Abstract
Plants utilize sunlight to drive photosynthetic energy conversion in the chloroplast thylakoid membrane. Here are located four major photosynthetic complexes, about which we have great knowledge in terms of structure and function. However, much less we know about auxiliary proteins, such as transporters, ensuring an optimum function and turnover of these complexes. The most prominent thylakoid transporter is the proton-translocating ATP-synthase. Recently, four additional transporters have been identified in the thylakoid membrane of Arabidopsis thaliana, namely one copper-transporting P-ATPase, one chloride channel, one phosphate transporter, and one ATP/ADP carrier. Here, we review the current knowledge on the function and physiological role of these transporters during photosynthesis and light stress in plants. Subsequently, we make a survey on the outlook of thylakoid activities awaiting identification of responsible proteins. Such knowledge is necessary to understand the thylakoid network of transporters, and to design strategies for bioengineering crop plants in the future.
Key words: Arabidopsis, photosynthesis, light stress, thylakoid, photosystem, membrane transporter, ATPase, ion channel, carrier
Introduction
Plastids are unique organelles of plant cells. Among the different plastid types, chloroplasts perform oxygenic photosynthesis, and are crucial for cell metabolism and plant growth. Photosynthesis is also vital for aerobic and heterotrophic life on Earth, because it produces molecular oxygen and the carbohydrates that sustain every food chain. The light reactions of photosynthesis use very abundant resources on Earth, water and sunlight, to produce molecular oxygen. Nevertheless, the main purpose of these reactions is to provide chemical energy (ATP) and reducing power (NADPH), to drive the so-called dark reactions of photosynthesis in the chloroplast soluble stroma, resulting in incorporation of CO2 from the atmosphere into carbohydrates. Chloroplasts and other types of plastids use the products of photosynthetic reactions to synthesize fatty acids, amino acids, pigments and nucleic acids. The apparatus involved in the light reactions consists of four major multisubunit protein complexes, namely PSII, cytochrome b6f, PSI and the ATP synthase CF0F1.1 All four photosynthetic complexes are located in the thylakoid membrane, which is, therefore, also known as the photosynthetic membrane. The first three complexes are involved in sunlight absorption, electron extraction from water and transfer to NADP+, whereas the fourth complex uses the H+ gradient established across the thylakoid membrane to power ATP synthesis.2 We have gained great knowledge about the structure and function of photosynthetic complexes, using various biophysical, biochemical and genetic approaches.1,3 Furthermore, the recent crystal structures have provided refined models of protein-protein interactions, and arrangement of cofactors in these complexes.1,2 Their biogenesis, regulation, protection and repair must be rigorously performed to ensure photosynthetic efficiency during everchanging and even stressful environmental conditions.3 Crucial for these processes is an array of auxiliary proteins, including kinases, phosphatases, proteases, stress-induced and heat-shock proteins as well as solute and metabolite transporters. In order to understand the importance of auxiliary proteins for the photosynthetic process itself, but also in the functioning of other plastid types, dedicated efforts are required to identify and characterize them at the molecular level. This review focuses on solute transporters of the thylakoid membrane, a field that has, until recently, been largely unexplored as compared to that of transporters from other membrane systems, including the chloroplast envelope. The envelope transporters mediate communication between the chloroplast soluble stroma and the cytosol, and further on with the rest of the cell. The biochemical function of thylakoid transporters is to exchange solutes between the chloroplast stroma and thylakoid lumenal space. Their physiological role is expected to be directly or indirectly connected to photosynthesis.
Classification and Strategies for Identification of Plant Thylakoid Transporters
The most common transport proteins found in biological membranes belong to one of the following three categories, according to the TC system: channels/porins (TC #1), secondary transporters (TC #2), and primary transporters/pumps (TC #3).4 Channels/porins are non-energy consuming, since they transport down the concentration gradient, and are the fastest (107–108 molecules/s). The main types are voltage-gated ion channels, aquaporins and porins. Secondary transporters use the gradient of co-transported molecules for transport, and work at an intermediate rate (102–104 molecules/s). The two main types are antiporters (exchangers) and symporters (co-transporters). Primary transporters/ pumps consume energy (mainly ATP) for transport against the concentration gradient, and are the slowest (1–103 molecules/s). There are four types of ATP-utilizing pumps in biological membranes, namely ATP-binding cassette (ABC) transporters, F-type ATPases, vacuolar V-ATPases and P-type ATPases. Following the sequencing of the Arabidopsis thaliana genome,5 bioinformatics analyses indicated the existence of 1,000 membrane transporters,6 representing 4.5% of the total number of nuclear gene products. Most recently, it has been reported a distribution of the putative transporters from Arabidopsis into the three main TC categories as follows: 13% ion channels, 67% secondary transporters, and 18% ATP-dependent pumps.7 Transport proteins belonging to these categories are represented in the three chloroplast membrane compartments, namely the outer envelope, the inner envelope, and the thylakoid membrane. These chloroplast membranes are distinct subcellular compartments in several aspects. The outer chloroplast envelope membrane has been thought for a long time to be freely permeable to solutes, while the inner envelope and the thylakoid membrane are known to function as selective barriers. However, several porins with distinct substrate specificity have been identified in the outer envelope membrane.8,9 Many different chloroplast transporters from the inner envelope have been extensively studied and reviewed.6,8 This particular review is dedicated to thylakoid solute transporters, belonging to the three TC categories.
