Abstract
Background
Although inflammatory breast cancer (IBC) is recognized as the most lethal variant of locally advanced breast cancer, there are few molecular signatures of IBC that have been identified that can be used as targets to develop therapeutics to effectively inhibit the aggressive phenotype displayed by IBC tumors.
Methods
Real time PCR, western blotting, modified Boyden chamber invasion assays, vasculogenic mimicry assays, and gelatin zymography were used in the present studies. Agonists and antagonists of EP3 and EP4 and EP4 short hairpin RNA knockdown approaches were used as tools to assess the role of prostanoid receptors EP3 and EP4 in regulation of specific biological activities of IBC breast cancer cells.
Results
The present studies revealed that IBC breast cancer cell lines, SUM149 and SUM190, express high levels of Cox-2 mRNA and protein, produce abundant levels of PGE2 and produce both EP3 and EP4 receptor proteins. Studies using the EP4 antagonist GW627368X and shRNA molecular knockdown approaches revealed a role for EP4 in regulating invasion of IBC cells. EP3 but not EP4 regulates the ability of SUM149 cells to undergo vasculogenic mimicry (VM), which is the ability to form capillary-like structures, a characteristic exhibited by very aggressive tumor types. Inhibition of VM by sulprostone was associated with an inhibition of matrix metalloprotease-2 (MMP-2) enzyme activity.
Conclusions
The prostanoid receptors EP3 and EP4 differentially regulate activities exhibited by IBC cells that have been associated with the aggressive phenotype of this lethal variant of breast cancer. While EP4 regulates invasion, EP3 regulates VM and the associated increased MMP-2 enzyme activity.
Keywords: inflammatory breast cancer, cyclooxygenase-2, prostanoid receptor, EP3, EP4, PGE2, invasion, vasculogenic mimicry, MMP-2
Introduction
Inflammatory breast cancer (IBC) is the most aggressive subtype of locally advanced breast cancer, with the worst prognosis and shortest overall survival time of any variant of this disease (reviewed in 1). IBC commonly presents as a skin rash due to invasion of IBC tumor cells into dermal lymphatics. Due to this peculiar pattern of presentation, IBC is commonly misdiagnosed, delaying correct diagnosis. IBC patients often present with late stage disease (stage IIIb or stage IV) at the time of first accurate diagnosis. Since IBC does not commonly present as a lump, imaging modalities such as Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) rather than mammography and ultrasound are required for accurate diagnosis and staging. Although the standard of care for IBC patients for the past 30 years has been multimodality treatment combining chemotherapy, surgery, and radiation, there has been no change in the very low overall survival rate of 2.9 years for patients diagnosed with IBC during this time period (2). Studies performed over the past decade have defined few molecular characteristics of IBC tumors including the association between increased expression of RhoC guanosine triphosphatase (3) and loss of the tumor suppressor, CCN6/Wnt-1 induced secreted protein 3 (WISP-3) (4). Other studies have documented that IBC tumors have increased expression of genes including the cyclooxygenase-2 (Cox-2) enzyme (5), which produces prostaglandin E2 (PGE2). Based on observations described in the present studies that Cox-2 gene is highly upregulated in both SUM190 and SUM149 cell lines, the goal of the present studies was to define the role of the distinct prostanoid (EP) receptors, EP1, EP2, EP3 and EP4 that are known to mediate the biological activities of Cox-2 and PGE2. Using EP receptor agonists and antagonists as well as molecular knockdown approaches, the effects of inhibition of these receptors was evaluated for their potential as therapeutic agents to block activities associated with the aggressive phenotype of IBC.
Materials and Methods
Cell Lines and Conditions
The human MCF-7 and MDA-MB-231 breast cancer cell lines were obtained from American Type Cell Culture (Manassas, VA). MCF-7 is an estrogen receptor positive (ER+) breast cancer cell line and MDA-MB-231 cells were derived from a patient with triple negative basal-like breast cancer. MCF-7 and MDA-MB-231 cells were cultured in Dulbecco’s modified Eagles medium (DMEM/F12; Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) at 37°C under 5% CO2 in a humidified incubator. The SUM149 and SUM190 cell lines were provided by Dr. Stephen Ethier (Asterand, Detroit MI) and were cultured in Ham F12 Nutrient Mixture (Invitrogen) supplemented with 10% FBS, insulin (1 mg/ml; Sigma-Aldrich, St. Louis, MO), and hydrocortisone (1 mg/ml; Sigma-Aldrich). Cell lines were cultured at 37°C under 5% CO2 in a humidified incubator.
