Abstract
The cytosolic protein α-synuclein is enriched at the pre-synaptic terminals of almost all types of neurons in the central nervous system. α-Synuclein overexpression and the expression of three different mutants have been shown to sustain the pathogenesis of selected forms of Parkinson’s disease. The localization of the protein and the defects found in knocked out or transgenic animals suggest a role of α-synuclein in the regulation of synaptic efficiency. However, the precise function of the protein and the molecular mechanisms of its action are still unclear. At synapses the synaptic vesicle release cycle is a finely tuned process composed of sequential steps that require the interconnected participation of several proteins and cytoskeletal elements. Actin microfilaments are required for the regulation of synaptic vesicle mobilization between different functional pools, for their organization at the active zone and influence the exocytotic process. We recently identified actin as a possible target of α-synuclein function. Through its binding to actin and the regulation of actin dynamics, α-synuclein could participate in the tuning of the vesicle release process, thereby modulating synaptic function and plasticity.
Key words: α-synuclein, synaptic vesicles, exocytosis, actin cytoskeleton, synaptic plasticity
α-Synuclein in the Nerve Terminals
α-Synuclein is a small, soluble, intrinsically unfolded protein of 140 aminoacids1,2 that has attracted great interest in the last several years. Within neurons α-synuclein, although distributed to almost all subcellular compartments, is particularly enriched in the nucleus and in the pre-synaptic terminals3–5 where it is loosely associated with the distal pool of synaptic vesicles6–8 and with the lipid rafts of the plasma membrane.9 The property of α-synuclein to associate with membranes in vivo was also confirmed by in vitro studies, including binding to artificial membranes.10–13 At synapses, however, the distribution of the protein is highly dynamic and dependent on neuronal stimulation, with rapid exchanges taking place among neighbouring synapses.14 α-Synuclein is also important in pathology. In the brain of patients with various neurodegenerative disorders, known as α-synucleinopathies,15–18 the protein can undergo changes of conformation11,12,19 yielding deposits of proteinaceous material. Loss and malfunction of synapses develops at the onset of neurodegenerative processes and much effort has been put in understanding the role of α-synuclein in synaptic function/dysfunction.
α-Synuclein and the Synaptic Vesicle Cycle
Both the high presynaptic concentration of α-synuclein and its association with synaptic vesicles suggest a physiological role of the protein in the modulation of neurotransmitter release. At rest, most synaptic vesicles are not freely diffusible in the cytoplasm. Rather, they are clustered and tethered in a pool separated from the active zones by a meshwork of actin microfilaments and other cytoskeletal components. This pool of vesicles is referred to as the reserve pool (RP). The exit of vesicles from the RP is increased by neuronal activity, vesicles are tracked to the active sites of fusion, where they can dock and undergo ATP-dependent priming reactions by which they become prepared for exocytosis. Vesicles docked at the plasma membrane and already primed form the readily releasable pool (RRP).20,21 The transition from one pool to the other is regulated in a complex manner by a variety of signal transduction pathways modulating the interactions of synaptic vesicles with other cellular components which govern their trafficking toward the active zones and their docking. The subsequent exocytosis determined by the fusion of the vesicle membrane with the plasma membrane is usually triggered by elevations of the cytosolic free Ca2+ levels ([Ca2+]c).
