Abstract
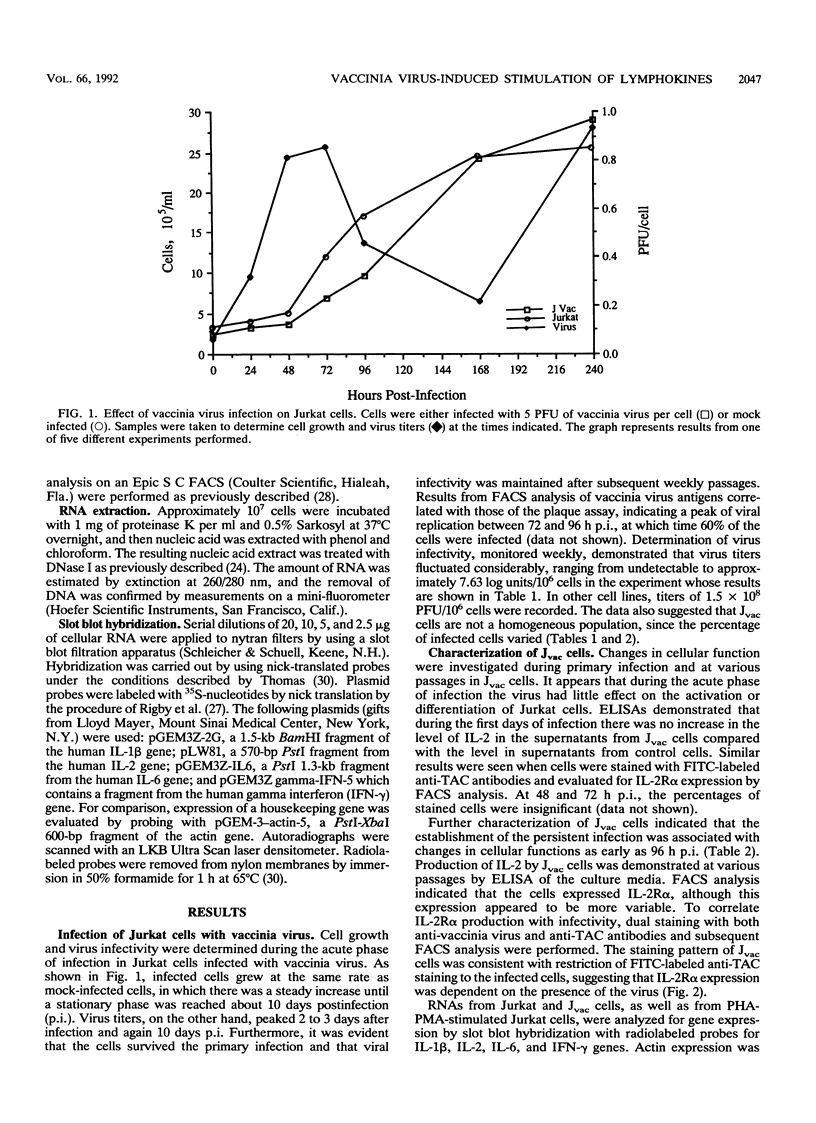
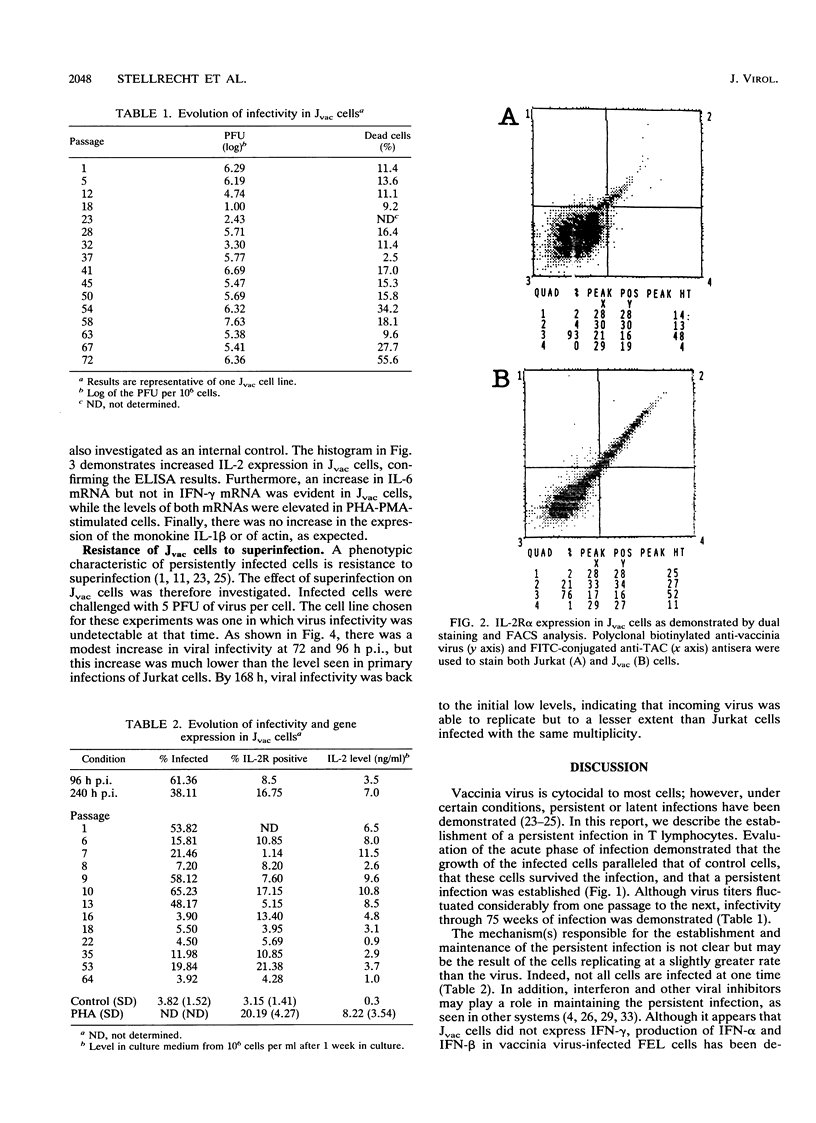
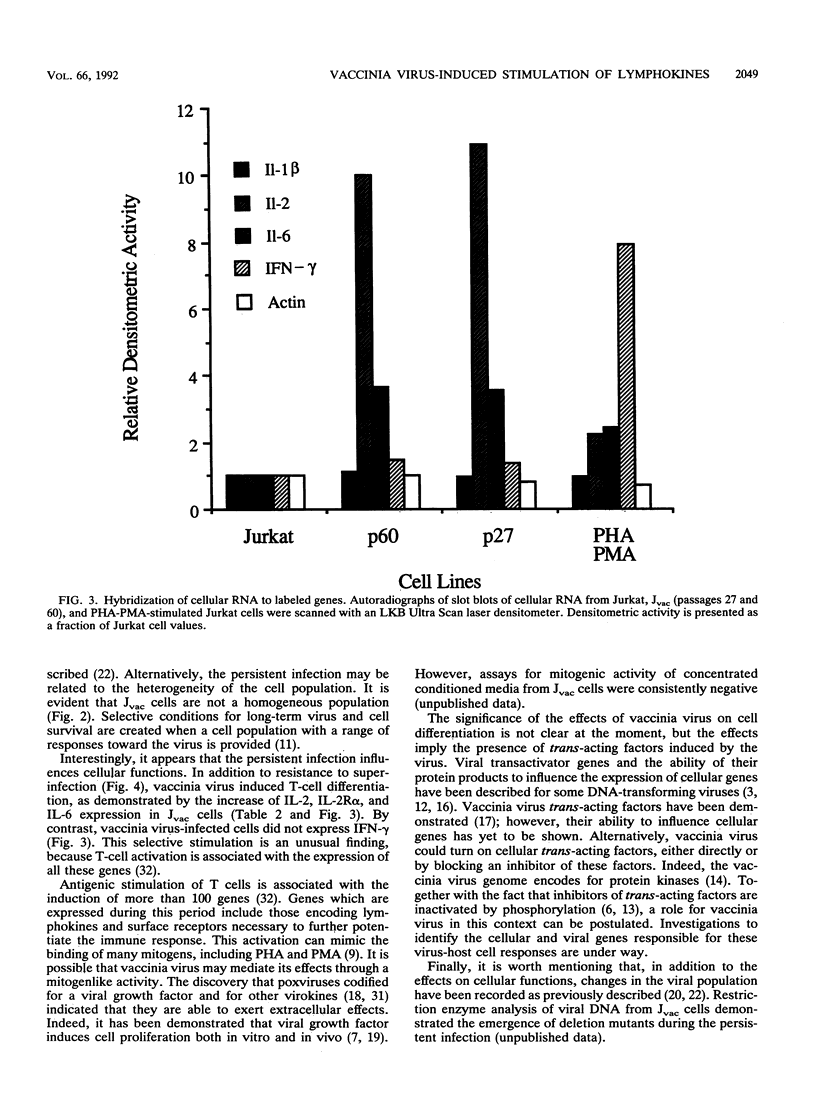
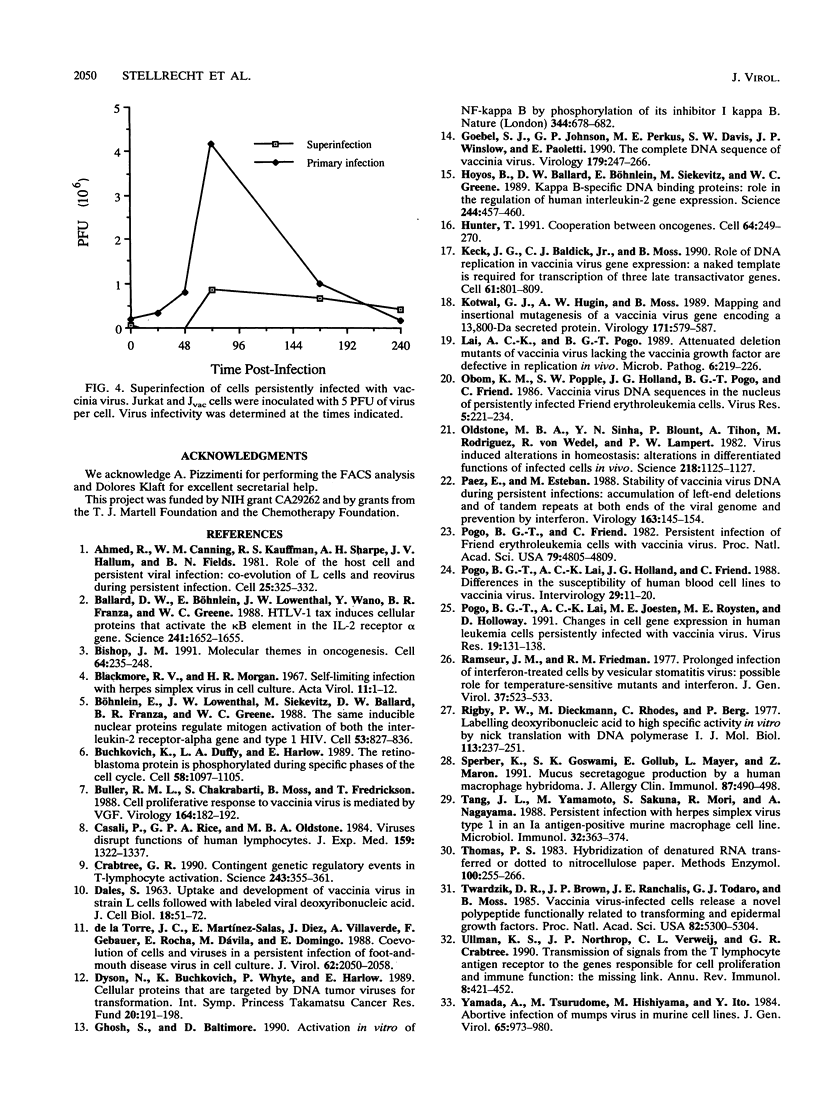
The response of the human CD4+ T-cell line Jurkat to infection with vaccinia virus was investigated. Virus titers peaked approximately 3 to 4 days after infection, while cell growth paralleled that of uninfected cells, indicating that growth rates were not appreciably affected by viral infection. Results from plaque assays and fluorescence-activated cell sorter (FACS) analyses of virus antigens demonstrated that a persistent infection in which the percentage of infected cells and the virus titers fluctuated from passage to passage was established. Further characterization of the persistent infection revealed that the virus influences cellular functions. Induction of interleukin-2 (IL-2) and IL-2 receptor alpha (IL-2R alpha) in Jvac cells was shown by enzyme-linked