Reagents
Hydrogen peroxide (30%), Triton X-100, and the monoclonal β-actin antibody were purchased from Sigma-Aldrich. Universal Vectastain Elite ABC (avidin-biotin-peroxidase complex) kit, hematoxylin, 3-amino-9-ethyl-carbazole (AEC), Aqua-Mount, and rabbit and mouse IgG antibodies were purchased from Vector Laboratories (Burlingame, CA). Matrigel was purchased from BD Biosciences (Bedford, MA). Hema-3 was purchased from Fisher Scientific (Middleton, VA). Gelatin zymogram gels, non-reducing sample buffer, 10x zymogram renaturing buffer, 10x zymogram development buffer, Coomassie blue-R250, and Coomassie blue destaining solution were purchased from Bio-Rad Laboratories (Hercules, CA). PGE2, arachidonic acid, PGE1 alcohol, butaprost, sulprostone, COX-2 monoclonal antibody, EP3 and EP4 receptor polyclonal antibodies were purchased from Cayman Chemical (Ann Arbor, MI). AH6809 and GW627368X were synthesized as previously described (6,7). EP4 primers were purchased from Sigma-Genosys (St. Louis, MO). Stock solutions of PGE2 (10 mM), arachidonic acid (10 mM), PGE1 alcohol (1 mM), butaprost (1 mM), AH6809 (10 mM), GW627368X (10 mM) and sulprostone (10 mM) were prepared indimethyl sulfoxide and stored at − 20°C. All reagents were diluted in culture medium to the indicated final concentration.
Real-time PCR Arrays
Cox-2 gene expression was evaluated using RT2Profiler™ PCR Arrays (SABioscience, Frederick, MD). RNA was isolated from subconfluent SUM149, SUM190, MCF-7, and MDA-MB-231 breast tumor cells using Trizol reagent (Invitrogen). Complementary DNA (cDNA) was isolated using the RT2 First Strand Kit, and then added to the RT SYBR Green qPCR Master Mix. This mixture was then aliquoted into each well of 96-well PCR array plates containing predispensed gene-specific primer sets. Thermal cycling was performed using an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA). The threshold cycle (Ct) for each well was calculated using SuperArray software. Five housekeeping genes with an additional three quality controls are included in each array.
Western Blot Analysis of Cox-2, and EP Receptors
Western blots were performed on protein lysates obtained from exponentially growing SUM149, SUM190, MCF-7 and MDA-MB-231 breast cancer cells. Cell pellets were lysed in 1% NP-40 lysis buffer. Equal amounts of protein were separated using 12% polyacrylamide gels (Bio-Rad) for COX-2 or using 15% polyacrylamide gels (Bio-Rad) for EP3 and EP4. Proteins were then transferred to nitrocellulose membranes (GE Healthcare, Piscataway, NJ), blocked for non-specific binding and then probed with monoclonal COX-2 antibody or polyclonal EP3 and EP4 antibodies followed by incubation with anti-mouse or anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Levels of COX-2, EP3 and EP4 receptor protein expression were normalized to β-actin, which was used as a loading control. Protein bands were visualized by enhanced chemiluminescence (GE Healthcare). Experiments were repeated three times and representative Western blots are shown.
PGE2 Production By Human Breast Cancer Cells Measured by Enzyme Immunoassay
Cells were plated at 1.0 × 106 cells/well in 100 mm dishes in 10 ml of medium and 24 hrs later, cells were placed in serum-free medium. At 24 hrs, media was added to human breast tumor cells that either did or did not contain arachidonic acid (10 μM). After 30 min incubation, culture medium was isolated, centrifuged and concentrated using spin columns with 10-kDa cutoff filters (Millipore, Bedford, MA). The amount of PGE2 in the concentrated supernatants was determined using a commercial enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. PGE2 concentrations were normalized to cell number and expressed as pg/ml/106 cells. The amount of PGE2 produced in the presence of arachidonic acid is designated as the amount of exogenous PGE2 and the amount of PGE2 produced in the absence of arachidonic acid represents that amount of endogenous PGE2 produced by human breast tumor cell lines. The experiments were performed in triplicate and repeated twice.
