Abstract
CD8+ memory T cells endanger allograft survival by causing acute and chronic rejection and prevent tolerance induction. We explored the role of CD27:CD70 T-cell costimulatory pathway in alloreactive CD8+/CD4+ T-cell activation. CD27-deficient (CD27−/−) and wild-type (WT) B6 mice rejected BALB/c cardiac allografts at similar tempo, with or without depletion of CD4+ or CD8+ T cells, suggesting that CD27 is not essential during primary T-cell alloimmune responses. To dissect the role of CD27 in primed effector and memory alloreactive T cells, CD27−/− or WT mice were challenged with BALB/c hearts either 10 or 40 days after sensitization with donor-type skin grafts. Compared to WT controls, allograft survival was prolonged in day 40- but not day 10-sensitized CD27−/− recipients. Improved allograft survival was accompanied by diminished secondary responsiveness of memory CD8+ T cells, which resulted from deficiency in memory formation rather than their lack of secondary expansion. Chronic allograft vascu-lopathy and fibrosis were diminished in CD27−/− recipients of class I- but not class II-mismatched hearts as compared to WT controls. These data establish a novel role for CD27 as an important costimulatory molecule for alloreactive CD8+ memory T cells in acute and chronic allograft rejection.
Keywords: CD8, CD4, costimulation, memory T cells, transplantation
Introduction
Human transplant recipients harbor significant numbers of alloreactive memory T cells generated by previous encounter to alloantigens, via heterologous immunity or homeostatic proliferation as a result of lymphoablation (1,2). Alloreactive memory T cells contribute to both acute and chronic allograft rejection, indicating that current im-munosuppressive therapies do not inhibit the generation or maintenance of T-cell memory (3–5). Immunologic memory is thought to be one of the major barriers that preclude the induction of antigen-specific tolerance in clinical transplantation (6). Donor-reactive memory T cells can precipitate costimulatory blockade-resistant allograft rejection (7–9). Inability of classical costimulatory blockade to induce long-term allograft survival in the presence of memory T cells prompted investigators to block activation pathways unique to generation and the recall of memory T cells.
The CD27:CD70 pathway, a member of the costimulatory TNF:TNFR superfamily, plays an important role in the formation of effector and memory T cells, likely by enhancing T-cell survival (10). CD27-deficient (CD27−/−) mice show reduced numbers of CD4+ and CD8+ T cells in lungs and spleens after primary and secondary infection with influenza A (11). Thus, CD27 controls the accumulation of CD4+ and CD8+ effector T cells at the site of infection. Costimulatory effects of CD27 on both CD4+ and CD8+ T cells were also revealed in CD70-transgenic mice, which demonstrated increased numbers of CD4+ and CD8+ T cells with effector phenotype and accumulation of CD8+ effector T cells upon challenge to influenza virus (12). Interestingly, upon infection with in-fluenza virus, secondary CD8+ T-cell responses are reduced in CD27−/− mice (11,13). Many observations argue for a role of CD27:CD70 interactions in memory T-cell formation, as the formation of influenza virus-specific memory CD8+ T cells was reduced in CD27−/− mice, as was the formation of ovalbumin-specific memory CD8+ T cells in mice treated with anti-CD70 antibody (14). Conversely, contraction of virus-specific CD8+ effector T cells was reduced in CD70-transgenic mice, resulting in increased memory cell formation (12). As CD27−/− memory CD8+ T cells were also deficient in secondary expansion, CD27 may contribute to memory cell differentiation. In contrast, the role of CD27:CD70 signaling in CD4+ T-cell memory is relatively unexplored (10).
Despite evidence for the role of the CD27:CD70 pathway in CD8+ T-cell-mediated immune responses to viral infections, the effects on CD8+ T-cell-mediated alloimmune responses are not completely understood. Using a complete MHC-mismatched murine model of cardiac transplantation, we recently demonstrated that anti-CD70 mAb induces long-term survival of allografts in CD28-deficient recipients by inhibiting the activation of effector and memory CD8+ T cells (15). The aim of this study was to use an established murine model of sensitized recipients of cardiac allografts (9) to further dissect the role of the CD27:CD70 pathway on effector and memory T-cell responses to al-loantigens, with particular focus on the effects of CD27 signaling.
Materials and Methods
Animals
C57BL/6 (B6, H2b), BALB/c (H2d), B6.C-H2bm12/KhEg (bm12), CBA/Caj (CBA) and B6.C-H2bm1/By (bm1) mice were purchased at The Jackson Laboratory, Bar Harbor, ME. CD27−/− B6 mice were obtained from the Netherlands Cancer Institute, Amsterdam (11) and maintained as a breeding colony in our animal facility. All mice were used at 8 to 12 weeks of age and housed in accordance with institutional and National Institutes of Health guidelines.
Heart and skin transplantation
To presensitize prospective cardiac allograft recipients, full-thickness skin grafts from BALB/c donors (~1cm2) were transplanted onto the dorsal thorax of wild-type (WT) or CD27−/− B6 mice. 10 or 40 days after skin transplantation, recipients were rechallenged with vascularized cardiac allografts from donor-type (BALB/c) or third-party (CBA) animals (9). Cardiac allografts were heterotopically transplanted, using microsurgical techniques (16).
Antibodies and in vivo treatment protocol
For CD4 or CD8 T-cell depletion, mice received 0.1 mg anti-CD4 (GK1.5) or anti-CD8 (GK2.43) depleting mAbs (Bioexpress Cell Culture, West Lebanon, NH) i.p. at day –6, –3 and –1 before heart transplantation, ensuring >95% depletion of the respective cell type in the peripheral blood on the day of transplantation. Cell counts start to recover by ~2 weeks after the last injection, with complete recovery occurring within 10 weeks (15,17–19).
For NK cell depletion, mice received 0.2 mg anti-NK mAb (PK136; Bioex-press Cell Culture, West Lebanon, NH) at day –3, –-2 and –1 before heart transplantation, ensuring >95% depletion of NK cells in the peripheral blood on day 0 and day 7 posttransplantation.
