Abstract
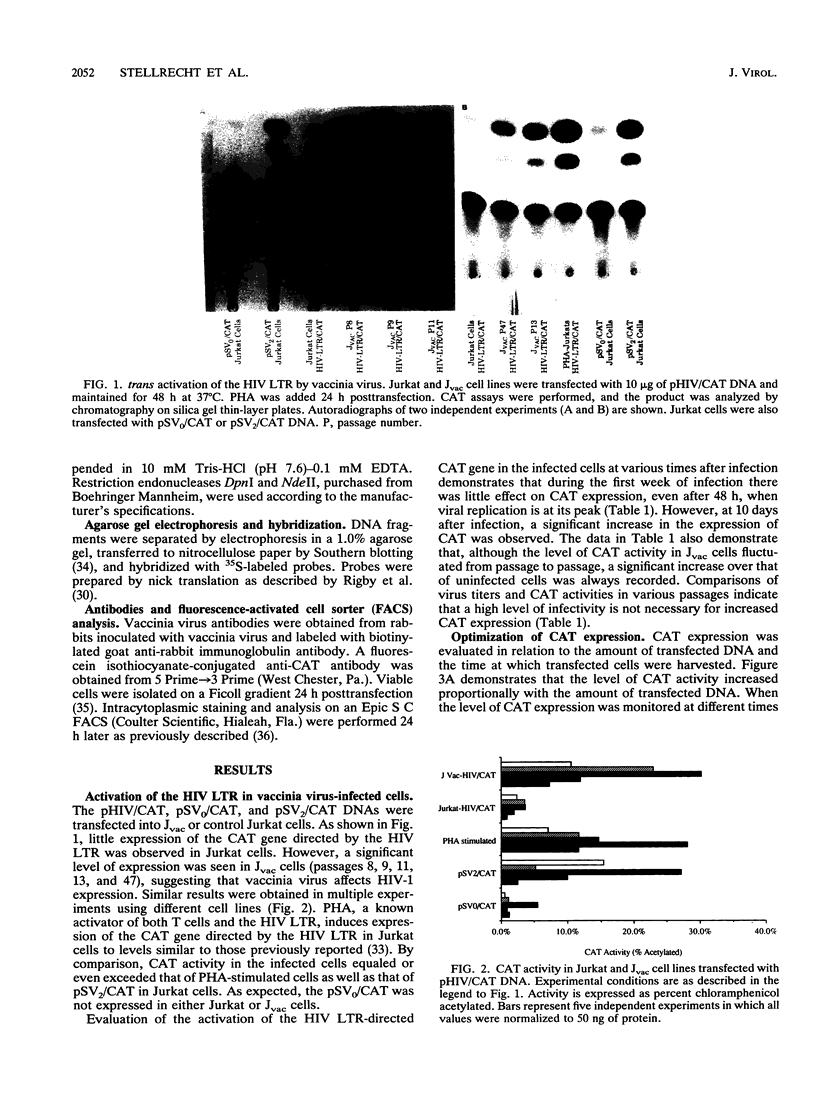
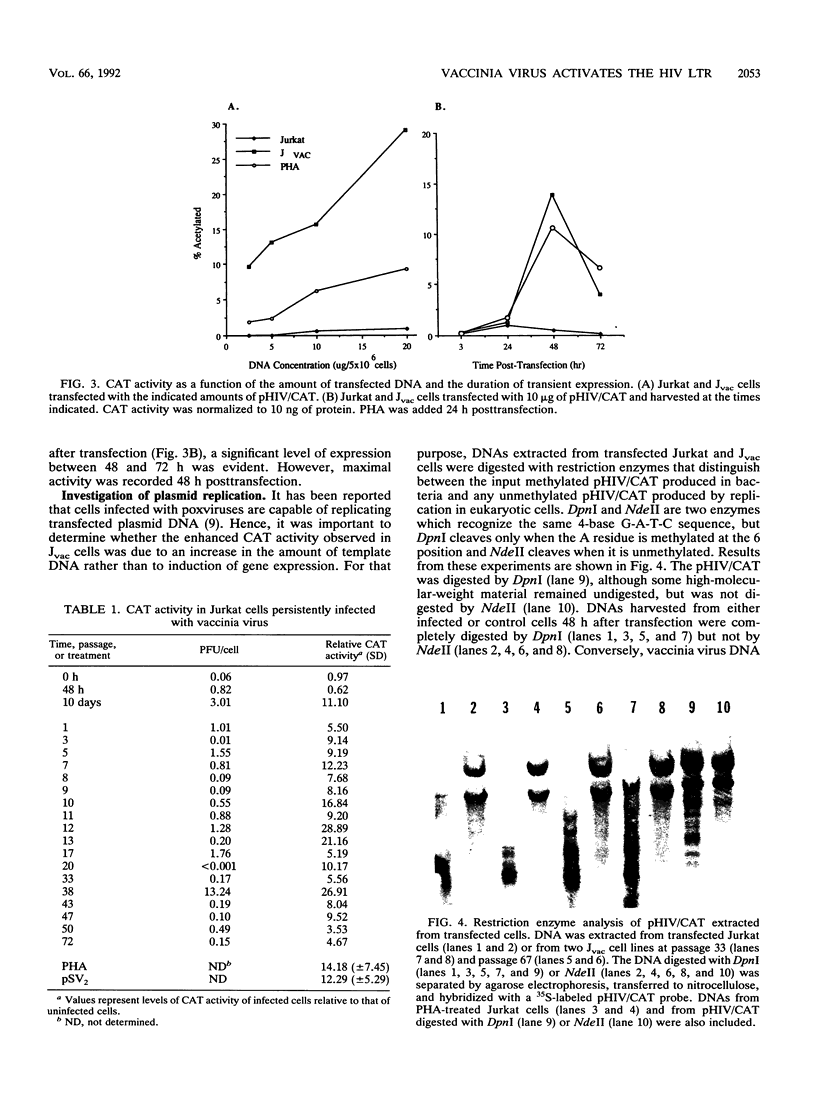
A variety of DNA viruses are known to activate gene expression directed by the long terminal repeat (LTR) of human immunodeficiency virus type 1 (HIV-1). In light of the proposed use of recombinant vaccinia virus for HIV-1 vaccines, evaluation of the role of vaccinia virus in HIV-1 activation is warranted. To investigate whether vaccinia virus induces HIV LTR-directed gene expression, transient expression assays in Jurkat cells persistently infected with vaccinia virus (Jvac) using plasmid DNA containing the LTR linked to the bacterial chloramphenicol acetyltransferase (CAT) gene were performed. CAT activity in Jvac cells was always recorded, although the level appears to fluctuate independently of virus titers. Dual intracytoplasmic staining and fluorescence-activated cell sorter analysis showed that CAT activity was expressed in the infected cells. CAT expression was not due to plasmid replication, since plasmid DNA extracted from Jvac cells 48 h after transfection was restricted only by enzymes which recognize methylated sequences, indicating a prokaryotic source for the DNA. These findings suggest that a factor(s) present in vaccinia virus-infected cells is capable of activating the LTR of HIV-1.
Full text
PDF
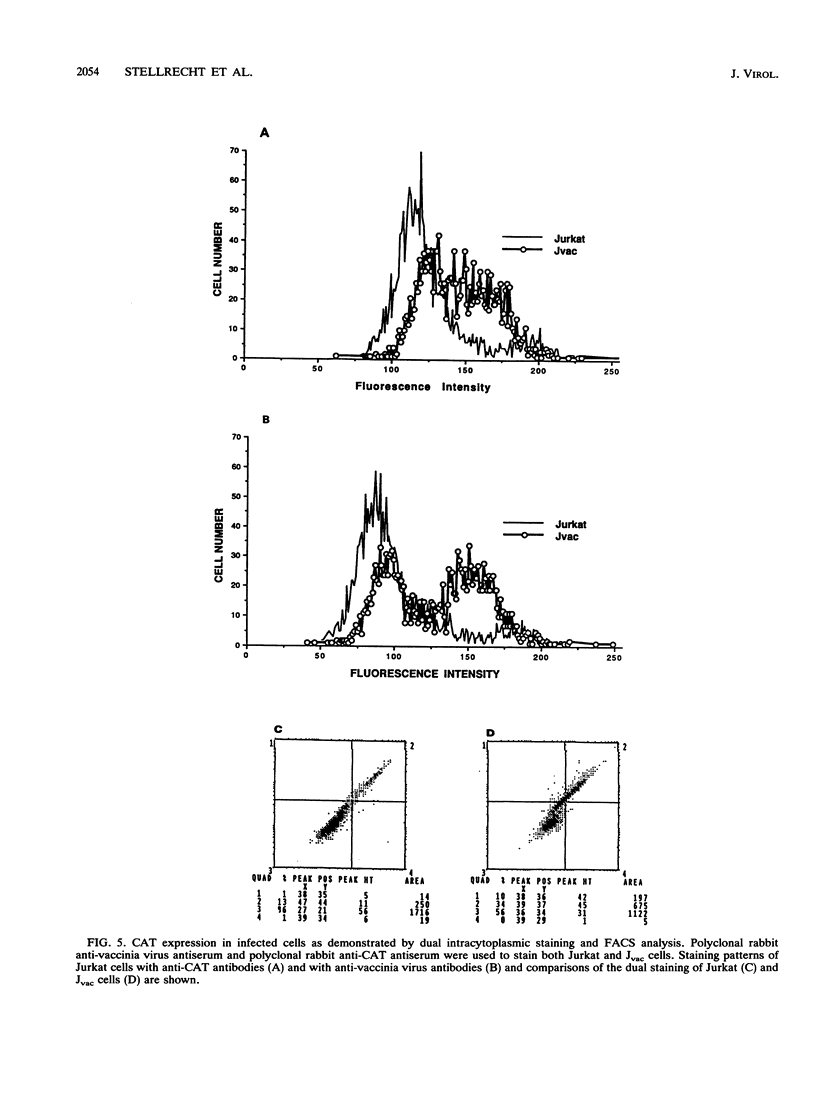


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht M. A., DeLuca N. A., Byrn R. A., Schaffer P. A., Hammer S. M. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J Virol. 