Effects of EP Receptor Agonists and Antagonists On Invasion Of An Artificial Basement Membrane By Human Breast Cancer Cells
The effects of PGE2, the EP2 agonist butaprost, the EP4 agonist PGE1 alcohol, the EP1/EP2 antagonist AH6809, the EP3 agonist sulprostone and the selective EP4 antagonist GW627368X on in vitro invasion of SUM149 IBC cells through a Matrigel basement membrane compared to MDA-MB-231 breast cancer cells was determined based on the number of cells that invaded through transwell inserts coated with the artificial basement membrane, Matrigel. Neither the MCF-7 nor the SUM190 cells were used in these assays since they have negligible invasive activity. SUM149 and MDA-MB-231 cells were trypsinized, resuspended in serum-supplemented medium and counted. Cells were then washed three times with serum-free medium. Plates (6-well) compatible with transwell inserts with 8 μm pore-size polycarbonate filters (Fisher Scientific) were coated with Matrigel in cold serum-free DMEM/F12 at a final concentration of 0.7 mg/ml and placed at room temperature for 40 min. Cells (in 500 μl serum-free medium) were added into the transwell inserts and incubated for 72 hrs in absence or presence of PGE2 (1 μM), the EP2 agonist butaprost (0.1, 1, 10 μM), the EP4 agonist, PGE1 alcohol (0.1, 1, 10 μM), the EP1/EP2 antagonist AH6809 (0.1, 1, 10 μM), the EP3 agonist sulprostone (0.1, 1, 10 μM) or the EP4 antagonist, GW627368X (0.1, 1, 10 μM). As a control, 10% fetal bovine serum (FBS) was used to evaluate the baseline extent of invasion of the different cell lines. After incubation, non-invading cells on the uppersurface of the filter were removed with cotton swabs. Cells that had passed through the pores onto the lower side of the filter were fixed, stained with Hema-3 stain (Fisher Scientific), and quantitated. The experiments were performed in triplicate and repeated twice.
Effects Of EP4 shRNA Knockdown On Invasion Of SUM149 Cells
The shRNA constructs against PTGER4 (EP4) were obtained from OriGene (Rockville, MD). A pRS plasmid containing EP4 shRNA and the negative control for the shRNA (empty vector) under the control of the mammalian U6 promoter was used to generate the stable transfectants. To obtain optimal EP4 knockdown, two different EP4 constructs designated as T1340309 (TTAAGTGTCTCACTAAAGCATGAAATGTG) and T1340312 (GCGCTGCTCCGCATGCACCGCCAGTTCAT) were used in these transfection studies. Negative control shRNA and the T1240309 or the T1340312 EP4 shRNA vectors were added to OptiMEM media. Lipofectamine 2000 was mixed with OptiMEM medium (Invitrogen, Carlsbad, CA) and these two solutions were then mixed together and incubated for 20 min. The mixture of Lipofectamine, OptimMEM and EP4 shRNA constructs or vector control shRNA were then added by single drops to SUM149 cells. At 24 hrs following transfection, medium was replaced and cells were cultured for an additional 72 hrs. Transfected SUM149 cells were transferred to Ham’s F-12 Nutrient Mixture supplemented with 10% FBS, insulin (1 mg/ml), and hydrocortisone (1 mg/ml; Sigma-Aldrich), with the addition of 2 μg/ml puromycin and were grown for 4 weeks, with limited dilution carried out so that each puromycin-resistant colony was derived from a single cell. Colonies were then isolated using cloning rings and grown in 6-well plates. Western Blotting was used to confirm knockdown of EP4. SUM149/Clone 1 is used to designate a clonally derived cell line containing stable transfection of EP4 shRNA that has knockdown of EP4 receptor protein as assessed by Western blotting. SUM149/Vector 5 are designated as the clonally derived cell line that has stable integration of the scrambled shRNA vector plasmid as determined by Western blotting to confirm the presence of EP4 receptor protein, which was similar to non-transfected control SUM149 cells. Studies were then performed to evaluate the effect of EP4 shRNA knockdown on invasion by SUM149 IBC cells using methods described above.
Effects of The EP3 Agonist Sulprostone On Vasculogenic Mimicry (VM) Exhibited By Breast Cancer Cells
MDA-MB-231 and SUM149 (4 × 104) were added to the top of twenty four-well plates coated with Matrigel (300 μl) in serum-free DMEM/F12 medium. Cells were treated with sulprostone or GW627368X at concentrations of 0.1, 1, 10 μM or with DMSO vehicle. To highlight the matrix-associated vascular channels formed, cells were stained with Periodic Acid Schiff (PAS) reagent. Representative photographs were taken at 24 hrs at 10× magnification. Each experiment was repeated three times.
Effects of Sulprostone On MMP-2 Activity Assessed By Zymography
Supernatants collected from MDA-MB-231 and SUM149 that had undergone VM were collected, centrifuged and concentrated using spin columns with 10-kDa-cutoff filters. Aliquots of 20 μl of conditioned medium was mixed (1:1) with non-reducing sample buffer, incubated at 37°C for 15 min, and then applied to a gelatin zymogram gel. After electrophoresis, gels were incubated for 3 hr in zymogram renaturing buffer, followed by an overnight incubation in zymogram development buffer at 37°C. Gels were stained with Coomassie blue-R250 for 3 hr and then destained. MMP-2 (gelatinase) activity was visible as clear bands against the dark blue background, indicating proteolysis of the substrate protein, gelatin.