Donor-specific antibodies (DSA)
Donor-type BALB/c splenocytes were incubated over 30 minutes with recipient sera in various dilution steps and afterward stained with fluorochrome-labeled mAbs against mouse IgG1 and IgG2a (BD Biosciences, San Jose, CA). Amount of DSA was assessed by measuring the percentage of IgG1-or IgG2a-bearing splenocytes.
Histology
Cardiac allografts were harvested at 8 weeks after transplantation, fixed in 10% formalin, embedded in paraffin, coronarly sectioned, stained with hematoxylin/eosin, Elastica-van Gieson’s and Masson’s trichrome dyes and analyzed by light microscopy (20,21). The degree of rejection was determined according to International Society of Heart and Lung Transplantation guidelines (22). The severity of vasculopathy was graded according to the percentage of luminal occlusion by intimal thickening with a scoring system described previously (20,21,23,24).
ELISPOT assay
ELISPOT assays were performed using mouse IFN-γ/IL-4 ELISPOT Kits (BD Biosciences). 0.5 × 106 unselected splenocytes or 0.1–0.25 × 106 enriched CD4+ or CD8+ T cells derived from splenocytes of CD27−/− or WT B6 recipient mice were used as responder cells and restimulated with 0.5 × 106 irradiated splenocytes from naïve donor-type (BALB/c) or third-party (CBA) mice. To obtain enriched (>93%) CD4+ or CD8+ T cells, splenocytes were purified by MACS using a CD4 or CD8a T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
Flow cytometry
Unselected splenocytes from allograft recipients were stained with fluorochrome-labeled mAbs against CD4, CD8, CD25, CD44 and CD62L (BD Biosciences). Percentages of effector-memory CD4+ or CD8+ T cells expressing the CD44highCD62Llow phenotype were calculated as described (25).
Statistics
Kaplan–Meier survival graphs were constructed, and the log-rank comparisons of the groups were used to calculate p-values. Student’s t-test was used for comparison of means between two groups or one-way ANOVA if more than two groups were present. Data were expressed as mean ± standard error of the mean.
Results
CD27 does not affect priming of naïve CD4+ or CD8+ cells during alloimmune responses
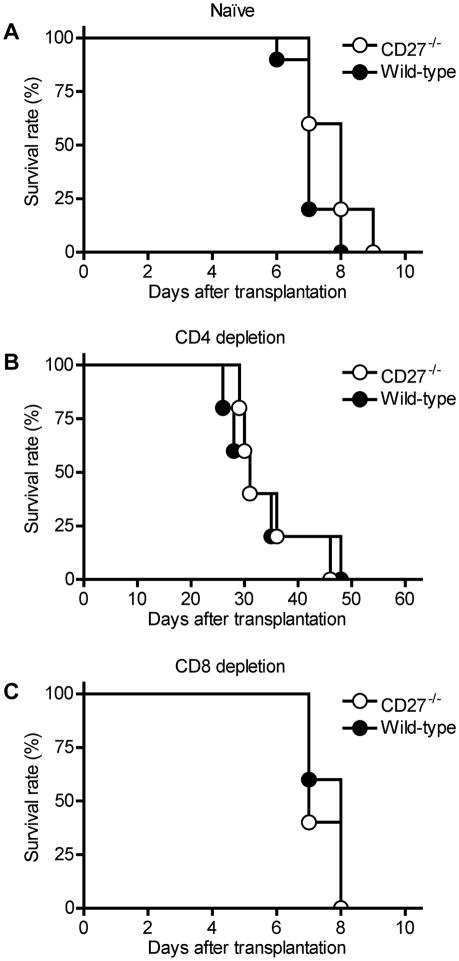
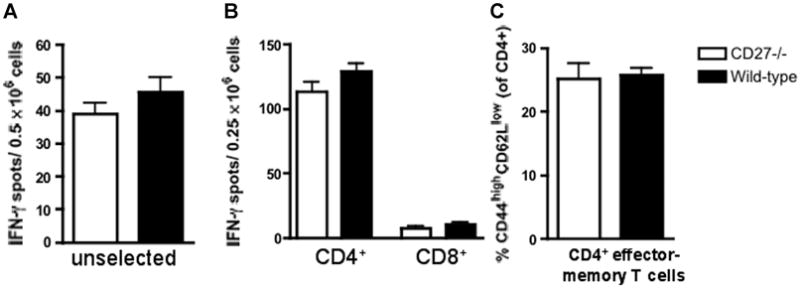
To investigate the role of the CD27:CD70 pathway in acute cardiac rejection, we used CD27−/− and WT B6 mice as recipients of fully MHC-mismatched allografts from BALB/c donor animals. CD27−/− and WT mice rejected allografts at a similar tempo (Figure 1A). To dissect the effects of CD27-deficiency on CD4+ and CD8+ T-cell-mediated allo-graft rejection, BALB/c allografts were transplanted into CD27−/− or WT animals after pretreatment with depleting anti-CD4 or anti-CD8 mAb (15,17–19). Transient depletion of CD4+ T cells resulted in a marked but equal prolongation of allograft survival in both CD27−/− and WT recipients (Figure 1B). Compared to untreated animals, depletion of CD8+ T cells did not affect allograft survival in any recipients (Figure 1C). Interestingly, CD27−/− mice showed higher frequencies of alloreactive IFN-γ- and IL-4-producing splenocytes and CD8+ effector-memory cells when compared to WT animals 7 days after transplantation (Figure 2).
Figure 1. BALB/c cardiac allograft survival in CD27−/− and wild-type (WT) B6 recipients.
(A) Both CD27−/− (n = 5) and WT recipients (n = 5) rejected allografts at a similar tempo. (B) Depletion of CD4+ cells in both CD27−/− (n = 5) and WT recipients (n = 5) significantly prolonged allograft survival, but there was no difference in allograft survival between the two groups. (C) Depletion of CD8+ cells had no effect on allograft survival in both CD27−/− (n = 5) and WT recipients (n = 5).
