Abstract
The molecular basis of breast cancer progression to metastasis and the role of estrogen receptor (ER) signaling in this process remain poorly understood. Emerging evidence suggests that ER participates in extranuclear signaling in addition to genomic functions. Recent studies identified proline-, glutamic acid-, and leucine-rich protein-1 (PELP1), as one of the components of ER signalosome in the cytoplasm. PELP1 expression is deregulated in metastatic breast tumors. We examined the mechanism and significance of ER-PELP1-mediated extranuclear signals in the cytoskeletal remodeling and metastasis. Using estrogen dendrimer conjugate (EDC) that uniquely activate ER-extranuclear signaling and by using model cells that stably express PELP1 shRNA, we demonstrate that PELP1 is required for optimal activation of ER-extranuclear actions. Using a yeast two hybrid screen, we identified ILK1 as a novel PELP1-binding protein. Activation of extranuclear signaling by EDC uniquely enhanced E2-mediated ruffles and filopodia-like structures. Using dominant -negative and -active reagents, we found that estrogen-mediated extranuclear signaling promotes cytoskeleton reorganization via ER-Src-PELP1-PI3K-ILK1 pathway. Utilizing in vitro Boyden chamber assays and in vivo xenograft assays, we found that ER-extranuclear actions contribute to cell migration. Collectively, our results suggest that ER-extranuclear actions play a role in cell motility/metastasis, establishing for the first time that endogenous PELP1 serves as a critical component of ER-extranuclear actions leading to cell motility/invasion and that the ER-Src-PELP1-ILK1 pathway represents a novel therapeutic target for preventing the emergence of ER-positive metastasis.
Keywords: estrogen receptor, coactivator, PELP1, breast cancer, extranuclear signaling
Introduction
The estrogen receptor (ER) is implicated in breast cancer progression. The majority of human breast cancers start out as ER positive (1) and a large portion of metastases retain their ER (2). While initial endocrine therapy has a positive effect on the treatment of advanced metastatic disease (3), acquired resistance to endocrine therapies frequently occurs, with tumors recurring as metastases, which is the leading cause of death from breast cancer. Tumor metastasis consists of a series of discrete biological processes that move tumor cells from the primary neoplasm to a distant location and involves a multi-step cascade (4). The process of migration is orchestrated through the activation of biochemical pathways that involves multiple cytoskeleton proteins (5). Even though substantial information is available on the process of metastasis, the role of E2-ER signaling in breast metastasis remains controversial.
ER-extranuclear signaling has been linked to rapid responses to E2 through stimulation of the Src kinase, mitogen-activated protein kinase (MAPK), and phosphatidylinositol-3-kinase (PI3K) pathways in the cytosol. Emerging evidence suggests that ER participates in extranuclear signaling via formation of a multiprotein complex collectively called a “signalsome” (6). The use of novel ligands that uniquely activate extranuclear signals demonstrated that extranuclear pathways have distinct biological outcomes (7). The molecular mechanism(s) of ER-extranuclear signaling, and the pathobiology of ER-extranuclear actions remain unknown.
Proline-, glutamic acid-, leucine-rich protein-1 (PELP1) (8) is also known as modulator of the nongenomic actions of the estrogen receptor, MNAR (9). PELP1 plays important roles both in the genomic (10) and the non-genomic actions of the ER (11, 12). Recent evidence also suggests that PELP1 couples ER to several signaling pathways such as Src-MAPK, PI3K-AKT, and EGFR-STAT3 (12, 13) and that PELP1 expression is deregulated in metastatic breast tumors (14). Although these studies suggested that PELP1 has tumorigenic potential, whether PELP1-mediated extranuclear signaling plays a role in cell invasion and/or metastasis has not yet been defined.
In this study, we examined whether PELP1-mediated ER-extranuclear signaling play a role in cytoskeletal remodeling, invasion and metastasis. Our results suggest that ER-extranuclear signaling has the potential to contribute to the tumor cell motility and that targeting the ER-PELP1 axis represents a novel therapeutic target to combat breast cancer progression to metastasis in ER-positive breast tumors.
Materials and Methods
Cell cultures and reagents
MCF7 cells were purchased from American-type culture collection (ATCC, Manassas, VA). ZR75 cells were maintained as described previously (15). Antibodies against vinculin, actin and the steroid hormone 17β estradiol were purchased from Sigma Chemical Co (St. Louis, MO). GFP-epitope antibody was purchased from Clontech (Mountain View, CA) and anti–T7-epitope antibody was purchased from EMD BioSciences (Gibbstown, NJ). PELP1 antibody was purchased from Bethyl laboratories (Montgomery, TX). Antibodies against phospho-AKT, phospho-MAPK, phospho-GSK3, phospho-Src and PI3K inhibitor LY294002 were purchased from Cell Signaling (Beverly, MA). Dasatinib was obtained from Bristol-Myers Squibb Pharmaceutical Research Institute (Princeton, New Jersey). MCF7 cells stably expressing PELP1-shRNA were generated using FuGENE-6 transfection (Roche, Indianapolis, IN) and by G418 selection (500μg/mL). PELP-specific shRNA (cat # KH19454N) and control shRNA vectors were purchased from SuperArray (Frederick, MD). MCF7-PELP1cyto cells were earlier described (12).