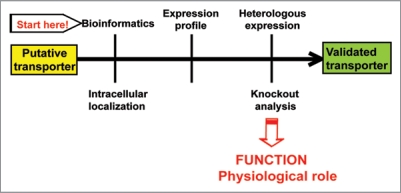
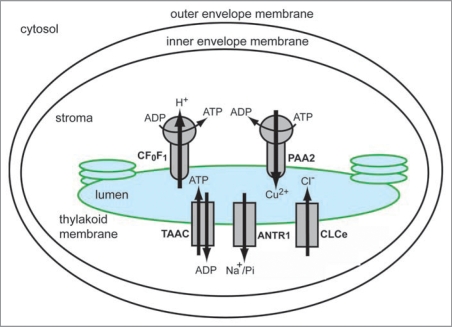
Mass-spectrometry-based proteomics has been a very successful approach in identifying a tremendous number of chloroplast proteins.10–12 Notably, most of them were soluble, peripherally bound or integral proteins with 1–2 predicted transmembrane domains. The identification of chloroplast transporters using large-scale proteomics has been much more successful in the case of the inner envelope membrane10 than of the thylakoid membrane, and hampered by the fact that, this type of proteins are highly hydrophobic, polytopic and maybe present in much lower abundance (10,000 fold, and in some cases 100,000 fold) than photosynthetic proteins. To illustrate this problem, we provide below some numbers extracted from several proteomics reviews,6,13 and from the current release of the Plant Proteome DataBase (PPDB, http://ppdb.tc.cornell.edu/). In Arabidopsis, plastids are predicted to contain around 15% of the nuclear gene products, corresponding to 4,919 proteins. Among them, 660 integral membrane proteins are predicted as envelope proteins, including 100–150 putative transporters. For comparison, a total of 1,367 proteins have been experimentally identified in plastids using proteomics, including 45 envelope transporters. Using the same approach, a total of 384 proteins have been identified in thylakoid membranes, including the highly abundant CF0F1, as the most prominent membrane transporter identified. To our knowledge, there is no information available about the number of predicted thylakoid transporters. The most likely explanation is that the available prediction programs cannot distinguish between targeting peptides for envelope and thylakoids, which has led to assigning an envelope location to most of the putative chloroplast transporters. In the last five years, four new solute transporters have been identified in addition to CF0F1 in thylakoids using various methods, and most recently one of them also using proteomics14 (Table 1). Therefore, it is necessary that using bioinformatics-based tools to find putative transporters in model plants with sequenced genome, and subsequently use a functional genomics approach for experimental validation (Fig. 1).6,13,15 Arabidopsis has been an excellent model plant to identify and characterize transporters from chloroplasts and other compartments with the help of available genomic information and a wide variety of genetic tools. This includes localization studies using GFP-constructs, immunological techniques, in vitro organellar import, expression studies using quantitative RT-PCR, functional studies using heterologous expression and activity assays, and phenotypic analyses of knockout mutants. Such genetic techniques have been used to identify and characterize in Arabidopsis the thylakoid transporters described below, namely the Cu2+-transporting P-ATPase PAA2, the chloride channel CLCe, the thylakoid ATP/ADP carrier TAAC, and the Na+-dependent Pi transporter ANTR1 (Table 1 and Fig. 2).
Table 1.
Thylakoid solute transporters from Arabidopsis thaliana
| Category name (thylakoid members) | AGI number | Location method | Substrate specificity | Physiological role |
| Primary transporters | ||||
| ATPase alpha subunit, CF1-atpA | AtCg00120 | |||
| ATPase beta subunit, CF1-atpB | AtCg00480 | |||
| ATPase gamma subunit, CF1-atpC | At4g04640 | ATP, ADP | ||
| ATPase delta subunit, CF1-atpD | At4g09650 | Photophosphorylation, ATP supply for CO2 fixation and other energy-dependent chloroplast processes | ||
| ATPase epsilon subunit, CF1-atpE | AtCg00470 | P | ||
| ATPase I subunit, CF0-I-atpF | AtCg00130 | H+ | ||
| ATPase II subunit, CF0-II-atpG | At4g32260 | |||
| ATPase III subunit CF0-II-atpH | AtCg00140 | |||
| ATPase IV subunit, CF0-IV-atpI | AtCg00150 | |||
| Cu2+-transporting ATPase (AtPAA2) | At5g21930 | GFP, CI | Cu2+ | Copper supply for photosynthetic electron transport |
| Channels | ||||
| Chloride channel (AtCLCe) | At4g35440 | GFP, WB | Cl-? NO2−? | Nitrate homeostasis in chloroplasts, Balance transthylakoid proton gradient? |
| Secondary transporters | ||||
| Anion transporter 1 (AtANTR1/AtPHT4;1) | At2g29650 | GFP, WB | Pi | Pi recycling to the stroma, Photoprotection? |
| Thylakoid ATP/ADP carrier (AtTAAC) | At5g01500 | GFP, P, WB, IG | ATP, ADP | ATP supply for thylakoid biogenesis, turnover, Photoprotection |
The transporters identified in the thylakoid membrane are classified according to the Transport Classification Database (http://www.tcdb.org/). The accession numbers in Arabidopsis (Arabidopsis gene index, AGI) are indicated. The thylakoid location is based on data obtained using proteomics (P), labeling with green fluorescent protein (GFP), immunogold electron microscopy (IG), western blotting (WB) or in vitro chloroplast import (CI). The ‘?’ symbol indicates that the corresponding information remains to be verified. References for localization and substrate specificity are provided in the text, describing each thylakoid transporter.