Figure 2. Frequency of IFN-γ(Th1)- and IL-4 (Th2)-producing alloreactive T cells, as assessed by ELISPOT (A, B) and percentage of alloreactive CD4+ and CD8+ effector-memory cells, as measured by flow cytometry, in CD27−/− and wild-type (WT) B6 recipients 7 days after transplantation of BALB/c hearts.
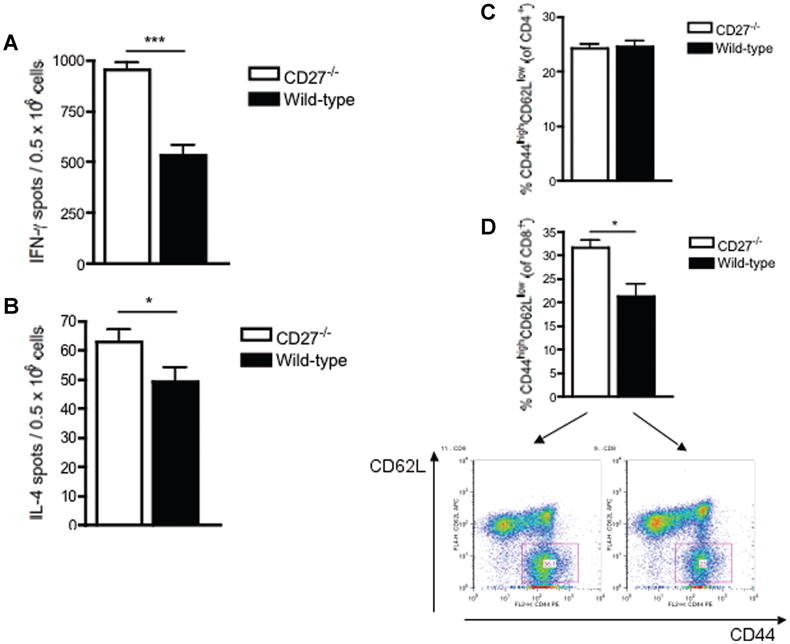
(A, B) The frequency of alloreactive IFN-γ- and IL-4-producing splenocytes was significantly increased in CD27−/− recipients compared to WT recipients. (C, D) While the frequency of alloreactive CD4+ effector-memory cells did not differ between CD27−/− and WT recipients, the frequency of CD8+ effector-memory T cells was significantly higher in CD27−/− recipients compared to WT recipients. Data and density plots are representative of two independent experiments (n = 3/3 each).
CD27 is crucial for activation of alloreactive CD8+ but not CD4+ memory T cells
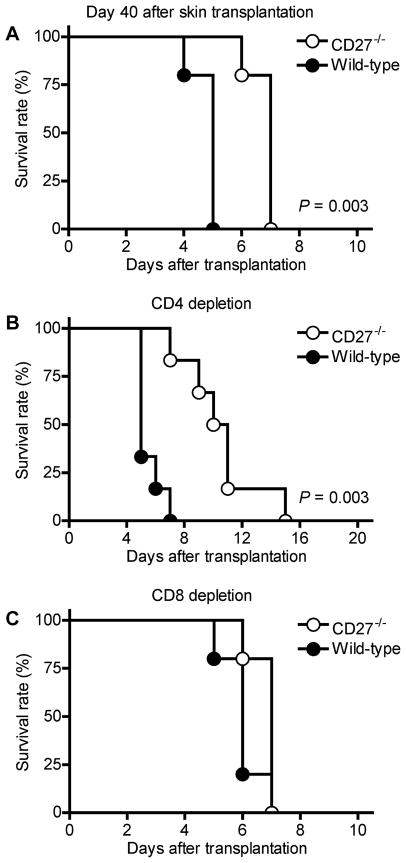
To determine the differential role of CD27 signaling to alloreactive effector and memory T cells, CD27−/− and WT B6 mice were sensitized with skin allografts from BALB/c donors 10 days (to generate effector-type alloreactive T cells) or 40 days (to generate memory-type alloreactive T cells) prior to transplantation of BALB/c cardiac allografts (9). CD27−/− and WT recipients sensitized on day –10 rejected heart allografts at a similar tempo (mean survival time [MST] = 2 vs. 2 days; n = 5/5; NS), suggesting that primed alloreactive effector T cells do not require CD27-costimulation for their activation. In contrast, cardiac allografts survived longer in CD27−/− recipients sensitized on day –40, compared to corresponding WT recipients (Figure 3A). This survival benefit was not observed in fully MHC-mismatched third-party cardiac allografts from CBA donors against which no specific memory cells were generated after sensitization with BALB/c skin grafts (MST 7 vs. 7 days; n = 5/5; NS). To determine whether the improved survival of BALB/c cardiac allografts in day 40-sensitized CD27−/− recipients is based on impaired function of CD4+ or CD8+ memory cells, CD4+ or CD8+ memory T cells were targeted with depleting mAb in day 40-sensitized CD27−/− and WT recipients of BALB/c hearts. Efficiency of CD4+ and CD8+ memory T-cell depletion was confirmed using Flow Cytometry (day 0 CD4: 0.04 ± 0.008%; CD8: 0.03 ± 0.009%; n = 8 and day 7 post cardiac transplantation CD4: 0.38 ± 0.23%; CD8: 0.52 ± 0.12%; n = 5; peripheral blood). Improved allograft survival in CD27−/−compared to WT animals was not only preserved, but even enhanced after CD4+ T-cell depletion (Figure 3B). In contrast, CD8+ T-cell depletion did not affect allograft survival in CD27−/− animals, as CD8+ memory T cells are not functional in these mice, but increased allograft survival in WT animals, in which intact CD8+ memory T cells mediate rejection (Figure 3C).
Figure 3. BALB/c cardiac allograft survival in CD27−/− and wild-type (WT) B6 recipients presensitized with donor-type skin transplantation 40 days prior to cardiac transplantation.