Plasmids, generation of mutants and transfection
PELP1-Src mutant (PELP1SrcMT) that contains a mutation in SH2-binding site and the Src phosphorylation site Y920F was generated by site-directed mutagenesis using Quick Change Lightning Mutagenesis kit (Stratagene, La Jolla, CA). Plasmids for the myr-p110 subunit of PI3K (active PI3K) and SrcY527F (active Src kinase) were described earlier (16, 17). RFP-myr-p110 (Active PI3K) was constructed by PCR based cloning of open reading frame of myr-p110 into pDsRed-monomer-N1 vector. Expression vector for ILK1 dominant active (DA) (ILKS423D) and dominant negative (DN) (ILKE359K) were provided by Dr. Dedhar (Department of Cancer Genetics, Vancouver, BC, Canada ) (18). The plasmid for Active CDC42 [pcDNA3-EGFP-cdc42 (Q61L)] was received from Addgene (#12600) (19).
Yeast two hybrid library screening and bait construction
PELP1 bait was constructed by amplifying DNA corresponding to amino acids 600-866 via polymerase chain reaction (PCR) and subcloning into Gal4-binding domain vectors (pGBD vector; Clontech, Mountain View, CA). pGBD-PELP1 (aa600-866) was used as a bait to screen a mammary gland cDNA library fused to a Gal4 activation domain (Clontech) as described (20).
Preparation of estrogen dendrimers
The estrogen dendrimer conjugates (EDC) were prepared following a published procedure by Katzenellenbogen et al. (7). A poly amido amine (PAMAM) dendrimer (G-6) was used for the preparation of EDCs. The EDCs were characterized by NMR analysis and the data were found to be consistent with that of the published report (7). A small aliquot was FITC labeled by using Sure Link Fluorescien (FITC) labeling kit (KPL Gaithersburg, MD).
Cell migration and metastasis assays
Wound healing, and Boyden chamber assays were performed as described (21). For determining in vivo metastatic potential, xenograft studies were performed as described (22). Briefly, 1 × 105 model cells in 100 μl PBS were injected into the tail vein or left cardiac ventricle of 5-6 week-old ovariectomized nude mice (n=5) that were each implanted with one E2 pellet (60 day release, 0.72 mg, Innovative Research of America). After 8 weeks, the mice were euthanized, and metastatic nodules on the surface of lung and liver were identified by color and counted under a dissecting microscope.
Western blotting and immunoprecipitation
Cell lysis, immunopreciptation and Western blot analysis with phospho antibodies were performed as described (23).
ILK kinase assays
Exogenously expressed GFP-ILK1 or endogenous ILK1 was immunoprecipitated and was used as a source of ILK enzyme. In vitro kinase assays using MBP protein were performed in HEPES buffer (50 mM HEPES, 10 mM MgCl2, 10 mM MnCl2, 1 mM NaF, 0.2 mM Na3VO4) containing immunoprecipitated ILK1 enzyme, 10 μCi of [γ-32P] ATP, and 25 μM ATP in 30 μl reaction.
Immunofluorescence studies
The immunofluorescence studies were performed as described previously (13). Secondary antibodies conjugated with Alexa 488 (green) or Alexa 546 (red), or Alexa 633 (Blue) dye was used to recognize different primary antibodies (Molecular Probes). The F-actin status was analyzed by phalloidin staining.
Results
PELP1 knock down affects E2-ER-mediated extranuclear signaling and cytoskeletal reorganization
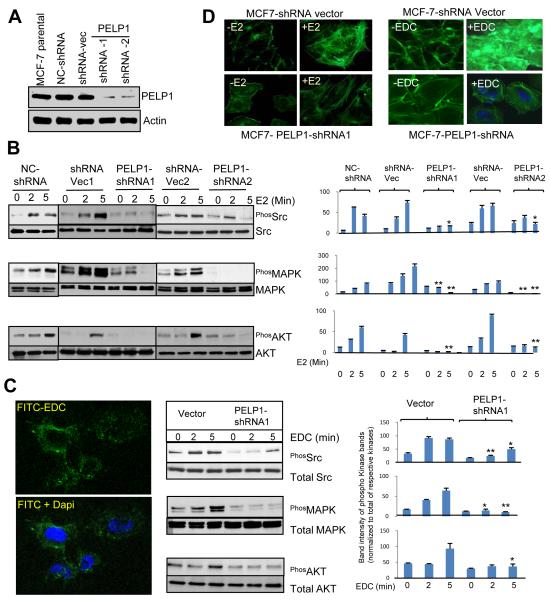
To study the in vivo significance of PELP1 in the extranuclear actions of ER, we established MCF7 breast cancer model cells that stably expressed PELP1shRNA that specifically down regulate endogenous PELP1. MCF7 cells were transfected with shRNA vector and negative control (NC) shRNA vector (that express shRNA targeting scrambled artificial sequence with no sequence identity to the human genome) were used as a control. Western blot analysis of total lysates revealed that the PELP1-shRNA clones down regulated PELP1 expression to ~80 % of the level seen in the parental and the vector-transfected clones (Fig. 1A). To examine the significance of endogenous PELP1 in the activation of ER-extranuclear signaling pathways, we measured the activation of signaling pathways including Src, AKT and MAPK after treating cells with E2 for short time periods. Compared to shRNA vector transfected cells, PELP1-shRNA-expressing cells had significantly less Src, AKT and MAPK activation (Fig. 1B). To further establish the role of PELP1 in E2-mediated non-genomic actions, we used EDC (nanoparticles coated with estrogen) that uniquely localize in the membrane/cytoplasm (Fig. 1C, left panel), and preferably activate ER-extranuclear signaling (7). MCF-7 cells that express vector or PELP1 shRNA were treated with EDC for 2, 5 min and signaling was analyzed by phospho-specific antibodies. EDC addition uniquely promoted activation of Src and MAPK pathways. However, knock down of PELP1 by shRNA significantly affected the EDC-mediated increase in Src and MAPK activation (Fig. 1C, middle and right panels). These results suggest that E2-mediated extranuclear actions play a key role in the activation of Src and MAPK and that the functional PELP1 signaling axis is needed for E2-mediated extranuclear signaling. Because Src and PI3K play important role in cytoskeletal functions, cell attachment and migration, we asked whether E2-ER extranuclear actions contribute to cytoskeletal reorganization leading to cell migration. MCF7 cells that expressed vector or PELP1 shRNA were treated with either E2 or EDC for 10 min and cytoskeletal changes were analyzed by confocal microscopy. E2 or EDC addition uniquely promoted actin reorganization with filopodia and ruffle formations. However, knock down of PELP1 by shRNA substantially affected actin reorganization by E2 or EDC with little ruffles / filopodia formations and predominantly showed cortical actin and stress fibers (Fig. 1D). These studies demonstrate that ER-extranuclear actions have the potential to promote cytoskeletal changes leading ruffle and filopodia formation.