Figure 1.
Flow scheme for experimental validation of putative transporters using a functional genomics approach in Arabidopsis thaliana.
Figure 2.
Current picture of thylakoid solute transporters from Arabidopsis thaliana. Chloroplasts are structurally organized in three membrane compartments (outer envelope, inner envelope and thylakoid membrane), and two soluble compartments (stroma and thylakoid lumen). The diagram shows the following transporters, that have been localized to the thylakoid membrane, and functionally characterized: the H+-translocating ATP synthase CF0F1, the Cu2+-transporting P-type ATPase PAA2, the thylakoid ATP/ADP carrier TAAC, the Na+-dependent Pi transporter ANTR1, and the chloride channel CLCe.
The Chloroplast ATP-Synthase
The chloroplast ATP synthase CF0F1 completes the energy transduction of the electron transport chain from water to NADPH, by using the generated electrochemical H+ gradient to drive ATP synthesis from ADP and Pi, in a process known as photophosphorylation.1 This photosynthetic complex belongs to the F-type ATPase family of primary transporters. Paul D. Boyer, John E. Walker and Jens C. Skou were awarded in 1997 Nobel prize in Chemistry for the elucidation of the mechanism of ATP synthesis by F-ATPases. F0F1-ATPase contain multiple subunits arranged in a hydrophilic structure (F1) interacting with nucleotides, and coupling the ATP synthesis to the electron transport, and a hydrophobic structure (F0), forming a channel, which transports H+ across the membrane (Fig. 2). F0F1 can be viewed as an enzymatic machinery combining mechanical, electrical and chemical aspects. The electrochemical motive force powers the electrical rotary motor F0, driving in its turn the chemical motor F1 to produce ATP. The latest insights into the rotary mechanism of this machinery have been reviewed,16 revealing torque generation and an elastic mechanical-power transmission from F0 to F1. The elastic power transmission between the two motors has been proposed to smooth their cooperation without the need for fine tunning.
F0F1-ATPases are present in both prokaryotic and eukaryotic organisms, localized in energy-transducing membranes, such as the cytoplasmic membrane of some bacteria, the mitochondrial inner membrane, and the chloroplast thylakoid membrane. The plant CF0F1 has not yet been crystallized, though recently a composite model was obtained using both structural and available biochemical information. The modelled CF0F1 consists of different crystallized subunits of the F0F1 from bovine mitochondria and yeast.1 Six subunits of the CF0F1 are encoded in the chloroplast genome, while three subunits are nuclear gene products (Table 1). The nuclear-encoded gamma subunit CF1-atpC has been shown to be crucial for photosynthesis, since its inactivation by T-DNA insertion mutagenesis destabilized the entire CF0F1 complex, and abolished the photophosphorylation reaction.18 Instead, a high level of H+ accumulation was observed in the thylakoid lumen, leading to swollen thylakoids and unusually high levels of dissipation of excess light energy as heat. Although there are no reports on mutagenesis of other major subunits of the CF0F1 from Arabidopsis, it is expected that the effects would be very similar to those observed for the gamma subunit.
The Thylakoid Copper-Transporting P-Type ATPase
Copper is a metal component of proteins located in the thylakoid lumen, including plastocyanin and polyphenol oxidase, and also a cofactor of stromal Cu/Zn SOD involved in scavenging of reactive oxygen species. Transport of Cu2+ ions across chloroplast envelope and thylakoid membranes has been reported in pea,18,19 and several proteins have been identified in Arabidopsis as members of the metal ion-transporting P-type ATPase superfamily.18,20,21 Among them, PAA1 and PAA2 have most similar sequences (43% identity).21 Using GFP-based fluorescence microscopy, PAA1 was localized to the envelope, and PAA2 to the thylakoid membrane (Fig. 2).21 In the same report, the thylakoid location of PAA2 was also confirmed using in vitro chloroplast import and fractionation (Table 1). Most recently, a soybean homologue of Arabidopsis PAA2 has been localized to the thylakoid membrane using peptide-specific antibodies, immunofluorescence and immunogold electron microscopy.22 PAA1 and PAA2 use the energy of ATP hydrolysis for transport and work, most likely, in series, as follows: PAA1 transport Cu2+ ions into the chloroplast, and then PAA2 transports these ions across the thylakoid membrane, into the lumen, where copper plays an essential role in the photosynthetic electron transport through plastocyanin.18,21 The phenotypes reported for the paa1 and paa2 knockout mutants support the distinct location and function of the two proteins. The paa1 mutant displays an impaired import of copper into the chloroplast, resulting not only in insufficient formation of holoplastocyanin and deficiency in photosynthetic electron transport, but also in the lack of stromal Cu/Zn SOD in green tissues.18,21 The paa2 mutant displayed a deficient photosynthetic electron transport, but with no effects on the expression of the Cu/Zn SOD. The double paa1-2 mutant was found to be seedling lethal,21 pointing to the importance of the two P-ATPases in the supply of copper for photosynthesis and protection against reactive oxygen species.