(A) Allografts survived significantly longer in CD27−/− recipients (n = 5) compared to WT recipients (n = 5). (B) Improved allo-graft survival in CD27−/− recipients was preserved and further enhanced after depletion of CD4+ T cells (n = 5). (C) Allograft survival did not differ between CD27−/− (n = 5) and WT (n = 5) recipients after CD8+ T-cell depletion.
To provide further evidence for the presence and activity of CD8+ memory T cells independent of the effectors generated from naïve CD8+ precursors and CD4+ T cells, we administered 500 μg of CTLA4-Ig to day 40-sensitized and CD4-depleted CD27−/− or WT recipients before transplantation of BALB/c cardiac allografts. CTLA4-Ig effectively prevents activation of naïve T cells, but not proliferation and maintenance of memory CD8+ T cells, which are CD28-independent. CTLA4-Ig prolonged allograft survival in sensitized CD4-depleted CD27−/−, but not WT mice (MST 16 vs. 6 days; n = 6/4; p = 0.01). These data confirm the lack of efficacy of CTLA4-Ig on memory CD8+ T cells in sensitized WT mice and the importance of CD27 for the generation of functional CD8+ memory T cells. Taken together, these results reveal a crucial role of CD27 for alloreactive memory CD8+ T-cell function.
To evaluate the role of CD27 costimulation in providing memory T-cell help to B-cell-mediated production of DSA, we measured DSA (IgG1, IgG2a) in the sera of CD27−/−and WT recipients of BALB/c cardiac allografts 47 days after skin sensitization/7 days after heart transplantation. The amount of both types of DSA was not different between CD27−/− and WT animals (Figure 4).
Figure 4. Donor-specific antibodies (DSA) production in day 40 skin-sensitized CD27−/− and wild-type B6 recipients 7 days after cardiac transplantation, assessed by flow cytometry.
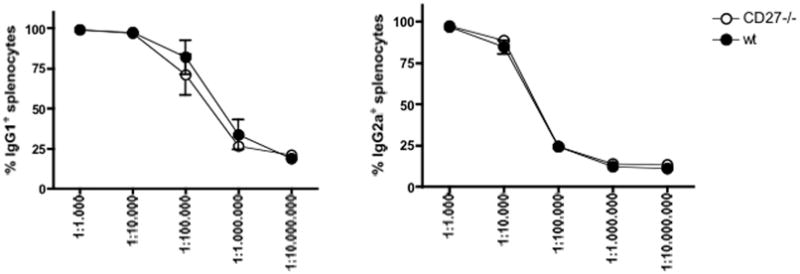
(A) CD27 deficiency did not affect production of IgG1-type DSA. (B) CD27 deficiency did not affect production of IgG2a-type DSA. Data are shown as percentage of IgG1- or IgG2a-loaded splenocytes, and are representative of two independent experiments (n = 3/3 each).
Finally, allograft survival in NK cell-depleted mice is similar to that in undepleted mice in both CD27−/− (MST 6 vs. 7 days; n = 5/5; NS) and WT recipients (MST 4 vs. 5 days; n = 5/5; NS). In contrast, transient double depletion of CD4+ and CD8+ T cells in sensitized WT animals strongly prolonged cardiac allograft survival as compared to undepleted mice (MST 17 vs. 5 days; n = 5/5; p = 0.0035), establishing T cells as the major mediators of acute rejection in this model.
CD27 deficiency suppresses secondary responsiveness of alloreactive CD8+ memory T cells by hampering their generation/survival
Secondary responsiveness is determined by the number of antigen-specific memory cells formed and their capacity for secondary expansion. To determine the mechanisms by which CD27 deficiency prolongs allograft survival in the memory phase of the alloimmune response, we determined the frequency of IFN-γ (Th1)- or IL-4 (Th2)-producing alloreactive T cells in sensitized CD27−/− and WT B6 recipients before (to determine memory formation) and after transplantation of BALB/c hearts (to determine secondary expansion) by ELISPOT assays. Forty days after skin transplantation, the frequency of alloreactive IFN-γ-producing splenocytes was lower in CD27−/− than in WT recipients (Figure 5A), but there was no difference between the two groups in response to third-party cells from CBA mice to which the animals were not sensitized (49 ± 2 spots/0.5 × 106 cells [CD27−/−] vs. 34 ± 6 [WT]; n = 2/2). These findings again confirm the effect of CD27 deficiency on memory-type rather than primary alloimmune responses.
Figure 5. Frequency of IFN-γ(Th1)- and IL-4 (Th2)-producing alloreactive T cells in day 40 skin-sensitized CD27−/− and wild-type (WT) B6 recipients prior to and 4 and 7 days after cardiac transplantation, assessed by ELISPOT.
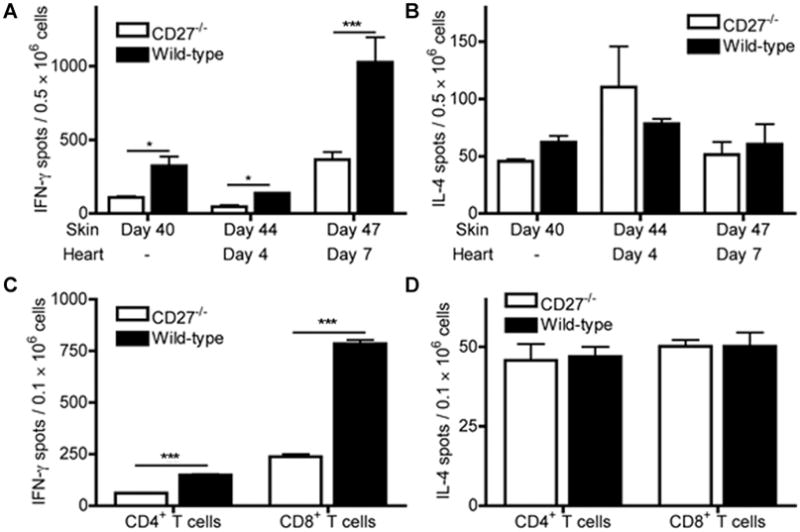
(A) The frequency of alloreactive IFN-γ-producing splenocytes was significantly decreased in CD27−/− recipients compared to WT recipients on day 40 after skin sensitization and 4 and 7 days after challenge with donor-type cardiac transplantation. (B) The frequency of alloreactive IL-4-producing splenocytes did not differ between CD27−/− and WT recipients at any of above time points. (C) The frequency of IFN-γ-producing CD4+ T cells purified from splenocytes was lower in CD27−/− animals than in WT controls on day 40 after skin transplantation; however, the decline in frequency of IFN-γ-producing CD8+ memory T cells purified from same splenocytes was markedly more enhanced in CD27−/− mice as compared to WT controls. (D) The frequency of donor-specific IL-4-producing cells among purified CD4+ or CD8+ T cells derived from day 40-sensitized splenocytes did not differ between CD27−/− and WT mice. Data are representative of three independent experiments (n = 3/3 each) and indicate the mean of quintuplicate results in each experiment. *p < 0.05; ***p < 0.001.