Figure 1.
Activation of ER-extranuclear signaling promotes actin reorganization. A, MCF7 control or MCF7-PELP1-shRNA cells were lysed and expression of PELP1 was analyzed by Western blotting. B, MCF7 vector control and MCF7-PELP1-shRNA cells were cultured in 5% DCC serum containing medium treated with or without E2. The activation of signaling pathways was analyzed by Western blotting of total protein lysates with phospho-specific antibodies. Densitometric analysis of the western blots of phospho bands from triplicate samples were performed and corrected with the values of respective total bands. Each column is an average of triple determinations. Bars, SEM. *, p<0.05. **, P<0.001. C, MCF7 cells were treated with FITC-labeled EDC for 45 min and localization of EDC was analyzed by confocal microscopy (left panel). MCF7 and MCF7-PELP1-shRNA cells were treated with EDC and activation of signaling pathways was analyzed by Western blotting (C, middle panel). Quantitation of the bands was as described in Fig. B legend (C, right panel). D, MCF7 or MCF7-PELP1-shRNA cells were treated either with E2 or EDC and the F-actin status was analyzed by phalloidin staining and visualized by confocal microscopy.
Src kinase plays a critical role in PELP1-mediated E2 extranuclear signaling leading to cytoskeletal reorganization
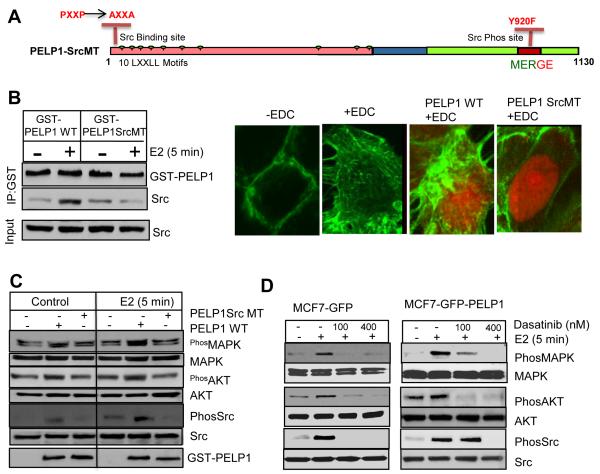
PELP1 acts as a scaffolding protein coupling ER with Src kinase leading to activation of ER-Src-MAPK and ER-Src-AKT pathways (11). Earlier studies also revealed that PELP1 interacts with c-Src SH3 domain via its N-terminal PXXP motif and Src phosphorylates PELP1 at C terminal (tyrosine 920) domain (9). To establish the significance of Src kinase in PELP1-mediated E2-ER extranuclear signaling, we generated a PELP1 mutant construct (PELP1SrcMT) that contains a mutation in the Src-SH3 binding site on PELP1 (ProXXPro is mutated to AlaXXAla) and a mutation in Src phosphorylation site (Tyr 920 is mutated to Phe, Fig. 2A). The PELP1SrcMT mutant is unable to interact with Src kinase and thus functions as a dominant negative mutant of PELP1. As expected, PELP1 WT but not the PELP1SrcMT interacted with Src kinase (Fig. 2B). Transient expression of PELP1SrcMT substantially effected the E2-mediated cytoskeletal reorganization in a dominant negative fashion (Fig. 2B, right panel) and also interfered with E2-mediated activation of Src and MAPK (Fig. 2C). Since Src kinase appears to play a key role in E2 extranuclear signaling, we examined effect of inhibition of Src kinase using dasatinib, a well-established orally available inhibitor of Src family tyrosine kinases (24). For these studies, we used MCF7 control cells or MCF7-PELP1WT model cells that overexpress PELP1 and exhibit increased E2-ER extranuclear signaling. Pharmacological inhibition of Src kinase using dasatinib abolished the E2-mediated activation of AKT and MAPK pathways both in MCF7 as well as in PELP1-overexpressing MCF7 cells (Fig. 2D). Collectively, these results suggest that Src kinase play an important role in PELP1-mediated E2 extranuclear actions.
Figure 2.