1989 May;63(5):1861–1868. doi: 10.1128/jvi.63.5.1861-1868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Peterlin B. M., Luciw P. A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Ciccarone V. C., Chrivia J., Hardy K. J., Young H. A. Identification of enhancer-like elements in human IFN-gamma genomic DNA. J Immunol. 1990 Jan 15;144(2):725–730. [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. Sequence-nonspecific replication of transfected plasmid DNA in poxvirus-infected cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):614–618. doi: 10.1073/pnas.83.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorozynski A., Anderson A. Deaths in vaccine trials trigger French inquiry. Science. 1991 Apr 26;252(5005):501–502. doi: 10.1126/science.2020851. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Hügin A. W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989 Aug;171(2):579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Obom K. M., Popple S. W., Holland J. G., Pogo B. G., Friend C. Vaccinia virus DNA sequences in the nucleus of persistently infected Friend erythroleukemia cells. Virus Res. 1986 Aug;5(2-3):221–234. doi: 10.1016/0168-1702(86)90020-1. [DOI] [PubMed] [Google Scholar]
- Picard O., Giral P., Defer M. C., Fouchard M., Morel M., Meyohas M. C., Lebas J., Imbert J. C., Frottier J., Salaun J. J. AIDS vaccine therapy: phase I trial. Lancet. 1990 Jul 21;336(8708):179–179. doi: 10.1016/0140-6736(90)91699-b. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Friend C. Persistent infection of Friend erythroleukemia cells with vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4805–4809. doi: 10.1073/pnas.79.15.