Lower secondary responsiveness of memory cells was also confirmed in recipients of BALB/c cardiac allografts following sensitization with BALB/c skin grafts (Figure 5A). However, CD27 deficiency did not affect the secondary expansion of memory cells, as measured by fold increase of the frequency of IFN-γ-producing splenocytes between days 40 and 47 (3.3-fold increase in CD27−/− vs. 3.1-fold increase in WT recipients).
To determine the dominant cell type producing IFN-γand IL-4, splenocytes were separated into CD4+ and CD8+ T cells at day 40 post skin transplantation. Although frequency of IFN-γ-producing CD4+ T cells was lower in CD27−/− recipients, the depressed overall frequency of IFN-γ-producing cells in CD27−/− recipients was mainly due to a significantly decreased number of IFN-γ-producing CD8+ memory T cells (Figure 5C). In contrast, the frequency of IL-4-producing cells did not differ between CD27−/− and WT recipients at any time point, neither among unselected splenocytes (Figure 5B) nor among purified CD4+ and CD8+ splenocytes (Figure 5D).
To assess the impact of CD27 on the generation of al-loreactive CD4+ and CD8+ effector-memory cells, the percentage of CD4+ and CD8+ cells with effector-memory (CD44highCD62Llow) and, in case of CD8+ cells, also central-memory (CD44highCD62Lhigh) phenotype were measured by Flow Cytometry. Being not significantly different in naïve animals (2.1 ± 0.04 vs. 2.2 ± 0.24%; n = 3/3; NS), CD8+ effector-memory T cells were significantly lower in CD27−/− recipients compared to WT recipients at any time point post skin sensitization (Figures 6A and B). Interestingly, the extent of the effect of CD27 deficiency on the percentage of CD8+ effector-memory cells was similar in all three groups as compared to WT controls: 1.7-fold decrease at 40 days post skin sensitization, 2.2-fold decrease at 44 days post skin transplantation/4 days post heart transplantation; and 2-fold decrease at 47 days post skin transplantation/7 days post heart transplantation. All in all, these data confirm the crucial role of CD27 for alloreactive CD8+ memory formation/survival rather than their secondary expansion. CD8+ central-memory T cells did not significantly differ between CD27−/− and WT animals at day 40 post skin transplantation, but CD27−/− animals showed lower CD8+ central-memory cells both at day 44 post skin transplantation/day 4 post heart transplantation (13.9 ± 1.7 vs. 23.2 ± 0.9%; n = 3/3; p = 0.009) and at day 47 post skin transplantation/day 7 post heart transplantation (21.3 ± 1.5 vs. 27.4 ± 1.2%; n = 3/3; p = 0.03), compared to WT animals, resulting in a net decrease of the entire CD8+ memory T-cell pool in CD27−/− animals (day 44 post heart transplantation/day 4 post heart transplantation: 16.0 ± 1.8 vs. 27.9 ± 1.2%; n = 3/3; p = 0.005; day 47 post skin transplantation/day 7 post heart transplantation: 34.8 ± 2.5 vs. 54.2 ± 1.9%; n = 3/3; p = 0.003).
Figure 6. Frequency of alloreactive CD4+ and CD8+ effector-memory T cells in day 40-sensitized CD27−/− and wild-type (WT) recipients prior to and 4 and 7 days after the cardiac transplantation.
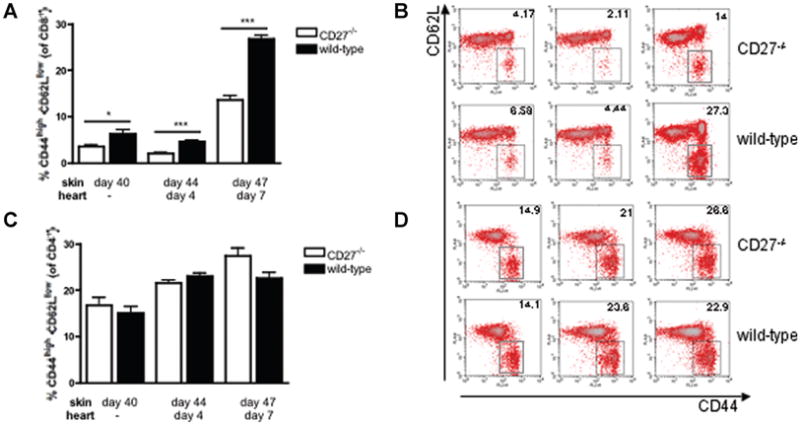
(A) and (B) The frequency of CD8+ effector-memory T cells was significantly decreased in CD27−/− recipients compared to WT recipients at any time point examined. (C) and (D) The frequency of CD4+ effector-memory T cells did not significantly differ between CD27−/− and WT recipients over the studied time course. The density plots shown are representative of at least three separate experiments (n = 3/3 each). *p < 0.05; ***p < 0.001.
In contrast, frequencies of CD4+ effector-memory T cells did not differ between CD27−/− and WT recipients at any time point (Figures 6C and D), suggesting a key role of CD27 for the generation/activation of CD8+ effector-memory T cells.