Extensive evidence, obtained by the study of α-synuclein knock-out mice and other experimental models, indicates that α-synuclein has a role in the trafficking of synaptic vesicles and in the regulation of the exocytic process. A knock-out lineage22,23 showed an increase in synaptic paired-pulse facilitation, suggesting α-synuclein to act as an activity-dependent, negative regulator of release that would restrict the traffic of synaptic vesicles from the RP to the release sites. These results appear consistent with the increased refilling of the RRP observed in the striatum of mice null for the α-synuclein gene or expressing its A30P mutated form,24 which also show a reduced size of the RP.25
α-Synuclein dissociation from synaptic vesicles was shown to occur only after exocytosis and to increase with the number of action potentials, suggesting a role in the fusion process that however would occur only at low firing rate.14 An activity dependence of the α-synuclein regulatory effect appears also consistent with the observation that the inhibition of RRP refilling diminishes with repeated stimulation, possibly because α-synuclein disperses from nerve terminals upon increased synaptic activity. Finally, in PC12 cells overexpressing α-synuclein, as well as in chromaffin cells derived from mice overexpressing wild-type (wt) or A30P α-synuclein, catecolamine release was reduced, with accumulation of dense-core vesicles docked at the plasma membrane, suggesting an inhibition of the priming of neurosecretory vesicles.26
Further studies on knock-out mouse models yielded results apparently inconsistent with the above interpretation and suggested different functions for the protein. Working with double α/β-synuclein knock-out mice, Chandra et al.27 showed differences neither in the size of vesicle pools nor in synaptic transmission, but only in the levels of the signalling molecules complexin and 14-3-3 proteins, indicating that α-synucleins are not essential components of the synaptic machinery, but may contribute to more subtle regulatory phenomena. Cabin et al.,23 working with a different knock-out mice lineage, showed an impaired response to tetanic stimulation due to deficient refilling of the RRP during extensive stimulation, possibly due to a reduction in the RP pool. The decrease of RP was also observed in neurons treated with α-synuclein antisense oligonucleotides.28
Studies of mouse and cellular models suggested that α-synuclein may also act as a positive regulator of synaptic transmission, by modulating the late steps of exocytosis. In cystein string protein alpha (CSPα) knock-out mice, in fact, transgenic expression of α-synuclein was shown to rescue the assembly and function of the exocytic SNAREs29, preventing neurodegeneration and abolishing lethality that take place in this lineage. In hippocampal neurons in culture, the introduction of α-synuclein, possibly acting synergistically with Ca2+ and synaptic receptors, enhanced both spontaneous and evoked neurotransmitter release, while its deletion by antisense oligonucleotides blocked the potentiation of synaptic transmission.30 The analysis of the frequency and amplitude of synaptic currents in CA1 cells revealed stronger responses in wt as compared to α-synuclein knock-out animals, however only when the release probability was high,31 pointing again to a regulatory role of α-synuclein restricted to highly demanding conditions. Finally, α-synuclein was found to interact with soluble players affecting the exocytic cycle, such as phospholipase D2,32 and the family of the Rab small GTPases.33 The functional significance of these interactions remained however undefined.
α-Synuclein Interaction with the Actin Cytoskeleton
The interaction of α-synuclein with cytoskeletal components such as microtubules34,35 and actin36 has been recently reported, raising the possibility that such interactions play a role in mediating its function. A partial co-localization of α-synuclein with actin was first reported in two neuronal cell lines.37 Based on these data we wondered whether a modulation of microfilament assembly could be a molecular mechanism by which α-synuclein regulates synaptic vesicle trafficking and discharge. We found that α-synuclein directly interacts with actin and is able to regulate its dynamics. Moreover, these effects were profoundly altered by the pathogenetic A30P mutation.38
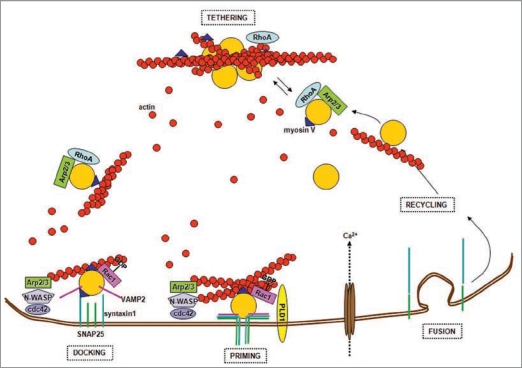
In the past, modulation of the vesicle release cycle by actin had been proposed to take place by multiple mechanisms. Regulated release of synaptic vesicles is accompanied by a fine reorganization of the peripheral microfilaments, which tether the RP. In the subplasmalemma region actin could work as a physical barrier opposing exocytosis, binding synaptic vesicles and hindering their movement.39 On the other hand, actin has a scaffolding role40 and is required to cluster molecular complexes necessary for vesicle fusion at the release sites.41–43 Therefore exocytosis could not occur without at least a minimal actin structure. Actin microfilaments could also provide tracks along which vesicles travel to reach their targets at the terminal, carrying them ahead by waves of actin polymerization44 (Fig. 1).
