be possible explanations for this inconsistency in findings.
This study had a number of limitations. First, the sample size was small. Second, exhaled NO levels are known to vary due to multiple reasons, e.g. individual difference, different analyzer, etc [9, 43]. To maintain consistency, all measurements were obtained by one data collector using a single analyzer located in the same room between the hours of 9am and 5pm. Nevertheless, large individual variances in exhaled NO were consistent across subject groups. Third, we did not investigate reproducibility of the multiple flow rates maneuver due to the potential to cause fatigue, especially in participants with active pulmonary sarcoidosis with visible evidence of shortness of breath (an average of 33 minutes was required to obtain seven measurements). In a previous study enrolling patients with various stages of chronic obstructive pulmonary disease (COPD), a high level of reproducibility of the multiple flow rates maneuver was reported (Intra-class correlation coefficient 0.96) [13]. Nevertheless, testing reproducibility is recommended. Fourth, almost 70% of patients with sarcoidosis were prescribed ICS at enrollment which makes it difficult to compare our findings to studies where subjects were not prescribed these medications [37, 39, 40]. However, airway hyperreactivity is common in sarcoidosis [44-47] and ICS are commonly prescribed to manage this problem. Sixth, in healthy non-smokers subject screening was based on self-reported health information. Therefore, it is possible that unreported information influenced exhaled NO levels, e.g. smoking, alcohol use. Finally, the follow-up period was limited due to the pilot feasibility nature of the present study. Extending the follow-up period with further measurements may provide more valuable insight regarding changes in exhaled NO during the disease progress in patients with sarcoidosis. It is possible that our negative results might be due to variability in the disease course in pulmonary sarcoidosis [3]. Also, poor correlations have been reported between physiological aberrations and histological severity in patients with sarcoidosis[42]. Considering this variability, it may not have been possible to detect a pattern of correlation in this pilot study with a relatively short term follow and small sample.
In the present study, due to a large inter-subject variability and difficulty controlling potential confounders, particularly use of the ICS, monitoring exhaled NO did not appear to be a clinically useful method of detecting disease progression in patients with sarcoidosis. However, given the sample size and length of follow-up, it is premature to conclude that exhaled NO has no utility in patients with pulmonary sarcoidosis. A longitudinal study that controlled potential confounders, especially the effect of ICS, would be challenging, but worth further consideration.
Table 5.
Clinical outcomes in 6 patients with active sarcoidosis.
| No. | Follow-up interval (weeks) | Disease activity | Changes in treatment | Other new system involvement |
|---|---|---|---|---|
| 1 | 16 | Inactive | Continue methotrexate therapy | No |
| 2 | 20 | Active | Discontinue methotrexate due to side effect | No |
| Increase dose of prednisone | ||||
| 3 | 57 | Inactive | Continue methotrexate | No |
| Discontinue prednisone | ||||
| 4 | 29 | Inactive | Discontinue treatment | No |
| 5 | 32 | Inactive | Continue methotrexate | No |
| Taper off prednisone | ||||
| 6 | 24 | Active | Increase dose of methotrexate | No |
Note. Clinical outcomes at follow-up visit 16-57 weeks after the last measurement.
Acknowledgement
This study was performed at Dorothy P. & Richard P. Simmons Center for Interstitial Lung Disease and Comprehensive Lung Center at University of Pittsburgh Medical Center. The authors wish to thank clinical staff for their sincere help in subject recruitment. The authors also acknowledge all participants for their support for the study.
Funding provided by the NIH, National Institute of Nursing Research, U.S. Public Health Service (F31 NR009457 and T32 NR008857) and Leslie Hoffman Endowed Acute Care Research Award, University of Pittsburgh
Contributor Information
JiYeon Choi, University of Pittsburgh School of Nursing, Pittsburgh, PA.
Leslie A. Hoffman, Department of Acute/Tertiary Care University of Pittsburgh School of Nursing, Pittsburgh, PA.
Jigme M. Sethi, Division of Pulmonary Allergy and Critical Care Medicine, Department of Medicine University of Pittsburgh School of Medicine, Pittsburgh, PA.