Statistical Analyses
Two-tailed Student’s t-tests were performed to compare the amount of PGE2 produced by the different human breast tumor cell types in the presence and absence of arachidonic acid. Two-tailed Student’s t-tests were performed to evaluate the number of invaded cells between untreated cells and those treated with PGE2, butaprost, PGE1 alcohol, AH6809 and GW627368X and to compare the effect of transfection of EP4 shRNA on invasion of SUM149 tumor cells. A p value of <0.05 was considered statistically significant.
Results
Comparative Analysis of Cox-2 mRNA In Breast Cancer Cells
Both the SUM149 and SUM190 IBC breast tumor cells express high levels of the Cox-2 gene. The levels of Cox-2 are 2,500 and 11,000-fold increased in SUM149 (Figure 1A, clear bar) and SUM190 IBC cells (Figure 1A, hatched bar), respectively, compared to the triple negative basal-like MDA-MB-231 breast cancer cells that express very low levels of Cox-2 mRNA or the PTGS-2 null MCF-7 breast cancer cell line which served as the negative control for these studies.
Figure 1. Cox-2 mRNA, Protein And EP Receptor Protein Production By Breast Cancer Cells.
Figure 1A. Cox-2 Gene Expression. The SUM190 and SUM149 IBC tumor cells expressed Cox-2 at high levels compared to Cox-2 mRNA levels expressed by MCF-7 and MDA-MB-231 breast cancer cells.
Figure 1B. Western Blot Of Cox-2, EP3 And EP4 Receptors. SUM149 and SUM190 IBC cells produce high levels of Cox-2 protein. All cell lines produce both EP3 and EP4 receptor proteins, with greater levels of EP4 proteins produced by SUM190 and SUM149 cells compared to the MCF-7 and MDA-MB-231 breast tumor cells.
Figure 1C. PGE2 Production. Both SUM190 and SUM149 cells produced significantly greater levels of endogenous PGE2 (p<0.05) that either MCF-7 or MDA-MB-231 breast cancer cells. Addition of arachidonic acid significantly increase PGE2 production by SUM190 and SUM149 IBC cells.
Western Blot Analysis Of Cox-2, EP3 and EP4 Receptor Proteins In Breast Cancer Cells
Both SUM149 and SUM190 IBC breast cancer cells produce high levels of Cox-2 protein compared to the low/undetectable levels of Cox-2 protein produced by the two non-IBC breast cancer cells (Figure 1B). All cell lines examined produced EP3 and EP4 receptor proteins. The IBC cell lines produced greater levels of protein for both of these receptor, with higher levels of EP4 than EP3 produced by both SUM149 and SUM190 cells. (Figure 1B).
PGE2 Production By Breast Cancer Cells
Both SUM190 and SUM149 cells produced significantly greater amounts of PGE2 (p<0.05) compared to either MCF-7 or MDA-MB-231 cells, with the SUM190 cells (Figure 1C,) producing more PGE2 compared to the SUM149 cells. Addition of the Cox-2 enzymatic substrate, arachidonic acid to IBC cells stimulated a significantly increased production of PGE2.
Effects Of EP3 Receptor Agonist And EP4 Antagonist On Invasion Of A Basement Membrane Matrix By Breast Cancer Cells
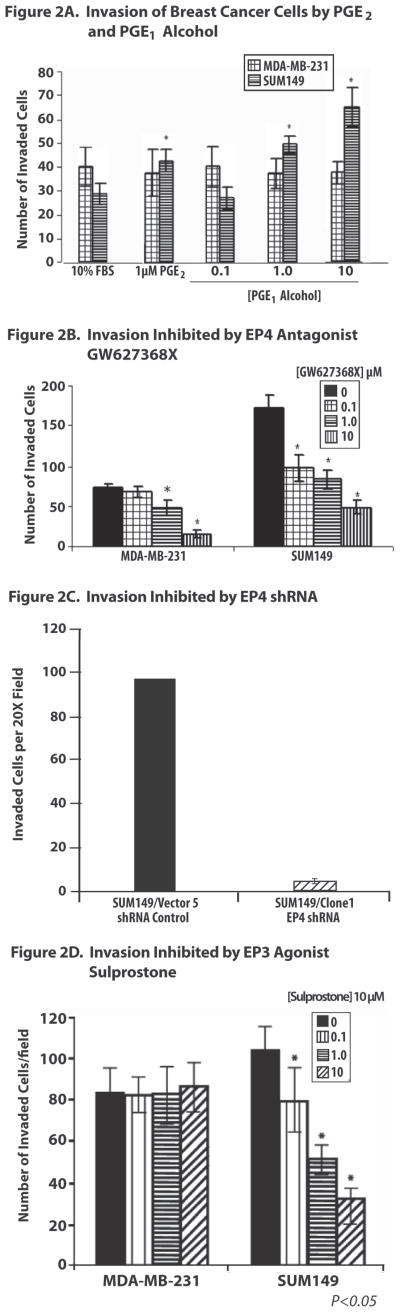