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Lai A. C., Joesten M. E., Royston M. E., Holloway D. Changes in cell gene expression in human leukemic cells persistently infected with vaccinia virus. Virus Res. 1991 May;19(2-3):131–138. doi: 10.1016/0168-1702(91)90040-3. [DOI] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., James W. D., Jones T. S., Brown C., Burke D. S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987 Mar 12;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Trans-activation of the human immunodeficiency virus long terminal repeat sequences, expressed in an adenovirus vector, by the adenovirus E1A 13S protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4200–4204. doi: 10.1073/pnas.85.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sperber K., Bauer J., Pizzimenti A., Najfeld V., Mayer L. Identification of subpopulations of human macrophages through the generation of human macrophage hybridomas. J Immunol Methods. 1990 May 8;129(1):31–40. doi: 10.1016/0022-1759(90)90417-t. [DOI] [PubMed] [Google Scholar]
- Stellrecht K. A., Sperber K., Pogo B. G. Stimulation of lymphokines in Jurkat cells persistently infected with vaccinia virus. J Virol. 1992 Apr;66(4):2046–2050. doi: 10.1128/jvi.66.4.2046-2050.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. The molecular pathogenesis of poxviruses. Curr Top Microbiol Immunol. 1990;163:125–151. doi: 10.1007/978-3-642-75605-4_5. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Robinson W. S. Hepatitis B virus X gene can transactivate heterologous viral sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2046–2050. doi: 10.1073/pnas.86.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]