The Thylakoid Chloride Channel
In animal and plant cells, anion channels/transporters play a role in the maintenance of electrochemical gradient and in signaling pathways allowing adaptation to stress of both biotic and abiotic type.23 Channels from the plasma membrane are best characterized, using electrophysiological techniques, but, the proteins responsible are in most cases so far not identified. Although anion currents have not been measured in Arabidopsis photosynthetic membranes, such activities have been previously reported in the higher plant Peperomia metallica.24 Chloride channel (CLC) family comprises seven members in Arabidopsis, localized to various membrane compartments, namely vacuoles (AtCLCa), Golgi (AtCLCd and AtCLCf) and thylakoid membrane (AtCLCe).25,26 The vacuolar AtCLCa member was shown to function as a 2NO3−/H+ exchanger, and therefore, proposed to play a role in nitrate homeostasis.27 Most recently, it has been reported that ATP binding to this protein regulates its transport activity.28 The AtCLCe member has been localized using GFP-based fluorescence microscopy and peptide-specific antibodies to the thylakoid membrane of chloroplasts.29 In agreement with this subcellular localization, AtCLCe expression is higher in green tissues compared with roots, and the clce mutants display a phenotype related to photosynthetic activity.
AtClCe has been predicted to function as a voltage-gated chloride channel in thylakoids (Fig. 2), supporting very specific biological functions, but not in anion accumulation. Most recently, it has been proposed that, in chloroplasts, Cl− and, most probably NO2− are good candidates as counter-anions able to compensate for the excess positive charges in the thylakoid lumen.30 In this situation, alterations in ionic strength or osmotic properties of chloroplast compartments may, in turn, directly or indirectly increase the nitrite level, most probably in the cytosol, as exhibited by both clce and clca mutants. This might suggest a role for AtClCe in nitrite translocation from the stroma into the thylakoid lumen, taking over from the nitrite transporter of the chloroplast envelope.31 The similar phenotype of clce and clca indicates interconnectiong roles of AtClCa and AtClCe in nitrate homeostasis, involving two different endocellular membranes.30 It remains to be demonstrated, for example, by measuring lumenal pH of the clce mutants whether CLCe activity plays also a role in balancing the trans-thylakoid proton gradient (Table 1).
The Thylakoid Pi Transporter
Pi is an essential nutrient for plant growth, since it is a key component of nucleotides, nucleic acids, proteins, and many metabolic compounds in the cell. At the same time, it plays regulatory roles, including modulation of photosynthesis. Its concentration in the plant cell has to be maintained within relatively narrow limits, and this is achieved through transport and metabolic recycling across cellular membranes. Combination of experimental evidence and genome sequence analysis indicate that plants contain a wide variety of Pi transporter genes. Five distinct families of secondary Pi transporters have been so far characterized, namely the H+-coupled Pi transporters PHT1 to PHT4, and the plastidic Pi transporters pPT, each of them being composed of multiple members.32–34
The thylakoid Pi transporter belongs to the most recently characterized PHT family, PHT4.34 PHT4 members share sequence similarity with a mammalian anion transporter family, known as SLC17 or type I Pi transporters, and therefore, PHT4 has been initially annotated as the ANTR family. However, in contrast to SLC17 transporters, which have a broad substrate specificity (Pi, organic anions and chloride), PHT4 members specifically transport Pi.34,35 PHT4;1, alias ANTR1, has been characterized using two expression systems: as a H+-dependent Pi transporter in yeast and as a Na+-dependent one in Escherichia coli. Five of the six PHT4 members are targeted to plastids, and the sixth one to the Golgi.34
Using GFP-based fluorescence microscopy, PHT4;1 has been localized to the Arabidopsis chloroplasts.36 Later on, its intrachloroplast location has been addressed using two specific peptidedirected antibodies, which could distinguish between the envelope PHT4;4 and the thylakoid PHT4;1 protein.35 PHT4;1 has been shown to have a circadian rhythm-regulated expression pattern.37
In plants, PHT4;1 is proposed to recirculate Pi from the thylakoid lumen to the stroma (Fig. 2), whereas PHT4;4 has been proposed to supply stroma with cytosolic Pi.34 The function of PHT4;1 has not yet been studied in Arabidopsis, but it is likely that it can transport Pi in or out of the thylakoid lumen, depending on the direction of the H+ or Na+ ions gradient. Whether these ions are co-transported with Pi has not been demonstrated either. Interestingly, most recently, a Na+/H+ antiporter (Nhas3) has been localized to the thylakoid membrane of Synechocystis sp. PCC 6803,38 and proposed to function as a dissipator of the H+ gradient generated during photosynthesis. Its Arabidopsis homologue remains to be identified. Phenotypic analyses of knockout mutants of PHT4;1 will hopefully reveal the physiological role of this protein during photosynthesis and plant growth.