Neither the MCF-7 nor the SUM190 breast cancer cells exhibit a robust invasive response, therefore, only SUM149 and MDA-MB-231 cells were analyzed in these studies. SUM149 invasion was significantly increased by PGE2 (1 μM) and the EP4 agonist, PGE1 alcohol (0.1 μM, 1 μM and 10 μM) (p<0.05) (Figure 2A). The selective EP2 agonist butaprost and the EP1/EP2 mixed antagonist AH6809 had no effect on invasion by either SUM149 or MDA-MB-231 cells (data not shown). Both the EP3 agonist sulprostone and the EP4 antagonist GW627368X significantly (p<0.05) decreased the number of SUM149 cells that exhibited invasion at all concentrations tested (0.1–10 μM) (Figure 2B and D). The EP4 antagonist GW627368X inhibited invasion by SUM149 cells by 66% and the EP3 agonist sulprostone inhibited invasion of SUM149 cells by approximately 40%. Knockdown approaches to assess the role of EP4 in SUM149 invasion revealed that a stable clone of SUM149 cells containing EP4 shRNA designated as SUM149/Clone 1 exhibited significantly inhibited invasion, which was diminished by 95% of the SUM149 cells containing a scrambled shRNA vector used as the control cell line (Figure 2C). Neither PGE2 nor PGE1-alcohol altered invasion by MDA-MB-231 cells. While the EP4 antagonist GW627368X inhibited invasion of MDA-MB-231 cells at concentrations of 1 μM and 10 μM, the EP3 agonist, sulprostone, had no effect on invasion by these cells at any concentration examined. At the cell densities used for the invasion assay, the EP4 antagonist GW627368X and the EP3 agonist sulprostone did not induce more than 15% growth inhibition of SUM149 cells, demonstrating that EP receptor-mediated suppression of invasion was not due to non-specific growth inhibition of these breast cancer cells by either of these compounds.
Figure 2. Effects Of EP Receptor Agonists And Antagonists On Invasion Of Breast Cancer Cells.
Figure 2A. Effect of PGE2 and EP4 Agonist PGE1-Alcohol on Invasion. PGE2 and the EP4 agonist PGE1-alcohol induced significantly increased invasion of SUM149 cells but had no effect on invasion of MDA-MB-231 cells.
Figure 2B. Effect Of GW627368X On Invasion. GW627368X, induced a significant dose dependent inhibition of invasion beginning at the lowest concentration of 0.1 μM, compared to the inhibition of invasion by MDA-MB-231 by GW627368X at higher concentrations.
Figure 2C. Effect Of EP4 shRNA On Invasion. The presence of EP4 shRNA in SUM149/Clone 1 abolished 95% of invasion.
Figure 2D. Effect Of Sulprostone On Invasion. Sulprostone inhibited invasion by SUM149 cells in a dose dependent manner starting at 0.1 μM, with no effect of this agent on invasion by MDA-MB-231 cells.
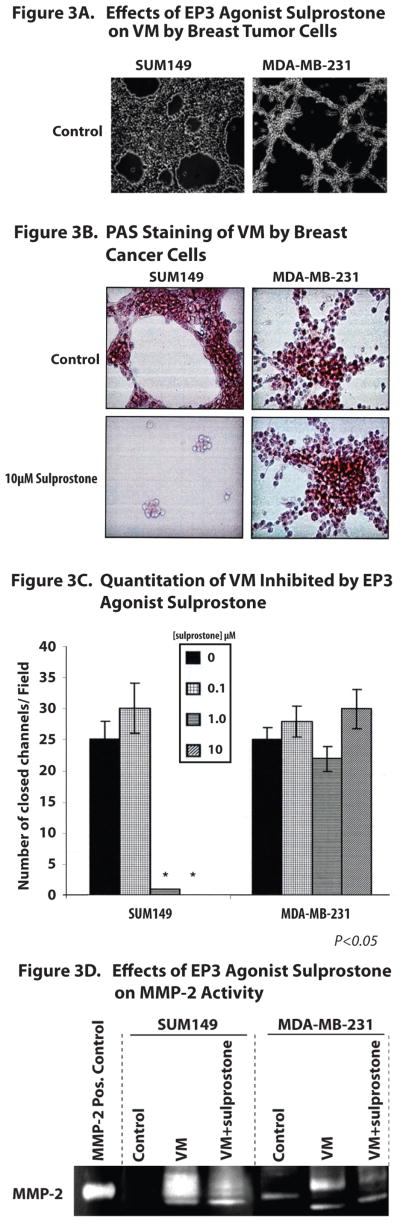
Effects of EP3 Agonist Sulprostone and EP4 Antagonist GW627368X On Vasculogenic Mimicry (VM) By Breast Cancer Cells
Tumor cells derived from very aggressive tumors display phenotypic plasticity, which includes their ability to form matrix-rich capillary like networks when placed in 3-dimensional culture in the absence of endothelial cells and fibroblasts, in a process defined as vasculogenic mimicry (VM) (8,9). We observed that SUM149 and MDA-MB-231 breast cancer cells but not SUM190 or MCF-7 cells undergo VM when placed onto a Matrigel matrix (Figure 3A and B). Phase contrast microscopy (Figure 3A) and PAS staining (Figure 3B) were used to directly image the capillary-like structures that have closed ends formed by aggressive breast cancer cells. The effects of agents on the formation of these closed structures can be evaluated to provide a quantitative analysis of the relative inhibitory effects of different agents. Using this approach, we found that VM was inhibited by the EP3 agonist