CD27 deficiency prevents chronic allograft vasculopathy and fibrosis in a model of CD8+ T-cell-mediated chronic allograft rejection
Chronic inflammation as in chronic viral infections has been associated with exhaustion of effector CD8+ T cells and in failure of memory CD8+ T-cell development, a process attributed to chronic CD70-driven costimulation (26). Thus, the effects of CD27 deficiency were evaluated in models of chronic allograft rejection, using CD27−/− and WT B6 mice as recipients of cardiac allografts from bm1 (single MHC-class I-mismatch) or bm12 (single MHC-class II-mismatch) donors. Chronic allograft rejection is primarily mediated by alloreactive CD8+ (bm1) or CD4+ (bm12) effector-memory T cells (27,28). As expected, CD27−/− and WT B6 recipients accepted bm1 and bm12 allografts indefinitely (both models: MST >56 vs. >56 days; n = 6/6; NS). Despite similar allograft survival, there was no or only minimal evidence of chronic allograft vasculopathy in two-thirds of CD27−/− recipients of bm1 hearts at day 56 posttransplantation (representative example Figure 7A, left panel), whereas bm1 allografts in WT recipients showed thickened intima and mild-to-severe occlusion of many intramyocardial and epicardial arteries (Figure 7A, right panel), with a clear overall trend toward lower vasculopathy scores in CD27−/− recipients compared to WT recipients (Figure 7C). In contrast, bm12 allografts showed various degrees of arteriosclerosis in both CD27−/− and WT recipients, with no difference in the resulting vasculopathy scores at day 56 posttransplantation (Figure 7C). Interstitial fibrosis of bm1 allografts was very mild in CD27−/−recipients (Figure 7B, left panel), but strongly distinctive in allograft epi- and myocardium of WT recipients (Figure 7B, right panel). In contrast, a similarly severe degree of interstitial fibrosis was observed in both CD27−/− and WT recipients of bm12 allografts (Figure 7D).
Figure 7. Histological findings of single MHC class I (bm1)- or class II (bm12)-mismatched allografts in B6 CD27−/− and wild-type (WT) recipients.
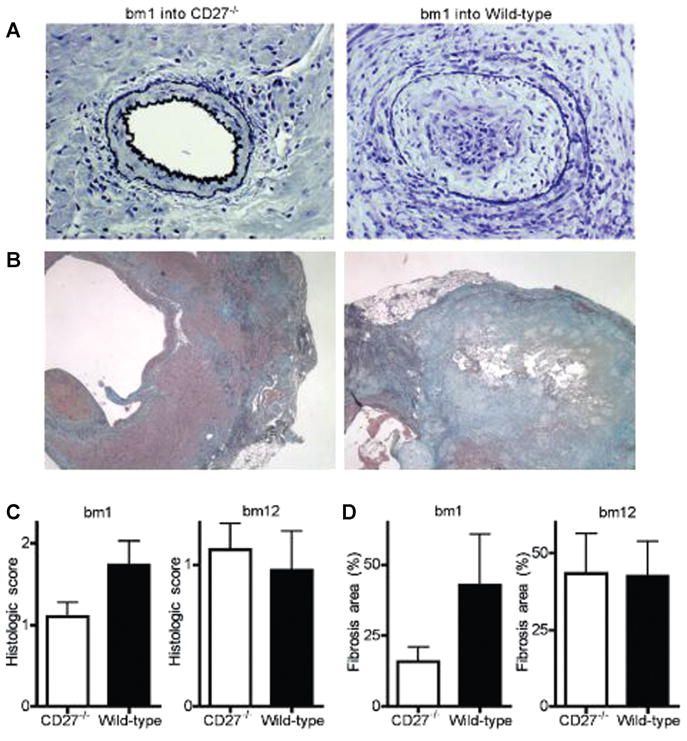
(A) Elastica-van Gieson’s staining of bm1 allografts shows essentially normal vessels in CD27−/−recipients compared to advanced arteriosclerosis in WT recipients (× 200). (B) Masson’s trichrome stainings of bm1 allografts show only mild epicardial fibrosis in CD27−/− recipients, but severe epicardial and in-tramyocardial fibrosis in WT recipients. (C) Vasculopathy score. Bm1 but not bm12 allografts display a lower degree of graft arteriosclerosis in CD27−/− recipients compared to WT recipients. (D) Fibrosis rate. Bm1 but not bm12 allografts display a lower degree of fibrosis in CD27−/−recipients compared to WT recipients.
On day 56 posttransplantation, ELISPOT assays revealed decreased frequencies of IFN-γ-producing splenocytes in CD27−/− recipients of bm1 allografts as compared to WT recipients (Figure 8A), but there was no difference between CD27−/− and WT recipients of bm12 allografts (Figure 9A). Enriched CD4+ and CD8+ T cells (purity >93%) revealed CD8+ T cells to be the major source of IFN-γproduction in the bm1 model, and CD8+ IFN-γ-producing T cells to be decreased in CD27−/− mice (Figure 8B), whereas CD4+ T cells were the major source of IFN-γ production in the bm12 model and did not differ between CD27−/− and WT recipients (Figure 9B). Taken together, these data establish the primary role of CD8+ T cells in the bm1 model and the impact of CD27 molecule on their activation.
Figure 8. Frequencies of alloreactive IFN-γ-producing splenocytes and CD8+ memory T cells in CD27−/− and wild-type (WT) B6 recipients of bm1 allografts.