Src kinase is needed for optimal activation of PELP1-mediated E2 extranuclear actions. A, Schematic representation of PELP1 mutant that cannot bind or be phosphorylated by Src kinase. B, MCF7 cells were transfected with PELP1WT or PELP1SrcMT and treated with or without E2. The ability of the expressed proteins to interact with Src kinase was analyzed by immunoprecipitation (left panel). MCF7 control and MCF7 cells were transiently transfected with either PELP1WT or PELP1SrcMT were treated with EDC for 5 min. The status of F-actin was analyzed by confocal microscopy (right panel). C, MCF7 cells were transfected with PELP1WT or PELP1SrcMT using the Amaxa nucleofection method and treated with or without E2. Activation of extranuclear signaling was measured by Western blotting. D, MCF7 or MCF7 cells that stably expressed PELP1 were treated with or without dasatinib and with or without E2. Activation of extranuclear signaling was measured by Western blotting.
Integrin linked kinase 1 is a novel PELP1-interacting protein
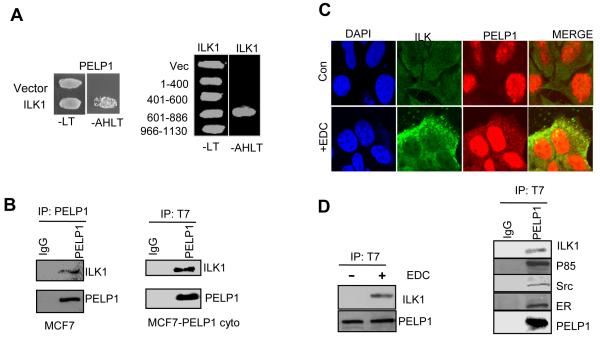
To identify the novel components of the PELP1 signalsome that contribute to ER-extranuclear signaling leading to cytoskeletal reorganization, we performed a yeast two-hybrid screen using mammary gland cDNA expression library. One of the positive clone sequences matched with that of ILK1. The specificity of ILK1 and PELP1 interaction was confirmed further using co-transformation followed by a survival assay in selection medium using yeast cells that stably expressed histidine, tryptophan, leucine nutrient reporter genes under the control of GAL response elements. The GBD-PELP1 and GAD-ILK1 transformed colonies grew in medium lacking adenosine, histidine, tryptophan, and leucine, whereas the cells co-transformed with the control GBD vector and GAD-ILK1 did not grow (Fig. 3A, left panel). Deletion experiments revealed that the possible interaction of ILK1 and PELP1 involved amino acids 601-886 (Fig. 3A, right panel). To further verify the interaction between ILK1 and PELP1, we transfected T7-tagged PELP1 and GFP-tagged ILK1 into MCF7 cells. Total lysates were subjected to immunoprecipitation with PELP1-tagged antibody followed by western blotting with ILK1. Results showed that PELP1 can interact with ILK1 in vivo. (Fig. 3b, left panel). Similarly, the PELP1 and ILK1 interaction was also observed in PELP1cyto model cells that express PELP1 exclusively in the cytoplasm, suggesting the physiological significance of such an interaction in the cytoplasm (Fig. 3B, right panel). Confocal analysis of EDC-treated MCF7 cells showed colocalization of PELP1 with ILK1 upon EDC treatment (Fig. 3C). Coimmunoprecipitation assay results showed that PELP1 interaction with ILK1 is dependent on ligand (Fig. 3D, left panel). Immunoprecipitation of PELP1 also showed the presence of ILK1 in the precipitates along with Src, ER and P85 subunit of PI3K, which are the known PELP1 signalsome components (Fig. 3D, right panel). These data suggest that ILK1 is a novel component of the PELP1 signalsome.
Figure 3.
Integrin linked kinase 1 (ILK1) is a novel PELP1 binding protein A, Confirmation of PELP1 interaction with ILK1 is shown in a yeast-based growth assay (left panel). Identification of the domain of interaction between PELP1 and ILK1 using a yeast-based growth assay (right panel). B, MCF7 cells that express T7-tagged PELP1WT (left panel) or T7-PELPcyto mutant (right panel) were treated with EDC and the PELP1 and ILK1 interaction was confirmed by immunoprecipitation assay. C, MCF7 cells were treated with or without EDC for 5 min and the colocalization of PELP1 and ILK1 were analyzed by confocal microscopy. D, MCF7- T7-PELPcyto cells were treated with or without EDC and total protein lysates were immunoprecipitated with T7-tagged antibody. The presence of PELP1, Src, ILK1, ER, and p85 in the immunoprecipitates was analyzed by Western blotting.