Figure 1.
Actin and the synaptic vesicle cycle. Synaptic vesicles are tethered in the reserve pool (RP) by a network of actin filaments. Small GTPases, such as RhoA and Rac1, control the state of actin polymerization by governing pathways that involve phosphorylation/dephosphorylation of different actin-binding proteins. The WASP protein and the Arp2/3 complex promote the actin filament nucleation process necessary to build actin tracks for vesicles trafficking. Vesicles are transported to the active zone along actin filaments by myosin V, which also contributes to vesicles docking via its binding to syntaxin1. Primed vesicles are part of the ready releasable pool (RR P). Ca2+ influx and the assembly of the SNARE ternary complex formed by the α-helices of VAMP2, SNAP25 and syntaxin1, trigger vesicle exocytosis at the active zones plasma membrane. Therefore, actin regulates priming and fusion, directly affecting neurotransmitter release. In addition it constitutes the tracks that guide recycling vesicles back to the RP.
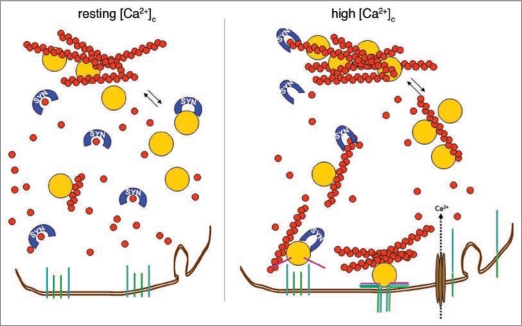
Our recent data indicate that α-synuclein is able to reduce the pool of polymerized actin available in the cell by sequestering actin monomers. This process appears to take place at basal [Ca2+]c levels (∼100 nM), but not at the high levels which can be reached upon heavy stimulation or at the extremely high levels (up to 100 µM) which accompany cell damage. Therefore, α-synuclein appears to modulate the state of actin polymerization depending on the [Ca2+]c in the pre-synaptic terminal, i.e., on its state of stimulation. According to our data, at high [Ca2+]c α-synuclein, by increasing the levels of polymerized actin with respect to the resting state, might contribute to the correct size and localization of RRP. Concomitantly, α-synuclein might finely tune the formation of the actin tracks along which the vesicles move to the release sites. Overall, α-synuclein appears therefore to play a major role in the regulation of vesicle exit from the RP and in their trafficking to the active zone (Fig. 2), as well as in the regulation of their exocytosis and recycling.
Figure 2.
Hypothetical model of α-synuclein action on actin dynamics. At synaptic terminals α-synuclein is associated with synaptic vesicles. At rest, when [Ca2+]c is around 100 nM, α-synuclein binds preferentially actin monomers, sequestering them and slowing down the polymerization of microfilaments. When [Ca2+]c rises upon cell stimulation, α-synuclein affinity for actin monomers decreases and binding to filaments prevails, shifting the balance toward polymerization. This series of events is expected to facilitate the maintenance of vesicles in the RP and, at the same time, to favour the formation of the tracks and the clustering of molecules necessary for vesicles docking and fusion to take place.
The consequences of these processes are probably multiple, concerning both the physiology and the pathology of synapses. On the one hand, by its direct binding to the synaptic vesicle membrane, to proteins crucial for the vesicle cycle and to various cytoskeletal elements, α-synuclein could be fundamental in neuronal plasticity and in conditions of high frequency stimulation; on the other hand, it could be important when neuronal responsiveness becomes weaker, as it happens, for example, in aging and in the early phases of neurodegeneration. Although the precise localization and the molecular environment of α-synuclein effects on actin dynamics are still to be determined, the significance of α-synuclein function in the complex scenario of the presynaptic compartment begins to be unravelled.