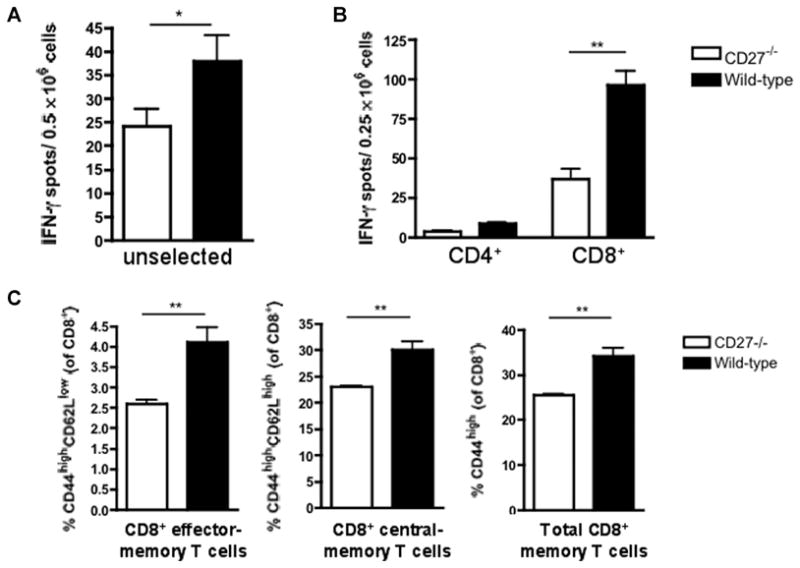
(A) The frequency of alloreactive IFN-γ-producing splenocytes was significantly decreased in CD27−/−recipients compared to WT recipients. (B) Alloreactive CD8+ T cells are the main source of IFN-γproduction. Frequency of CD8+ IFN-γ-producing splenocytes is significantly decreased in CD27−/− animals. (C) The frequency of CD8+ effector-memory and CD8+ central-memory cells as well as the overall CD8+ memory T-cell pool was significantly decreased in CD27−/− animals when compared to WT animals. Data are representative of two independent experiments (n = 3/3 each). ELISPOT data are shown as the mean of quintuplicate results in each experiment. *p < 0.05; **p < 0.01.
Figure 9. Frequencies of alloreactive IFN-γ-producing splenocytes and CD4+ memory T cells in CD27−/− and wild-type (WT) B6 recipients of bm12 allografts.
(A) The frequency of alloreactive IFN-γ-producing splenocytes was similar in CD27−/− and WT recipients. (B) Alloreactive CD4+ T cells are the main source of IFN-γproduction. Frequency of CD4+ IFN-γ-producing splenocytes are similar in CD27−/− and WT animals. (C) The frequency of CD4+ effector-memory cells is similar in CD27−/− and WT animals. Data are representative of two independent experiments (n = 3/3 each). ELISPOT data are shown as the mean of quintuplicate results in each experiment.
Flow Cytometry showed decreased CD8+ effector-memory and central-memory T cells and a decreased total CD8+ memory T-cell pool in CD27−/− recipients of bm1 al-lografts as compared to WT recipients (Figure 8C), whereas the frequencies of CD4+ effector-memory cells were similar in CD27−/− and WT recipients of bm12 allografts (Figure 9C). These mechanistic data establish the key role of memory CD8+ T cells in the bm1 model and underline the importance of CD27 for their activation.
Discussion
This study is the first to provide direct evidence for differential requirement of the CD27:CD70 costimulatory pathway for naïve versus primed memory CD8+ T cells in alloim-munity. We first asked whether activation of alloreactive CD8+/CD4+ T cells may have different CD27 costimulation requirements in naïve versus primed environments. To differentiate between effector and memory primed T cells, we used an established model in which mice are presen-sitized with donor-type skin allografts 10 or 40 days before cardiac engraftment (9,29–31). Our data demonstrate that CD27 signals are not essential for activation of naïve allore-active CD4+/CD8+ T cells, as both naïve CD27−/− and WT recipients rejected BALB/c hearts with the same tempo, and temporary depletion of CD4+ or CD8+ T cells did not result in differences in allograft survival between the two groups. These conclusions were supported by the frequencies of alloreactive splenocytes in naïve CD27−/− and WT controls on day 7 after cardiac transplantation, revealing more than adequate priming of CD27−/− recipients, and by similar alloresponses to third-party (CBA) splenocytes in CD27−/− and WT mice after 40 days of sensitization to BALB/c skin grafts. Although not completely excluding a role for CD27 signaling to naïve T cells in primary al-loimmune responses, these findings are consistent with previous data showing the dominant role of CD28 costim-ulation in priming of naïve T cells (32). In fact, our group previously demonstrated that blockade of CD70-induced indefinite graft survival in all CD28-deficient animals (15). These data suggest that the CD27:CD70 pathway is integral to the initiation of alloimmune responses in vivo, especially in the absence of CD28.
Moreover, our data show that CD27 signaling is required for optimal activation of alloreactive CD8+ memory T cells, as judged by survival benefit of cardiac allografts in day 40-sensitized CD27−/− recipients when compared to WT controls. Although the survival seemed biologically minor, its importance was underlined by the fact that it was further enhanced by depletion of memory CD4+ T cells, but completely disappeared when memory CD8+ T cells were eliminated. These findings may seem subtle, but they are biologically important and consistent with complementary and collective contribution of other costimulatory receptors such as 4–1BB and OX40 to formation of CD8+ memory T cells (33). Our mechanistic studies demonstrated diminished secondary responses of CD8+CD27- memory T cells to alloantigen, as demonstrated by declines in IFN-γproduction and expression of effector phenotypes. Our data confirm previous findings, albeit not in an alloimmune model, that secondary CD8+ T-cell responses are reduced in CD27−/− mice (11,13).
Secondary responsiveness of CD8+ memory T cells is determined by the number of antigen-specific memory cells formed, maintenance of memory T cells and their capacity for secondary expansion (3,6). CD27 deficiency may affect any or all of these steps. It is established that the magnitude of the expansion phase of the primary immune response and the degree of contraction determine the size of the generated memory T-cell pool. Our in vivo data, showing similar cardiac allograft survival rates in unprimed as well as day 10-sensitized CD27−/− and WT mice, and corresponding mechanistic studies point to no major differences in primary alloresponses or in the number of resultant effector T cells. Our in vivo and mechanistic data of day 40-sensitized mice clearly establish the importance of CD27 signaling for generation of memory CD8+ T cells. The decline in the number of alloantigen-specific memory CD8+ T cells in CD27−/− mice could be due to decreased survival or increased contraction of alloantigen-specific primed CD8+ T cells. In fact, contraction of virus-specific CD8+ effector T cells was reduced in CD70-transgenic mice, resulting in increased memory cell formation (12). While the maintenance of memory T cells is thought to be mainly dependent on cytokines such as IL-15, CD27 stimulation can promote survival of primed CD8+ T cells and thereby increase the allospecific memory T-cell pool (11,33,34). The vigor of recall response can be also attributed to the intrinsic antigen hyperresponsiveness of memory T cells.