ILK1 couples E2-mediated PELP1 signaling to cytoskeleton
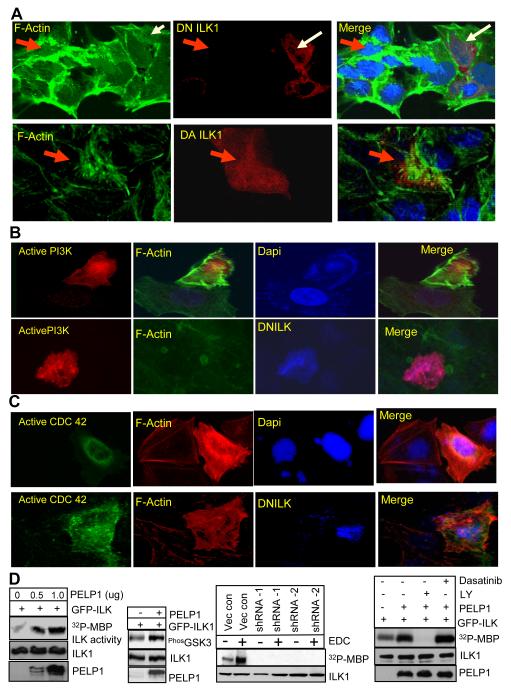
Since ILK1 is a novel component of PELP1 signalosome, we examined the significance of ILK1 in PELP1 signaling using dominant negative and active ILK1 constructs (18) and monitored the formation of motility related structures including formation of stress fibers, lamellipodia (membrane rufflings) and filopodia (microspikes). Expression of DN ILK1 into MCF7 cells significantly reduced the formation of actin structures by E2 treatment (Fig. 4A, upper panel). Accordingly, overexpression of dominant active ILK1 rescued formation of F-actin structures including ruffles and filopodia in MCF7-PELP1shRNA cells (Fig 4A, lower panel). Overexpression of Src kinase failed to rescue the cytoskeleton defects in PELP1 shRNA clones (data not shown), suggesting that PELP1 is downstream of Src kinase. Since Src kinase phosphorylation of PELP1 promotes downstream signaling by coupling with the PI3K axis, we introduced a membrane-targeted PI3K, RFP tagged, a myristoylated subunit of p110 that functions as an active PI3K, into PELP1 shRNA cells. Expression of membrane-targeted PI3K rescued the actin structures in PELP1 shRNA cells (Fig. 4B, upper panel). However, cotransfection of membrane targeted PI3K along with DN ILK1 inhibited active PI3K-mediated rescue of actin structures (Fig. 4B, bottom panel), indicating that ILK1 functions downstream of PI3K in the PELP1 signaling axis. We also measured whether the expression of active CDC42, a known downstream target of ILK1 rescues the phenotype. Overexpression of active CDC42 indeed restored the actin structures in PELP1 shRNA cells (Fig.4C, upper panel) and DN ILK1 failed to interfere with active CDC42-mediated restoration (Fig.4C, bottom panel). Such results suggest E2>PELP1>PI3K>ILK1>CDc42 as a signaling pathway that contribute to E2-mediated cytoskeleton changes.
Figure 4.
PELP1–ILK1 axis plays a productive role in E2-mediated cytoskeleton reorganization. Only one representative image for each experimental condition is shown and the results are representative of three independent replicates. A, MCF7 breast cancer cells were transfected with dominant negative (DN)-ILK1 (red). After 72 h, the cells were treated with EDC for 10 min and F-actin (green) status was analyzed by confocal microscopy (A, top panel). MCF7-PELP1 shRNA cells were transiently transfected with dominant active (DA)-ILK1. F-actin status was verified by confocal microscopy (A, Bottom panel). B, MCF7-PELP1 shRNA cells were transfected with constitutively active RFP-P110α subunit of PI3K (red) without (B, top panel) or with DN-ILK1 (B, bottom panel, blue color) and F-actin (green) changes were analyzed by confocal microscopy. C, MCF7-PELP1 shRNA cells were transiently transfected with DA-CDC42 (green) without (C, top panels) or with DN-ILK1 (C, bottom panels, blue) and F-actin status was verified by confocal microscopy. D, The ability of PELP1 to enhance ILK1 activity was measured by an in vitro kinase assay by incubating immunoprecipitated GFP-ILK1 with increasing of amounts of GST-PELP1 (D, left panel). MCF7 cells were transfected with ILK1 expression vector with or without PELP1 expression vector and the ability of PELP1 to enhance ILK1 downstream signaling was analyzed by Western blotting (D, left panel). MCF7 and MCF7-PELP1 shRNA cells were treated with or without EDC. ILK1 was immunoprecipitated and kinase activity was measured using an in vitro kinase assay (D, middle panel). Cells were transfected with ILK1 expression vector with or without PELP1 expression vector. After 72 hours, cells were treated with PI3K inhibitor (LY294002, 50 uM) or Src kinase inhibitor (dasatinib, 100 nM). ILK1 was immunopreciptated and the ILK1 activity was measured by an in vitro kinase assay (D, right panel).
Since earlier studies showed that PELP1 interactions with Src enhance Src kinase activity, we examined whether PELP1 interactions with ILK1 also modulate its kinase activity. We performed in vitro kinase assay using immunoprecipitated ILK1 as the enzyme source and baculovirus-purified GST-PELP1 as an activator. Incubation of ILK1 with GST-PELP1, but not GST alone, increased the ILK1 activity in in vitro assay (Fig. 4D, left panel). To examine PELP1 regulation of ILK1 in vivo, we transfected ILK1 with or without PELP1 in 293T cells and activation of the ILK substrate GSK3β was detected. Compared to vector transfection, cotransfection of PELP1 increased GSK3β phosphorylation (Fig. 4D, left panel). Treatment of MCF7 model cells with EDC that stimulate E2 extranuclear signaling, substantially increased ILK kinase activity, however, PELP1 knockdown substantially affect EDC-mediated activation of ILK1 (Fig. 4D, middle panel). To examine, whether PELP1 mediated activation of ILK1 dependent on functional Src or PI3K pathways, we have used inhibitors of these pathways. Results showed that treatment of cells with PI3K inhibitor LY294002 abolishes PELP1 mediated activation of ILK1; while Src inhibitor dasatinib has no effect on PELP1 mediated activation of ILK1 (Fig. 4D, right panel). Earlier studies have shown that ligand induced phosphorylation of PELP1 by Src is critical for PELP1 coupling to PI3K pathway by p85-SH2 domain (9). Similarly, overexpression of PELP1 (a situation that occurs in tumors) is also shown to constitutively activate PI3K pathway by p85-SH3 domain mediated interactions (12), suggesting that PELP1 can potentially interact with and activate PI3K via two distinct mechanisms. Since in this experiment (Fig. 4D), we have used PELP1 overexpression, inhibition of ILK1 activity by PELP1 in the presence of PI3K inhibitor but not in the presence of Src kinase inhibitor suggests that PELP1’s direct interactions with PI3K may lead to ILK1 activation. However, in the physiological context Src kinase does play a role in ligand mediated activation of ILK1 by promoting PELP1-PI3K-ILK1 complex formation.