The Thylakoid ATP/ADP Carrier
ATP is the most highly charged species transported across biological membranes. This nucleotide drives many different energydependent processes in chloroplasts, including phosphorylation, folding, import and degradation of various proteins.39 Alternatively, it is enzymatically converted to other nucleotides, such as GTP, which is subsequently used by GTP-binding proteins in mediating signal transduction and other chloroplast processes. There are two types of adenine nucleotide transporters represented in chloroplasts, which are structurally and phylogenetically different, namely the plastidic nucleotide translocators (of prokaryotic origin), and the mitochondrial carriers (eukaryote-specific).39 There are two identified envelope ATP/ADP translocators proposed to import ATP into the chloroplast at night, when the photosynthetic electron transport and CF0F1 are inactive.40 The proteins belonging to the mitochondrial carrier family were first recognized in the mitochondrial inner membrane. In addition to the mitochondrion, such carriers have also been found in the peroxisome, hydrogenosome, amyloplast and chloroplast.41 There are at least five AACs among the 58 predicted members of the mitochondrial carrier family in Arabidopsis, and some of them have been heterologously expressed and functionally characterized.42
The activity of the first chloroplast member of the mitochondrial carrier family was reported in the spinach thylakoid membrane, transporting ATP into the lumenal space.43 Bioinformatics searches for proteins responsible for this transthylakoid activity, led to one candidate in Arabidopsis, named thereafter AtTAAC (Table 1 and Fig. 2). The protein has been localized to the thylakoid membrane using immunogold labeling, western blotting, and most recently using proteomics.14,44 A recombinant AtTAAC protein has been expressed in Escherichia coli, and found functionally inserted into the cytoplasmic membrane. Based on uptake studies in intact cells as well as transport assay using Arabidopsis thylakoid membranes, an ATP/ADP exchange-type of transport has been demonstrated for the thylakoid carrier.
The AtTAAC gene is highly expressed in young photosynthetic organs, such as developing leaves, flower buds and green siliques.44 Therefore, a role for TAAC in thylakoid biogenesis was proposed. TAAC expression is strongly upregulated in leaves undergoing senescence or exposed to wounding, high light stress, oxidative stress, salt stress and desiccation, pointing to an additional role in supplying ATP for energy-dependent processes during thylakoid turnover. In the same report, a T-DNA insertion taac mutant displayed reduced ATP transport across the thylakoid membrane. Phenotypic analyses of this mutant indicated a better photoprotection during short term high light stress, and increased sensitivity to prolonged high light stress as compared to the wild-type plants (Yin L, Schoefs B, Spetea C, unpublished observations). The increased sensitivity is attributed to an impaired PSII repair cycle (see below).
The Critical Role of Thylakoid Transporters during High Light Stress in Plants
Plants have many different strategies to cope with fluctuations of light intensities in their environment, ensuring high levels of survival and productivity.45,46 A sustained exposure to excess radiance will, however, lead inevitably to photoinhibition, decline in photosynthetic efficiency and productivity.47,48 Various types of environmental stresses, such as cold, heat, salt and oxidative stress enhance the extent of photoinhibition.49
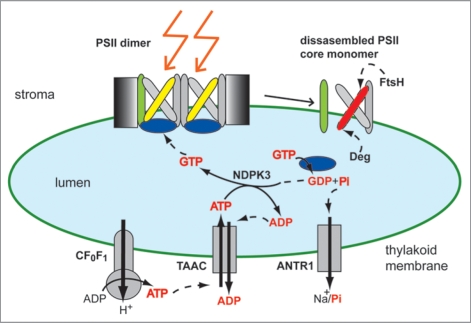
Among photosynthetic complexes, the water-oxidizing PSII complex has attracted a special attention, since its electron transport chain is inactivated, and the reaction center D1 subunit is oxidatively damaged, degraded and replaced under excess and even optimal light conditions.50,51 In order for the plant to survive, PSII complex undergoes a multi-step repair cycle to replace D1 and reactivate the electron transport chain in the complex.51,52 D1 protein degradation itself is a multi-step proteolytic event, requiring GTP and ATP, and performed by Deg and FtsH proteases.53–57 The PSII repair cycle is regulated by reversible phosphorylation of several core subunits, including D1.58 In Figure 3 are shown only the steps involving monomerization of the dimeric complex, and partial disassembly of the PSII core monomer to allow access of Deg and FtsH proteases to the proteolytic sites of the damaged D1 protein. During these steps, the transport activities of TAAC and ANTR1 are proposed to play a critical role, as explained below and illustrated in Figure 3.
Figure 3.
Schematic model of ATP transport and GTP-mediated signaling during high light stress. The active photosystem II (PSII ) is a multisubunit and dimeric complex, containing an intact reaction center D1 protein (yellow). As a result of high-light-induced inactivation, the D1 protein is oxidatively damaged (red), and needs to be replaced. The ATP-synthase CF0F1 supplies ATP in the stroma, from where it is translocated by the thylakoid ATP/ADP carrier (TAAC) into the lumen in exchange for ADP. The lumenal nucleoside diphosphate kinase NDPK3 converts ATP to GTP, which is subsequently bound and hydrolyzed by the PSII extrinsic subunit PsbO (blue). This leads to PsbO dissociation, partial disassembly of PSII complex (CP43 subunit shown in green), and D1 proteolysis by Deg and FtsH proteases in a highly controlled manner. The resulting phosphate (Pi) is exported back to the stroma by the Na+- dependent Pi transporter (ANTR1). The various nucleotides and Pi are highlighted as red text.