sulprostone, but was not altered by the EP4 antagonist GW627368X (Figure 3C). In contrast, neither sulprostone nor GW627368X had any effect on VM exhibited by MDA-MB-231 cells (Figures 3A, B and C) (data not shown for GGW627368X). Since MMP-2 activity has been reported to be increased following cells undergoing VM, we. therefore evaluated the effects of the EP3 agonist, sulprostone, on MMP-2 activity, as assessed by gelatin zymography. Under control conditions, SUM149 do not have detectable MMP-2 activity, however MMP-2 activity is increased following SUM149 undergoing VM, which was effectively inhibited by sulprostone (Figure 3D). In contrast, MDA-MB-231 cells produced MMP-2 under control conditions, which was increased when these cells undergo VM and was not altered by the EP3 agonist sulprostone. These results are consistent with the lack of inhibition of VM by sulprostone in MDA-MB-23 non-IBC breast tumor cells, as described above.
Figure 3. Effects Of EP3 Agonist Sulprostone, On Vasculogenic Mimicry And MMP-2 Activity By Breast Cancer Cells.
Figure 3A and B. Visualization Of Breast Cancer Cells Undergoing VM. Phase contrast microscopy (A) and light microscopy of breast cancer cells stained with Periodic Acid Schiff Reagent (B) allows visualization of SUM149 and MDA-MB-231 breast cancer cells undergoing VM. The EP3 agonist sulprostone blocked formation of capillary-like tubular structures by SUM149 cells but not by MDA-MB-231 cells.
Figure 3C. Quantitation Of Change In Number of Closed Channels In Breast Cancer Cells Undergoing VM. Analysis of changes in the number of closed channels of the capillary-like structures formed by breast cancer cells undergoing VM demonstrates that the EP3 agonist sulprostone blocks formation of capillary like structures formed by SUM149 IBC cells, but has no effect on VM exhibited by MDA-MB-231 cells.
Figure 3D. MMP-2 Activity Assessed By Gelatin Zymography. SUM149 cells under control conditions produce no MMP-2 activity, however VM, these breast cancer cells produce MMP-2 which is inhibited by the EP3 agonist sulprostone. In contrast, sulprostone had no effect on MMP-2 activity by MDA-MB-231 cells.
Discussion
Inflammatory breast cancer is the most aggressive variant of locally advanced breast cancer (1). Although studies have identified several genes that are over-expressed or lost in IBC tumors and in IBC cell lines, thus far few molecular signatures have been identified that have led to “druggable” targets that would allow development of therapeutic agents effective for treatment of IBC. Previous studies have reported that Cox-2 is elevated in IBC tumors (5). Our laboratory was the first to report the association of elevated Cox-2 mRNA and protein in breast tumors with evidence of invasion (12), Numerous studies have now validated Cox-2 as a critically important regulator of invasion, angiogenesis and metastasis (13–16). Additional studies performed in our laboratory demonstrated that the presence of Cox-2 cDNA directly regulates proliferation, invasion, anchorage independent growth in soft agar and production of the vascular endothelial growth factor (VEGF) by breast tumor cells in vitro (17) as well as in breast tumor xenografts in immunocompromised mice (18). Other studies have reported that Cox-2 drives the ability of cells to undergo VM (10,11).