PELP1 is needed for optimal cell migration promoted by E2 extranuclear actions
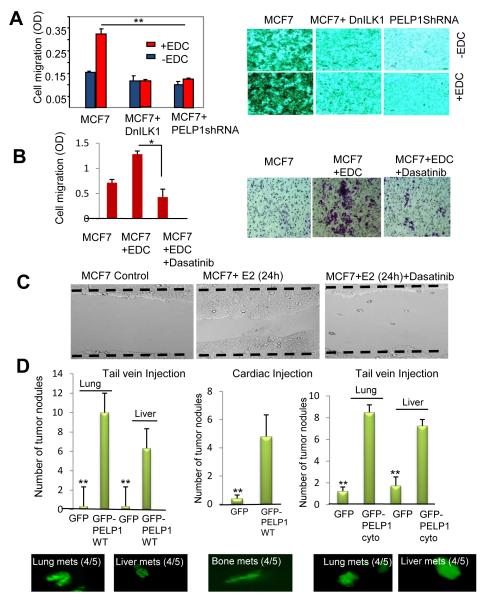
We examined whether E2-mediated extranuclear actions contribute to cell migration. In Boyden chamber assays, parental MCF7 cells showed low motility, and EDC further increased the migratory potential of those cells. The knockdown of PELP1 expression by siRNA substantially reduced EDC-mediated cell motility (Fig. 5A). Interestingly, model cells expressing DN ILK1 also failed to migrate upon EDC stimulation (Fig. 5A). We also examined whether EDC-mediated cell migratory potential can be blocked by pharmacological inhibition of Src kinase. Dasatanib effectively blocked the EDC-mediated cell migration in Boyden chamber assays (Fig. 5B). Similarly dasatinib also inhibited E2-mediated cell migration in would healing assays (Fig. 5C).
Figure 5.
E2-mediated extranuclear actions promote cell migration and metastasis. A, MCF7 cells, MCF7 cells transfected with dominant negative ILK1 (DN-ILK1), and MCF7 cells stably transfected with PELP1-shRNA were treated with or without EDC and the migratory potential was analyzed by using Boyden chamber assay. Photomicrographs of migrated cells in various treatments (right panel). Data shown are the means of ± SEM from three independent experiments performed in triplicate wells. **, p <0.001. B, MCF7 cells were treated with EDC in the presence or absence of Src inhibitor dasatinib (100 nM). The cell migratory potential was analyzed by using Boyden chamber assay. Data shown are the means of ± SEM from three independent experiments performed in triplicate wells. *, p <0.05. C, Wound healing assay was performed in the presence or absence of E2 and in presence or absence of dasatinib. D, ZR75 cells expressing GFPvector or GFP-PELP1WT were injected into nude mice either via tail vein (D, Left panel) or cardiac route (D, middle panel) and metastases were recorded after 8 weeks. MCF7 cells expressing control GFP-vector or GFP+PELP1cyto were injected into nude mice (n=5) via tail vein (D, right panel). Representative images of metastatic nodules as observed by fluorescence microscope are shown. The average number of tumor nodules is shown in the graph. Bars, SEM, **, P<0.0001.
PELP1 over expression enhances in vivo metastatic potential of ER-positive ZR75 cells
Since PELP1 expression is deregulated in metastatic tumors (14), we hypothesized that PELP1 overexpression may play a role in metastasis by promoting E2 extranuclear actions. We performed a proof-of-principle experiment using ER-positive ZR75 cells that exhibit poor metastasis in nude mice models. ZR75 cells were stably transfected with a GFP control or PELP1WT-GFP vector. PELP1WT-GFP cells had 3 fold higher expression of PELP1 than the control cells (data not shown). Mice injected with GFP control cells showed 0-1 metastatic nodules. However, PELP1-overexpressing cells had an increased propensity for metastases with 8-12 nodules identified in lungs (4 of 5 mice) and 6-8 nodules in liver (4 of 5 mice) (Fig. 5D). To validate these findings further, we also injected GFP-vector and GFP-PELP1WT cells via a cardiac route into nude mice. Earlier studies found that this route facilitates bone metastasis (25). GFP-PELP1WT-overexpressing cells, but not GFP vector-expressing cells, had metastases in the bone (Fig. 5D, middle panel). To examine the significance of PELP1 extranuclear signaling in metastasis, we have repeated xenograft assay using PELP1cyto cells (12) that uniquely express PELP1 in the cytoplasm and are shown to excessively promote ER extranuclear signaling. Similar to PELP1WT cells, PELP1cyto cells also showed increased propensity to metastasize compared to MCF7 control cells (Fig. 5D, right panel). These results further suggest that ER-extranuclear actions have potential to promote metastasis.