Thomas G. Zullo, Department of Acute and Tertiary Care University of Pittsburgh School of Nursing.
Kevin F. Gibson, Division of Pulmonary Allergy and Critical Care Medicine, Department of Medicine Clinical Director, Dorothy P. & Richard P. Simmons Center for Interstitial Lung Disease University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
- 1.Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60(5):565–82. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 3.Costabel U. Sarcoidosis: clinical update. Eur Respir J Suppl. 2001;32:56s–68s. [PubMed] [Google Scholar]
- 4.Raghu G. Interstitial Lung Diseases: A Clinical Overview and General Approach. In: Fishman A, et al., editors. Fishman's Pulmonary Disease and Disorders. McGraw Hill; New York: 1998. [Google Scholar]
- 5.Rybicki BA, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 6.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J. 2002;19(5):794–6. doi: 10.1183/09031936.02.00492002. [DOI] [PubMed] [Google Scholar]
- 7.Gustaffson L, Leone A, Persson M. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Comm. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 8.Kharitonov S, Barnes P. Exhaled Markers of Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 10.Tsoukias NM, George SC. A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol. 1998;85(2):653–66. doi: 10.1152/jappl.1998.85.2.653. [DOI] [PubMed] [Google Scholar]
- 11.George SC, et al. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004;96(3):831–9. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kharitonov S, Barnes P. Clinical aspects of exhaled nitric oxide. Eur Resp J. 2000;16:781–792. doi: 10.1183/09031936.00.16478100. [DOI] [PubMed] [Google Scholar]
- 13.Brindicci C, et al. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J. 2005;26(1):52–9. doi: 10.1183/09031936.04.00125304. [DOI] [PubMed] [Google Scholar]
- 14.Girgis RE, et al. Partitioning of alveolar and conducting airway nitric oxide in scleroderma lung disease. Am J Respir Crit Care Med. 2002;165(12):1587–91. doi: 10.1164/rccm.2104003. [DOI] [PubMed] [Google Scholar]
- 15.Lehtimaki L, et al. Extended exhaled NO measurement differentiates between alveolar and bronchial inflammation. AmJ Resp Crit Care Med. 2001;163:1557–1561. doi: 10.1164/ajrccm.163.7.2010171. [DOI] [PubMed] [Google Scholar]
- 16.van Veen IH, et al. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur Respir J. 2006;27(5):951–6. doi: 10.1183/09031936.06.00087905. [DOI] [PubMed] [Google Scholar]
- 17.Gelb AF, et al. Alveolar and airway sites of nitric oxide inflammation in treated asthma. Am J Respir Crit Care Med. 2004;170(7):737–41. doi: 10.1164/rccm.200403-408OC. [DOI] [PubMed] [Google Scholar]
- 18.Brindicci C, et al. Nitric oxide from peripheral lung is increased in idiopathic pulmonary fibrosis and sarcoidosis. Proceedings of the American Thoracic Society. 2005:A931. [Google Scholar]
- 19.Nunes H, et al. Imaging in sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):102–20. doi: 10.1055/s-2007-970336. [DOI] [PubMed] [Google Scholar]
- 20.Eakin EG, et al. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–24. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 21.Oxford Brooks University [2006 December 4];Oxford Brooks University School of Social Science and Law Department of Psychology Random Order Generator. Available from: http://www.brooks.ac.uk/schools/social/psych/order.html.
- 22.Lynch JP, 3rd, et al. Pulmonary sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):53–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- 23.Adding L, Gustaffson L. In: Physiology of nitric oxide, in Disease markers in exhaled breath. Marczin N, et al., editors. Marcel Dekker; NewYork Basel: 2003. pp. 29–72. [Google Scholar]
- 24.Lakari E, et al. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol. 2002;117(1):132–42. doi: 10.1309/w7t9-hw9v-v94b-r9km. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonov S. Exhaled markers of inflammatory lung diseases: ready for routine monitoring? Swiss Med Weekly. 2004;134:175–192. doi: 10.4414/smw.2004.10411. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, et al. Markers of lung disease in exhaled breath: nitric oxide. Biol Res Nurs. 2006;7(4):241–55. doi: 10.1177/1099800405286131. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administrations [2004 July 21];FDA Consumer Magazine Updates. 2003 Available from: http://www.fda.gov/fdac/departs/2003/403_upd.htm.