ATP synthesized by CF0F1 on the stromal side of the thylakoid membrane, is translocated by TAAC into the lumen, where it is converted to GTP by the nucleoside diphosphate kinase NDPK3.43 GTP binds to the extrinsic PsbO subunit of the PSII complex.43,59 PsbO hydrolyzes GTP to GDP and Pi, and is subsequently released from its docking site on PSII complex.59 This facilitates dissociation of the CP43 subunit of the PSII core monomer and subsequent D1 proteolysis.53,60 Notably, between the two PsbO isoforms in Arabidopsis, it has recently been reported that PsbO2 plays an essential role in D1 turnover during high-light stress, and has a higher GTPase activity than PsbO1.60–62 The thylakoid Pi transporter ANTR1,35 may play a role in exporting back to the stroma Pi generated during nucleotide metabolism, including GTP hydrolysis.
In addition to the critical role in disassembly during PSII repair, TAAC and ANTR1 may play a role in photoprotection during high light stress, more specifically in the thermal energy dissipation, a process known to be initiated by an increase in the transthylakoid H+ gradient, and subsequent acidification of the lumen.45 For related types of transporters, an electrogenic mechanism of transport has been demonstrated.63,64 To support an electrogenic transport mediated by TAAC, addition of uncouplers during transport assays in Escherichia coli as well as thylakoid membranes was found effective.44 Therefore, it appears as TAAC transport activity consumes a fraction of the H+ gradient across the thylakoid membrane. Notably, the taac mutants display higher levels of photoprotection than the wild type (Yin L, Schoefs B and Spetea C, unpublished observations), confirming that a larger fraction of the gradient becomes available for thermal dissipation of excess light energy. Higher levels of photoprotection were also detected for mutants lacking the thylakoid ANTR1 protein (Karlsson P, Spetea C, unpublished observations). Thus, it appears that TAAC and ANTR1 play a role to maintain steady and balanced electrochemical gradient across the thylakoid membrane.
Thylakoid Transporters Awaiting Identification
We have above reviewed the current knowledge available about thylakoid transporters. However, the list is far from complete. Below we review the evidence from the published literature for transport activities in the thylakoid membrane (highlighted in italics), awaiting identification of the responsible proteins.
Essential metals, including manganese, calcium and magnesium, are required for the operation of oxygenic photosynthesis. Therefore, it is expected that Arabidopsis mutants defective in the transport of these essential metals will have severe phenotypes. Manganese and calcium ions are components of the oxygenevolving complex, located on the lumenal side of PSII complex.65 One MntABC transport system has been identified in the plasma membrane of the photosynthetic bacterium Synechocystis sp. PCC 6803,66 but no homologues have been predicted in plant chloroplasts. There are reports supporting the existence of a calcium/proton antiporter in the thylakoid membrane of pea, working in a light-dependent manner, but the responsible protein has not been yet identified.67 Magnesium is the most abundant metal in the thylakoid membrane, since it is a component of chlorophyll, which is the major pigment involved in light harvesting and primary light reactions. In addition, efflux of magnesium ions from the thylakoid lumen has been proposed to participate in formation of the electrochemical gradient across the thylakoid membrane,68 however, the protein involved is unknown.
We have above reviewed the identification of an anion channel in the thylakoid membrane. The activity of various cation channels (potassium and divalent cations) has been reported in the thylakoid membrane using electrophysiology.69–72 By allowing specific ion fluxes across the thylakoid membrane, and subsequently balancing the light-induced H+ gradient across the membrane, these ion channels may either directly or indirectly modulate photosynthetic oxygen evolution activity.
Ascorbate is a multifunctional molecule, having roles as antioxidant, redox signaling modulator and enzyme cofactor, e.g., cofactor for violaxanthin de-epoxidase, an enzyme involved in the thermal dissipation of excess light energy, and its availability in the lumen can limit this process.73 Although there is evidence for ascorbate transport across pea thylakoid membrane, no such protein involved has been identified.74 The experimentally-determined lumenal proteome contains 81 proteins, participating not only in photosynthesis and photoprotection, but also in proteolysis, protein (un)folding and antioxidant response.75,76 The thylakoid lumen contains several proteases, namely Deg1 involved in proteolysis of the PSII reaction center D1 protein,57 the D1 processing peptidase CtpA, and the thylakoid processing peptidase, removing signal peptides from chloroplast lumenal proteins.75,77 This implies the existence of so far unidentified recycling transport pathways for the peptides and/ or amino acids generated during proteolysis, which will otherwise accumulate in the lumen.
Water is a substrate for the PSII-catalyzed oxidation to molecular oxygen, which takes place on the lumenal side of the thylakoid membrane. It is not clear how water is transported into the lumen, i.e., by free diffusion or via a specific protein (aquaporin), as reported in the case of other cellular compartments.78,79 Evidence for a carbonic anhydrase activity with a role in water oxidation, and recently the identification of Cah3 in the thylakoid lumen of the photosynthetic alga Chlamydomonas reinhardtii have been reported.80,81 This implies the presence of a channel/transporter either for bicarbonate or CO2, e.g., CO2 aquaporin as the one discovered in the envelope79 or as the bicarbonate (ABC-type) transporter from the chloroplast envelope of Chlamydomonas.82
In addition, there is increasing evidence for nucleotide-dependent reactions in the thylakoid lumen of plant chloroplasts, as based on the activity of NDPK3 and of the PsbO protein as a GTPase.43,59,60 TAAC is specific for adenine nucleotide transport,44 and therefore the question of a guanosine diphosphate transporter is raised.