Discussion
The pathological significance of ER extranuclear signaling and its role in the progression to metastasis of breast cancer remain unknown. In this study, using estrogen dendrimers, dominant negative reagents, and pharmacological inhibitors of ER-extranuclear signaling, we found that ER-extranuclear actions play an important role in cell motility and metastases. In addition, we established for the first time that endogenous PELP1 play a critical role in coupling ER-extranuclear signaling to cell motility via ER-Src-PELP1-ILK-Rac/CDC42 pathway.
The proto-oncogene c-Src is a multifunctional intracellular tyrosine kinase implicated in the regulation of a variety of processes including proliferation, differentiation, survival, and motility (26). Src interacts with multiple cellular factors including HER2, EGFR and ER and breast tumors overexpress Src kinase (27). PELP1 acts as a scaffolding protein coupling the ER with Src kinase leading to activation of the ER-Src-MAPK pathway (11). Our data suggest that PELP1 and Src kinase play an essential role in the activation of ER-extranuclear signaling leading to cytoskeleton reorganization and migration. Since breast tumors overexpress Src kinase, deregulation of PELP1 seen in breast tumors can contribute to activation of Src, leading to the progression to metastasis. Pharmacological inhibition of Src using dasatinib significantly inhibited E2-mediated extranuclear actions and reduced E2 mediated migratory potential. These results suggest that the ER-Src-PELP1 axis is a novel target for preventing the emergence of metastatic cells and that dasatinib may have therapeutic utility in blocking ER-positive metastases.
ERα has been implicated in breast cancer progression and majority of the human breast cancers start out as hormone-dependent. Some evidence suggests that the extranuclear effects of estrogen can regulate different cellular processes, such as proliferation, survival, apoptosis (28). Our results using EDC demonstrates that ER extranuclear signaling has potential to promote cytoskeletal changes, leading to increased cell migration. Findings from these studies also showed that E2 extranuclear signaling promotes formation of signaling complexes that contain PELP1, ER Src, and ILK1 and that extranuclear signaling from this axis play important roles in cytoskeletal rearrangements, motility and metastasis.
We identified ILK1 as a novel interacting protein of PELP1 and demonstrated that ILK1 functions as a downstream effector of ER-extranuclear signaling, leading to cytoskeletal reorganization. ILK1 is known to play an important role in cytoskeleton reorganization and in the activation of Rho GTPases (Rac and CDC42). These effects are reversible upon inhibition of ILK protein expression (29) The ability of PELP1 to modulate the ILK1 pathway and its potential deregulation in metastatic breast cancer suggest that the modulation of ILK1 pathway may represent one potential mechanism by which PELP1 promotes metastasis in breast cancer cells.
PELP1 is a key component of the ER signalsome in the cytoplasm and is shown to play a role in ER extranuclear actions (8, 9). PELP1 expression appears to be predominantly in the cytoplasm in a subset of breast tumors. Previous studies showed that PELP1 cytoplasmic locolization excessively promote ER-extranuclear signaling and that such deregulation contributed to tamoxifen therapy resistance (12). A recent study demonstrated that patients whose tumors had high levels of cytoplasmic PELP1 had a tendency to respond poorly to tamoxifen compared with patients whose tumors had low levels of cytoplasmic PELP1 (30). In this study, using ligands that uniquely activate ER extranuclear signaling (EDC) and PELP1shRNA or dominant mutants that block PELP1 signaling, we found that E2-driven PELP1-mediated ER-extranuclear actions can promote the cell migratory potential.
Endocrine therapy has also been shown to have a positive effect on the treatment of advanced metastatic disease (3). A few earlier studies suggested a negative effect of ER signaling on motility and invasion of cells (31, 32), while several recent studies showed a positive effect of ER signaling on motility (32, 33). Many metastatic tumors retain ER (34), and if primary tumors are ER positive, >80% of lymph node metastases and 65-70% of distant metastases retain ER (2, 35). A clinical correlation has also been reported between ER-positive tumors and the development of bone metastasis (36, 37). Similarly ER signaling has been shown to enhance lung metastasis by promoting host-compartment response (38). PELP1 expression is deregulated in metastastic tumors (14) and PELP1 protein expression is an independent prognostic predictor of shorter breast cancer-specific survival and its elevated expression is positively associated with markers of poor outcome (39). Our data suggest that ER-extranuclear signaling plays a role in metastasis and PELP1 deregulation commonly seen in metastastic tumors may play a role in metastasis by enhancing ER-extranuclear signaling.
In summary, our data provide the first evidence demonstrating the significance of ER-extranuclear signaling to the metastatic potential of breast cancer cells. Our findings also identified ILK1 as a novel component of ER-PELP1 signalsome that connects ER signaling to cytoskeleton. We hypothesize that the ER-Src-PELP1-PI3K-ILK1 pathway represents a novel target to prevent the emergence of ER-positive metastatic cells via blockage of ER-extranuclear signals in combination with endocrine therapy.
Acknowledgments
This study was supported by the grants NIH-CA0095681 (RKV), DOD-W81XWH-08-1-0604 (RKV), Komen- KG091267 (DC), NIH-CA075253 (LZS), and NIH -P30CA54174 (RKV and RRT).