- 28.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368–70. [PubMed] [Google Scholar]
- 29.Kharitonov SA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343(8890):133–5. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 30.Jatakanon A, et al. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharitonov S, Yates D, Barnes P. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. American Journal of Respiratory and Critical Care Medicine. 1996;153(1):454–7. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 32.Green RH, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 33.Pijnenburg MW, et al. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172(7):831–6. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 34.Smith AD, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 35.de Jongste JC, et al. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med. 2009;179(2):93–7. doi: 10.1164/rccm.200807-1010OC. [DOI] [PubMed] [Google Scholar]
- 36.Shaw DE, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(3):231–7. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell DM, et al. Exhaled nitric oxide and bronchoalveolar lavage nitrite/nitrate in active pulmonary sarcoidosis. Am J Respir Crit Care Med. 1997;156(6):1892–6. doi: 10.1164/ajrccm.156.6.9705013. [DOI] [PubMed] [Google Scholar]
- 38.Wilsher ML, et al. Exhaled nitric oxide in sarcoidosis. Thorax. 2005;60(11):967–70. doi: 10.1136/thx.2004.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziora D, Kaluska K, Kozielski J. An increase in exhaled nitric oxide is not associated with activity in pulmonary sarcoidosis. Eur Respir J. 2004;24(4):609–14. doi: 10.1183/09031936.04.00110803. [DOI] [PubMed] [Google Scholar]
- 40.Moodley YP, Chetty R, Lalloo UG. Nitric oxide levels in exhaled air and inducible nitric oxide synthase immunolocalization in pulmonary sarcoidosis. Eur Respir J. 1999;14(4):822–7. doi: 10.1034/j.1399-3003.1999.14d17.x. [DOI] [PubMed] [Google Scholar]
- 41.Kharitonov S, et al. Dose-dependent onset and duration of action of 100/400 mcg budesonide on exhaled nitric oxide and related changes in other markers of airway inflammation in mild asthma. American Journal of Respiratory and Critical Care Medicine. 2000;161:A186. [Google Scholar]
- 42.Bergin CJ, et al. Sarcoidosis: correlation of pulmonary parenchymal pattern at CT with results of pulmonary function tests. Radiology. 1989;171(3):619–24. doi: 10.1148/radiology.171.3.2717731. [DOI] [PubMed] [Google Scholar]
- 43.Borrill Z, et al. A comparison of exhaled nitric oxide measurements performed using three different analysers. Respir Med. 2006;100(8):1392–6. doi: 10.1016/j.rmed.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal AN, et al. Bronchial hyperresponsiveness in patients with sarcoidosis. J Assoc Physicians India. 2004;52:21–3. [PubMed] [Google Scholar]
- 45.Manresa Presas F, Romero Colomer P, Rodriguez Sanchon B. Bronchial hyperreactivity in fresh stage I sarcoidosis. Ann N Y Acad Sci. 1986;465:523–9. doi: 10.1111/j.1749-6632.1986.tb18529.x. [DOI] [PubMed] [Google Scholar]
- 46.Marcias S, et al. Aspecific bronchial hyperreactivity in pulmonary sarcoidosis. Sarcoidosis. 1994;11(2):118–22. [PubMed] [Google Scholar]
- 47.Shorr AF, Torrington KG, Hnatiuk OW. Endobronchial involvement and airway hyperreactivity in patients with sarcoidosis. Chest. 2001;120(3):881–6. doi: 10.1378/chest.120.3.881. [DOI] [PubMed] [Google Scholar]
- 48.Leach CL. Improved delivery of inhaled steroids to the large and small airways. Respir Med. 1998;92(Suppl A):3–8. doi: 10.1016/s0954-6111(98)90211-6. [DOI] [PubMed] [Google Scholar]