Since the proteins responsible for the above-described transport activities have not been so far identified using thylakoid membrane proteomics, bioinformatics combined with genetic strategies (Fig. 1) are required for their identification. On the other hand, there are several examples of thylakoid transporters found by mass-spectrometry-based proteomics, with representants from the three TC categories, namely ABC transporters, symporters and channels.14 Again, genetic strategies are required for elucidation of their biochemical function and physiological role in the optimal function of the thylakoid.
Concluding Remarks
Although the importance of solute transport for the photosynthetic machinery in the thylakoid membrane is obvious, our knowledge of the proteins involved and their mode of function and regulation of plant photosynthesis is limited. The gaps in our knowledge of thylakoid transporters lead to a challenging scientific question, which is at the same time of great importance from agricultural and ecological point of view. Detailed understanding of the thylakoid network of transporters will provide genetic solutions for bioingeneering of crop plants in the future.
Acknowledgements
The authors would like to acknowledge the Swedish Research Council, the Swedish Research Council for Environment, Agriculture and Space Planning (Formas) and Linköping University (to Cornelia Spetea), the French Ministére de l’Education Nationale de l’Enseignement Supérieur et de la Recherche, the Institut National de la Recherche Agronomique (INRA), the Centre National de la Recherche Scientifique (CNRS) and the University of Burgundy (to Benoît Schoefs).
Abbreviations
- ANTR
anion transporter
- ATP
adenosine triphosphate
- CF0F1
chloroplast ATP synthase
- CLC
chloride channel
- Cu/Zn SOD
copper/zinc superoxide dismutase
- GFP
green fluorescent protein
- GTP
guanosine triphosphate
- H+
proton
- NADPH
nicotinamide adenine dinucleotide phosphate
- PAA
P-type ATPase
- Pi
inorganic phosphate
- PS
photosystem
- TAAC
thylakoid ATP/ADP carrier
- TC
transport classification
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/10909
dio]Figures and Tables
References
- 1.Nelson N, Ben-Shem A. The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol. 2004;5:971–982. doi: 10.1038/nrm1525. [DOI] [PubMed] [Google Scholar]
- 2.Merchant S, Sawaya MR. The light reactions: a guide to recent acquisitions for the picture gallery. Plant Cell. 2005;217:648–663. doi: 10.1105/tpc.105.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson B, Salter AH, Barber J. Molecular genetics of photosynthesis. Oxford, UK: IRL Press; 1996. [Google Scholar]
- 4.Busch W, Saier MH., Jr The transporter classification (TC) system, 2002. Crit Rev Biochem Mol Biol. 2002;37:287–337. doi: 10.1080/10409230290771528. [DOI] [PubMed] [Google Scholar]
- 5.Arabidopsis Genome Initiative, authors. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 6.Weber AP, Schwacke R, Flügge UI. Solute transporters of the plastid envelope membrane. Annu Rev Plant Biol. 2005;56:133–164. doi: 10.1146/annurev.arplant.56.032604.144228. [DOI] [PubMed] [Google Scholar]
- 7.Nagata T, Iizumi S, Satoh K, Kikuchi S. Comparative molecular biological analysis of membrane transport genes in organisms. Plant Mol Biol. 2008;66:565–585. doi: 10.1007/s11103-007-9287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhaus HE, Wagner R. Solute pores, ion channels and metabolite transporters in the outer and inner envelope membranes of higher plant plastids. Biochim Biophys Acta. 2000;1465:307–323. doi: 10.1016/s0005-2736(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 9.Duy D, Soll J, Philippar K. Solute channels of the outer membrane: from bacteria to chloroplasts. Biol Chem. 2007;388:879–889. doi: 10.1515/BC.2007.120. [DOI] [PubMed] [Google Scholar]
- 10.Rolland N, Ferro M, Seigneurin-Berny D, Garin J, Douce R, Joyard J. Proteomics of chloroplast envelope membranes. Photosynth Res. 2003;78:205–230. doi: 10.1023/B:PRES.0000006891.12416.6c. [DOI] [PubMed] [Google Scholar]
- 11.Schröder WP, Kieselbach T. Update on chloroplast proteomics. Photosynth Res. 2003;78:181–193. doi: 10.1023/B:PRES.0000006914.52898.67. [DOI] [PubMed] [Google Scholar]
- 12.van Wijk KJ. Plastid proteomics. Plant Physiol Biochem. 2004;42:963–977. doi: 10.1016/j.plaphy.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Ephritikhine G, Ferro M, Rolland N. Plant membrane proteomics. Plant Physiol Biochem. 2004;42:943–962. doi: 10.1016/j.plaphy.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One. 2008;3:1994. doi: 10.1371/journal.pone.0001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbier-Brygoo H, Gaymard F, Rolland N, Joyard J. Strategies to identify transport systems in plants. Trends Plant Sci. 2001;26:577–585. doi: 10.1016/s1360-1385(01)02149-5. [DOI] [PubMed] [Google Scholar]
- 16.Junge W, Sielaff H, Engelbrecht S. Torque generation and elastic power transmission in the rotary F(0)F(1)-ATPase. Nature. 2009;459:364–370. doi: 10.1038/nature08145. [DOI] [PubMed] [Google Scholar]