Although numerous studies reported that selective Cox-2 inhibitors have anti-tumor and anti-angiogenesis activities and effectively block breast cancer progression (19,20), these agents have been removed from the market in the case of rofecoxib or have black box warnings in the case of celecoxib, due to the risk of cardiovascular side effects (21, 22). This has prompted a search for other approaches to block Cox-2/PGE2 associated activities. One alternative is to inhibit binding of PGE2 to its G protein coupled receptors, defined as the prostanoid receptors (EPs). There are 4 members of this receptor family, designated as EP1, EP2, EP3 and EP4. Binding of PGE2 to these distinct receptors stimulates different biological activities of cells depending upon the cell type as well as the distinct EP receptor(s) that are activated (23–26). While binding of PGE2 or the appropriate agonist to the EP1 receptor stimulates release of intracellular calcium (Ca2+) and signals through the protein kinase C (PKC) pathway, binding of PGE2 to the EP2 and EP4 receptors stimulates increased intracellular cyclic adenosine monophosphate (cAMP) and adenylate cyclase production (23). In contrast to the stimulatory activities of EP1, EP2 and EP4, EP3 is a negative regulator of the effects of PGE2, and has been suggested to play a role in inhibiting the effects of PGE2 through binding to an inhibitory subunit of the G protein, Gi, resulting in inhibition of adenylate cyclase. While it is clear that binding of PGE2 to the EP receptors stimulates signaling pathways that play important roles in regulating normal homeostatic functions as well as have a role in a variety of human diseases, the importance of these receptors to biological activities of tumor cells depends in part upon the profile of EP receptors present as well as on the species and tissue type under study. The availability of EP receptor agonists and antagonists provides the opportunity to evaluate the effects of blockade of the individual EP receptors on specific parameters associated with tumor growth and invasion. In addition, knockdown approaches can be used to define the role(s) of the EP receptors in regulating specific activities of tumor cells.
The present observations are the first to demonstrate that Cox-2, PGE2, and the EP3 and EP4 prostanoid receptors are all up-regulated in IBC cells, with less EP3 and EP4 receptor protein produced by the ER+ slowly growing minimally invasive MCF-7 breast cancer cells and the triple negative, rapidly proliferation, highly invasive and metastatic MDA-MB-231 breast cancer cells. These results suggest that EP3 and EP4 are the primary EP receptors that are active on IBC cells, with no apparent role of either the EP1 or EP2 receptors on any biological activity of IBC cells examined. Previous studies (28–31), including our own (27), have demonstrated that Cox-2 is involved in regulating invasion and metastasis. The present studies confirm that the EP4 receptor is primarily involved in regulating activities of IBC cells associated with migration, invasion and metastasis (28–31). In contrast to the studies elucidating the roles of EP4 in breast metastasis, significantly less is known about the specific role of the down regulatory EP3 receptor in activities of tumor cells. The present studies reveal that the MCF-7 and MDA-MB-231 breast cancer cells which either lack Cox-2 or are Cox-2low are relatively resistant to the inhibitory effects of the EP3 agonist sulprostone and the EP4 antagonist GW627368X on any parameter evaluated until concentrations of these agents are reached that are associated with off-target effects.
Our studies are the first to define a differential response of breast cancer cells to EP receptor agonists and antagonists, with identification of specific roles of EP4 and EP3 receptors in regulating invasion and VM, respectively, exhibited by IBC breast tumor cells. Based on the combination of pharmacologic and molecular knockdown approaches, the present studies reveal that the EP4 receptor pathway appears to have a significant and primary role in regulating invasion exhibited by the SUM149 IBC cells, which were derived from pleural effusion of an IBC patient. The present studies are also the first to report a role for EP3 in regulating the ability of IBC tumor cells to undergo VM, which has been associated with an aggressive phenotype of other tumors, including uveal melanoma and ovarian carcinoma. EP4 does not appear be involved in VM exhibited by SUM149 IBC cells. These studies also suggest that EP3 regulates MMP-2 activity during VM in IBC cells, which has not previously been reported.
Taken together, the present studies demonstrate that Cox-2 is upregulated in IBC tumor cells, which is consistent with previous reports that IBC is upregulated in IBC primary tumors (5). The present studies extend these previous observations and demonstrate that the EP3 and EP4 receptors are critically important in differential regulation of the aggressive phenotype of IBC cells characterized by their robust invasion, ability to undergo VM with associated increased production of the proteolytic enzyme, MMP-2. Agents that may directly or indirectly target the signaling pathways activated following binding of PGE2 to the EP3 and EP4 receptors may be important to evaluate for their effectiveness as a potential therapeutic agent for inhibiting the aggressive phenotype of IBC.
Acknowledgments
Sources of support: American Airlines-Komen Foundation Promise Grant KGO81287 (FMR, MC); NIH NCI CA-128797-02 (FMR).
The technical assistance of Kimberly Boley and Ross Pickei is acknowledged.