References
- (1).Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:195–216. [PubMed] [Google Scholar]
- (2).Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66:9308–15. doi: 10.1158/0008-5472.CAN-06-1769. [DOI] [PubMed] [Google Scholar]
- (3).Utsumi T, Kobayashi N, Hanada H. Recent perspectives of endocrine therapy for breast cancer. Breast Cancer. 2007;14:194–9. doi: 10.2325/jbcs.959. [DOI] [PubMed] [Google Scholar]
- (4).Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- (5).Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- (6).Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- (8).Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–5. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- (10).Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–23. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- (11).Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- (12).Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–32. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Manavathi B, Nair SS, Wang RA, Kumar R, Vadlamudi RK. Proline-, glutamic acid-, and leucine-rich protein-1 is essential in growth factor regulation of signal transducers and activators of transcription 3 activation. Cancer Res. 2005;65:5571–7. doi: 10.1158/0008-5472.CAN-04-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic Potential of the Nuclear Receptor Coregulator Proline-, Glutamic Acid-, Leucine-Rich Protein 1/Modulator of the Nongenomic Actions of the Estrogen Receptor. Cancer Res. 2007;67:5505–12. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Le SE, Zhu Q, Wang L, Bandyopadhyay A, Javelaud D, Mauviel A, et al. Transforming growth factor-beta suppresses the ability of Ski to inhibit tumor metastasis by inducing its degradation. Cancer Res. 2008;68:3277–85. doi: 10.1158/0008-5472.CAN-07-6793. [DOI] [PubMed] [Google Scholar]
- (16).Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–27. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–20. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- (18).Oloumi A, Syam S, Dedhar S. Modulation of Wnt3a-mediated nuclear beta-catenin accumulation and activation by integrin-linked kinase in mammalian cells. Oncogene. 2006;25:7747–57. doi: 10.1038/sj.onc.1209752. [DOI] [PubMed] [Google Scholar]
- (19).Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM. Activation of endogenous Cdc42 visualized in living cells. Science. 2004;305:1615–9. doi: 10.1126/science.1100367. [DOI] [PubMed] [Google Scholar]
- (20).Nair SS, Guo Z, Mueller JM, Koochekpour S, Qiu Y, Tekmal RR, et al. Proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor enhances androgen receptor functions through LIM-only coactivator, four-and-a-half LIM-only protein 2. Mol Endocrinol. 2007;21:613–24. doi: 10.1210/me.2006-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–60. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bandyopadhyay A, Agyin JK, Wang L, Tang Y, Lei X, Story BM, et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 2006;66:6714–21. doi: 10.1158/0008-5472.CAN-05-3565. [DOI] [PubMed] [Google Scholar]
- (23).Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- (24).Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- (25).Arguello F, Baggs RB, Frantz CN. A murine model of experimental metastasis to bone and bone marrow. Cancer Res. 1988;48:6876–81. [PubMed] [Google Scholar]
- (26).Trevino JG, Summy JM, Gallick GE. SRC inhibitors as potential therapeutic agents for human cancers. Mini Rev Med Chem. 2006;6:681–7. doi: 10.2174/138955706777435724. [DOI] [PubMed] [Google Scholar]
- (27).Russello SV, Shore SK. SRC in human carcinogenesis. Front Biosci. 2004;9:139–44. doi: 10.2741/1138. [DOI] [PubMed] [Google Scholar]
- (28).Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- (29).Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837–49. doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- (30).Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–30. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Rochefort H, Platet N, Hayashido Y, Derocq D, Lucas A, Cunat S, et al. Estrogen receptor mediated inhibition of cancer cell invasion and motility: an overview. J Steroid Biochem Mol Biol. 1998;65:163–8. doi: 10.1016/s0960-0760(98)00010-7. [DOI] [PubMed] [Google Scholar]
- (32).Sisci D, Aquila S, Middea E, Gentile M, Maggiolini M, Mastroianni F, et al. Fibronectin and type IV collagen activate ERalpha AF-1 by c-Src pathway: effect on breast cancer cell motility. Oncogene. 2004;23:8920–30. doi: 10.1038/sj.onc.1208098. [DOI] [PubMed] [Google Scholar]
- (33).Thompson EW, Reich R, Shima TB, Albini A, Graf J, Martin GR, et al. Differential regulation of growth and invasiveness of MCF-7 breast cancer cells by antiestrogens. Cancer Res. 1988;48:6764–8. [PubMed] [Google Scholar]
- (34).Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- (35).Zheng WQ, Lu J, Zheng JM, Hu FX, Ni CR. Variation of ER status between primary and metastatic breast cancer and relationship to p53 expression*. Steroids. 2001;66:905–10. doi: 10.1016/s0039-128x(01)00121-0. [DOI] [PubMed] [Google Scholar]
- (36).Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- (37).Wang J, Jarrett J, Huang CC, Satcher RL, Jr., Levenson AS. Identification of estrogen-responsive genes involved in breast cancer metastases to the bone. Clin Exp Metastasis. 2007;24:411–22. doi: 10.1007/s10585-007-9078-6. [DOI] [PubMed] [Google Scholar]
- (38).Banka CL, Lund CV, Nguyen MT, Pakchoian AJ, Mueller BM, Eliceiri BP. Estrogen induces lung metastasis through a host compartment-specific response. Cancer Res. 2006;66:3667–72. doi: 10.1158/0008-5472.CAN-05-4416. [DOI] [PubMed] [Google Scholar]
- (39).Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]