Acknowledgements
The original work reported in this article is supported by grants from the European Community (APOPIS-LSHM-CT-2003-503330), the Italian Ministry of Research (FIRB 2004 and PRIN 2006 Programs), the Telethon Fondazione Onlus (GGGP030234) and the CARIPLO Foundation (2006-0779).
Abbreviations
- RP
reserve pool
- RRP
readily releasable pool
- [Ca2+]c
cytosolic free calcium concentration
- wt
wild-type
- CSPα
cystein string protein alpha
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/10964
References
- 1.Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 3.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 4.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Letts. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 5.Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997;99:87–94. doi: 10.1016/s0165-3806(96)00210-6. [DOI] [PubMed] [Google Scholar]
- 6.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, et al. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha-synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Jeon H, Kandror KV. Alpha-synuclein is localized in a subpopulation of rat brain synaptic vesicles. Acta Neurobiol Exp (Wars) 2008;68:509–515. doi: 10.55782/ane-2008-1717. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Ueda K, et al. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 9.Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha-helix in folded alpha-Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 11.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 12.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 13.Jo E, McLaurin J, Yip CM, St. George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 14.Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Letts. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 16.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 17.Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- 18.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Lee SJ. Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem. 2002;277:48976–48983. doi: 10.1074/jbc.M208192200. [DOI] [PubMed] [Google Scholar]
- 20.Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Progr Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- 22.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alphasynuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 23.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yavich L, Oksman M, Tanila H, Kerokoski P, Hiltunen M, van Groen T, et al. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human alpha-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alphasynuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, et al. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin ED, Gonzalez-Garcia C, Milan M, Farinas I, Cena V. Stressor-related impairment of synaptic transmission in hippocampal slices from alphasynuclein knockout mice. Eur J Neurosci. 2004;20:3085–3091. doi: 10.1111/j.1460-9568.2004.03801.x. [DOI] [PubMed] [Google Scholar]
- 32.Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J Mol Biol. 2004;337:1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Dalfo E, Ferrer I. Alpha-synuclein binding to rab3a in multiple system atrophy. Neurosci Lett. 2005;380:170–175. doi: 10.1016/j.neulet.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, et al. Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J Alzheimers Dis. 2004;6:435–442. doi: 10.3233/jad-2004-6412. [DOI] [PubMed] [Google Scholar]
- 35.Lucking CB, Brice A. Alpha-synuclein and Parkinson’s disease. Cell Mol Life Sci. 2000;57:1894–1908. doi: 10.1007/PL00000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Gu G, Goodlett DR, Zhang T, Pan C, Montine TJ, et al. Analysis of alpha-synucleinassociated proteins by quantitative proteomics. J Biol Chem. 2004;279:39155–39164. doi: 10.1074/jbc.M405456200. [DOI] [PubMed] [Google Scholar]
- 37.Esposito A, Dohm CP, Kermer P, Bahr M, Wouters FS. alpha-Synuclein and its disease-related mutants interact differentially with the microtubule protein tau and associate with the actin cytoskeleton. Neurobiol Dis. 2007;26:521–531. doi: 10.1016/j.nbd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Sousa VL, Bellani S, Giannandrea M, Yousuf M, Valtorta F, Meldolesi J, Chieregatti E. {alpha}-synuclein and Its A30P mutant affect actin cytoskeletal structure and dynamics. Mol Biol Cell. 2009;20:3725–3739. doi: 10.1091/mbc.E08-03-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763:1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- 41.Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742:37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 43.Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/NWASP- induced actin filaments. Mol Biol Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levitan ES. Signaling for vesicle mobilization and synaptic plasticity. Mol Neurobiol. 2008;37:39–43. doi: 10.1007/s12035-008-8014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]