immunosorbent assay and FACS analysis, respectively. Hybridization of cellular RNA with cloned probes confirmed the increased IL-2 expression and demonstrated that Jvac cells also expressed more IL-6 but not gamma interferon (IFN-gamma) or IL-1 beta. Dual-antibody staining and FACS analysis for vaccinia virus antigens and IL-2R alpha indicated that IL-2R alpha expression was restricted to the infected cells. Jvac cells were also resistant to superinfection, an additional proof that persistent infection elicited phenotypic changes in the cell population.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Blackmore R. V., Morgan H. R. Self-limiting infection with herpes simplex virus in cell culture. Acta Virol. 1967 Jan;11(1):1–12. [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. Cellular proteins that are targetted by DNA tumor viruses for transformation. Princess Takamatsu Symp. 1989;20:191–198. [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Hügin A. W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989 Aug;171(2):579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Lai A. C., Pogo B. G. Attenuated deletion mutants of vaccinia virus lacking the vaccinia growth factor are defective in replication in vivo. Microb Pathog. 1989 Mar;6(3):219–226. doi: 10.1016/0882-4010(89)90071-5. [DOI] [PubMed] [Google Scholar]
- Obom K. M., Popple S. W., Holland J. G., Pogo B. G., Friend C. Vaccinia virus DNA sequences in the nucleus of persistently infected Friend erythroleukemia cells. Virus Res. 1986 Aug;5(2-3):221–234. doi: 10.1016/0168-1702(86)90020-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Sinha Y. N., Blount P., Tishon A., Rodriguez M., von Wedel R., Lampert P. W. Virus-induced alterations in homeostasis: alteration in differentiated functions of infected cells in vivo. Science. 1982 Dec 10;218(4577):1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Friend C. Persistent infection of Friend erythroleukemia cells with vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4805–4809. doi: 10.1073/pnas.79.15.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Lai A. C., Holland J. G., Friend C. Differences in the susceptibility of human blood cell lines to vaccinia virus. Intervirology. 1988;29(1):11–20. doi: 10.1159/000150024. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sperber K., Goswami S. K., Gollub E., Mayer L., Marom Z. Mucus secretagogue production by a human macrophage hybridoma. J Allergy Clin Immunol. 1991 Feb;87(2):490–498. doi: 10.1016/0091-6749(91)90007-b. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Yamada A., Tsurudome M., Hishiyama M., Ito Y. Abortive infection of mumps virus in murine cell lines. J Gen Virol. 1984 May;65(Pt 5):973–980. doi: 10.1099/0022-1317-65-5-973. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