Recent work suggests that in fact CD27:CD70 interaction may be important for the recall of CD8+ memory T cells (10). Our data in skin-sensitized hosts challenged with a donor-type cardiac allograft clearly establish lack of a role of CD27 signaling for secondary expansion of alloreactive memory CD8+ T cells.
The role of CD27:CD70 pathway in generation of memory CD8+ T cells in models of chronic viral infection in which CD70 is continuously upregulated on both dendritic cells (DCs) and T cells has been discussed controversially. Expression of CD70 on DCs resulted in continuous generation of CD8+ memory T cells (35), but transgenic expression of CD70 on T cells resulted in exhaustion of effector CD8+ T cells and in failure of memory CD8+ T-cell development (26). Prevention of chronic allograft vasculopathy (CAV) and fibrosis in CD27−/− recipients of bm1 allografts in a chronic cardiac transplantation model demonstrate the importance and continuous function of CD27 for CD8+ memory formation in this setting.
NK cells were recently shown to acquire memory cell-type features and mediate biological effects upon reactivation, as demonstrated in a murine model of cytomegalovirus (36). By depleting NK cells before cardiac transplantation in sensitized CD27−/− and WT mice, we demonstrated that NK cells, with or without memory function, are not relevant in this model of memory T-cell-mediated allograft rejection.
In conclusion, our data reveal that alloreactive memory CD8+ T-cell-mediated alloimmune responses have differential requirements for CD27 costimulation compared to their naïve counterparts and that targeting this molecular pathway may be an important novel target for clinical intervention. Further clarification of the mechanisms of CD27:CD70 interaction is required to allow for rational interventions for the induction of durable transplantation tolerance in humans.
Acknowledgments
This work was supported by National Institute of Health (NIH) Grant RO1 AI51559, RO1 AI23847 and AI42223. N.N. is a recipient of the American Heart Association Scientist Development Grant and NIH 1KO8AI064335-01A2. O.B. is funded by Research Fellowship Grants from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and The American Society of Transplantation.
References
- 1.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol. 2007;18:2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and home-ostatic proliferation as barriers to tolerance. Curr Opin Immunol. 2004;16:558–564. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Lakkis FG, Sayegh MH. Memory T cells: A hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 4.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: Potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 5.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 6.Valujskikh A. Targeting T-cell memory: Where do we stand? Curr Opin Organ Transplant. 2008;13:344–349. doi: 10.1097/MOT.0b013e3283061126. [DOI] [PubMed] [Google Scholar]
- 7.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 8.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allo-graft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: Therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 10.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 12.Arens R, Schepers K, Nolte MA, et al. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: A critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 15.Yamada A, Salama AD, Sho M, et al. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- 16.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Salama AD, Sho M, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada A, Kishimoto K, Dong VM, et al. CD28-independent cos-timulation of T cells in alloimmune responses. J Immunol. 2001;167:140–146. doi: 10.4049/jimmunol.167.1.140. [DOI] [PubMed] [Google Scholar]
- 19.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu K, Schonbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165:3506–3518. doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 21.Sayegh MH, Wu Z, Hancock WW, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant. 2003;3:381–389. doi: 10.1034/j.1600-6143.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 22.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 23.Lee RS, Womer KL, Yamada K, et al. The role of indirect recognition of donor MHC class II peptides in cardiac transplantation in miniature swine. J Heart Lung Transplant. 2001;20:172. doi: 10.1016/s1053-2498(00)00342-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Popoola J, Khandwala S, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117:660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 26.van Gisbergen KP, van Olffen RW, van Beek J, et al. Protective CD8 T cell memory is impaired during chronic CD70-driven costimula-tion. J Immunol. 2009;182:5352–5362. doi: 10.4049/jimmunol.0802809. [DOI] [PubMed] [Google Scholar]
- 27.Schulz M, Schuurman HJ, Joergensen J, et al. Acute rejection of vascular heart allografts by perforin-deficient mice. Eur J Immunol. 1995;25:474–480. doi: 10.1002/eji.1830250225. [DOI] [PubMed] [Google Scholar]
- 28.Nathenson SG, Geliebter J, Pfaffenbach GM, Zeff RA. Murine major histocompatibility complex class-I mutants: Molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- 29.Zhai Y, Meng L, Busuttil RW, Sayegh MH, Kupiec-Weglinski JW. Activation of alloreactive CD8+ T cells operates via CD4-dependent and CD4-independent mechanisms and is CD154 blockade sensitive. J Immunol. 2003;170:3024–3028. doi: 10.4049/jimmunol.170.6.3024. [DOI] [PubMed] [Google Scholar]
- 30.Zhai Y, Wang Y, Wu Z, Kupiec-Weglinski JW. Defective alloreactive CD8 T cell function and memory response in allograft recipients in the absence of CD4 help. J Immunol. 2007;179:4529–4534. doi: 10.4049/jimmunol.179.7.4529. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Wang Y, Gao F, Shen X, Zhai Y, Kupiec-Weglinski JW. Critical role of CD4 help in CD154 blockade-resistant memory CD8 T cell activation and allograft rejection in sensitized recipients. J Immunol. 2008;181:1096–1102. doi: 10.4049/jimmunol.181.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal A, Newell KA. The role of positive costimulatory molecules in transplantation and tolerance. Curr Opin Organ Transplant. 2008;13:366–372. doi: 10.1097/MOT.0b013e328306115b. [DOI] [PubMed] [Google Scholar]
- 33.Hendriks J, Xiao Y, Rossen JW, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 34.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller AM, Schildknecht A, Xiao Y, Van Den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]