Abbreviations
- Cox-2
cyclooxygenase
- DMEM
Dulbecco’s Minimal Essential Media
- EP
prostanoid receptor
- ER
estrogen receptor
- IBC
inflammatory breast cancer
- MMP-2
matrix metalloprotease-2
- MRI
magnetic resonance imaging
- PAS
Periodic Acid Schiff Stain
- PET
positron emission tomography
- PGE2
prostaglandin E2
- PR
progesterone receptor
- shRNA
short hairpin RNA
- WISP-3
Wnt-induced secreted protein-3
- VM
vasculogenic mimicry
References
- 1.Dawood S, Ueno NT, Cristofanilli M. The medical treatment of inflammatory breast cancer. Semin Oncol. 2008 Feb;35(1):64–71. doi: 10.1053/j.seminoncol.2007.11.012. Review. [DOI] [PubMed] [Google Scholar]
- 2.Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D, Hortobagyi GN. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40(4):321–9. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 3.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000 Oct 15;60(20):5832–8. [PubMed] [Google Scholar]
- 4.Kleer CG, Zhang Y, Pan Q, Gallagher G, Wu M, Wu ZF, Merajver SD. WISP3 and RhoC guanosine triphosphate cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110–115. doi: 10.1186/bcr755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004 Dec 1;10(23):7965–71. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 6.Bays DE. Xanthone derivates. 3,755,319. US Patents. 1973
- 7.Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, Brown J, Wise A, Chowdhury J, Pritchard S, Coote J, Noel LS, Kenakin T, Burns-Kurtis CL, Morrison V, Gray DW, Giles H. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006 Jun;148(3):326–39. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999 Sep;155(3):739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003 Jun;3(6):411–21. doi: 10.1038/nrc1092. Review. [DOI] [PubMed] [Google Scholar]
- 10.Basu GD, Liang WS, Stephan DA, Wegener LT, Conley CR, Pockaj BA, Mukherjee P. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8(6):R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. 2005;7(4):R422–35. doi: 10.1186/bcr1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrett ML, Harris RE, Joarder FS, Ross MS, Clausen KP, Robertson FM. Cyclooxygenase-2 Gene Expression in Human Breast Cancer. Intl J Oncol. 1997;10(3):503–507. doi: 10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- 13.Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclooxygenase 2 expression is associated with angio- genesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–434. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies G, Salter J, Hills M, Martin LA, Sacks N, Dowsett M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2651–2656. [PubMed] [Google Scholar]
- 15.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, Kristiansen G, Reles A, Siegert A, Guski H, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007 May 31;26(26):3789–96. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 17.Prosperi JR, Mallery SR, Kigerl KA, Erfurt AA, Robertson FM. Invasive and angiogenic phenotype of MCF-7 human breast tumor cells expressing human cyclooxygenase-2. Prostaglandins Other Lipid Mediat. 2004 Apr;73(3–4):249–64. doi: 10.1016/j.prostaglandins.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Robertson FM, Mallery SR, Bergdall-Costell VK, Cheng M, Pei P, Prosperi JR, Ferrari M. Cyclooxygenase-2 directly induces MCF-7 breast tumor cells to develop into exponentially growing, highly angiogenic and regionally invasive human ductal carcinoma xenografts. Anticancer Res. 2007 Mar–Apr;27(2):719–27. [PubMed] [Google Scholar]
- 19.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000 Mar 1;60(5):1306–11. [PubMed] [Google Scholar]
- 20.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004 Apr;31(2 Suppl 7):22–9. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001 Aug 22–29;286(8):954–9. doi: 10.1001/jama.286.8.954. Review. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002 Oct 5;360(9339):1071–3. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007 Apr 20;282(16):11613–7. doi: 10.1074/jbc.R600038200. Review. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. Scientific World Journal. 2007 Sep;Jan;7:1329–47. doi: 10.1100/tsw.2007.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol. 2000 Aug;83(4):279–85. doi: 10.1254/jjp.83.279. Review. [DOI] [PubMed] [Google Scholar]
- 26.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001 Jul;108(1):25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson FM, Simeone AM, Mazumdar A, Shah AH, McMurray JS, Ghosh S, Cristofanilli M. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Ther Oncol. 2008;7(4):299–312. [PubMed] [Google Scholar]
- 28.Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C. Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res. 2003 Oct 1;289(2):265–74. doi: 10.1016/s0014-4827(03)00269-6. [DOI] [PubMed] [Google Scholar]
- 29.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 2006 Oct 15;66(20):9794–7. doi: 10.1158/0008-5472.CAN-06-2067. Review. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006 Mar 15;66(6):2923–7. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Huang Y, Porta R, Yanagisawa K, Gonzalez A, Segi E, Johnson DH, Narumiya S, Carbone DP. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006 Oct 1;66(19):9665–72. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]