- 17.Bosco CD, Lezhneva L, Biehl A, Leister D, Strotmann H, Wanner G, Meurer J. Inactivation of the chloroplast ATP synthase gamma subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem. 2004;279:1060–1069. doi: 10.1074/jbc.M308435200. [DOI] [PubMed] [Google Scholar]
- 18.Shikanai T, Müller-Moulé P, Munekage Y, Niyogi KK, Pilon M. PAA1, a P-type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell. 2003;15:1333–1346. doi: 10.1105/tpc.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shingles R, Wimmers LE, McCarty RE. Copper transport across pea thylakoid membranes. Plant Physiol. 2004;135:145–151. doi: 10.1104/pp.103.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seigneurin-Berny D, Gravot A, Auroy P, Mazard C, Kraut A, Finazzi G, et al. HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J Biol Chem. 2006;281:2882–2892. doi: 10.1074/jbc.M508333200. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Ghany SE, Müller-Moulé P, Niyogi KK, Pilon M, Shikanai T. Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell. 2005;17:1233–1251. doi: 10.1105/tpc.104.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernal M, Testillano PS, Alfonso M, del Carmen Risueño M, Picorel R, Yruela I. Identification and subcellular localization of the soybean copper P1BATPase GmHMA8 transporter. J Struct Biol. 2007;158:46–58. doi: 10.1016/j.jsb.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 23.De Angeli A, Thomine S, Frachisse JM, Ephritikhine G, Gambale F, Barbier-Brygoo H. Anion channels and transporters in plant cell membranes. FEBS Lett. 2007;581:2367–2374. doi: 10.1016/j.febslet.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Schönknecht G, Hedrich R, Junge W, Raschke K. A voltage-dependent chloride channel in the photosynthetic membrane of a higher plant. Nature. 1988;336:589–592. [Google Scholar]
- 25.Hechenberger M, Schwappach B, Fischer WN, Frommer WB, Jentsch TJ, Steinmeyer K. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. J Biol Chem. 1996;271:33632–33638. doi: 10.1074/jbc.271.52.33632. [DOI] [PubMed] [Google Scholar]
- 26.De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H. Review. CLC-mediated anion transport in plant cells. Philos Trans R Soc Lond B Biol Sci. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geelen D, Lurin C, Bouchez D, Frachisse JM, Levievre F, Courtial B, et al. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 2000;21:259–267. doi: 10.1046/j.1365-313x.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- 28.De Angeli A, Moran O, Wege S, Filleur S, Ephritikhine G, Thomine S, et al. ATP binding to the C terminus of the Arabidopsis thaliana nitrate/proton antiporter, AtCLCa, regulates nitrate transport into plant vacuoles. J Biol Chem. 2009;284:26526–26532. doi: 10.1074/jbc.M109.005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marmagne A, Vinauger-Douard M, Monachello D, et al. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J Exp Bot. 2007;58:3385–3393. doi: 10.1093/jxb/erm187. [DOI] [PubMed] [Google Scholar]
- 30.Monachello D, Allot M, Oliva S, Krapp A, et al. Two anion transporters AtClCa and AtClCe fulfill interconnecting but not redundant roles in nitrate assimilation pathways. New Phytol. 2009;183:88–94. doi: 10.1111/j.1469-8137.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura M, Georgescu MN, Takahashi M. A nitrite transporter associated with nitrite uptake by higher plant chloroplasts. Plant Cell Physiol. 2007;48:1022–1035. doi: 10.1093/pcp/pcm073. [DOI] [PubMed] [Google Scholar]
- 32.Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- 33.Knappe S, Flügge UI, Fischer K. Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol. 2003;131:1178–1190. doi: 10.1104/pp.016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo B, Jin Y, Wussler C, Blancaflor EB, Motes CM, Versaw WK. Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol. 2008;177:889–898. doi: 10.1111/j.1469-8137.2007.02331.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz Pavón L, Lundh F, Lundin B, Mishra A, Persson BL, Spetea C. Arabidopsis ANTR1 is a thylakoid Na+-dependent phosphate transporter: functional characterization in Escherichia coli. J Biol Chem. 2008;283:13520–13527. doi: 10.1074/jbc.M709371200. [DOI] [PubMed] [Google Scholar]
- 36.Roth C, Menzel G, Petétot JM, Rochat-Hacker S, Poirier Y. Characterization of a protein of the plastid inner envelope having homology to animal inorganic phosphate, chloride and organic-anion transporters. Planta. 2005;218:406–416. doi: 10.1007/s00425-003-1121-5. [DOI] [PubMed] [Google Scholar]
- 37.Guo B, Irigoyen S, Fowler TB, Versaw WK. Differential expression and phylogenetic analysis suggest specialization of plastid-localized members of the PHT4 phosphate transporter family for photosynthetic and heterotrophic tissues. Plant Signal Behav. 2008;3:784–790. doi: 10.4161/psb.3.10.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsunekawa K, Shijuku T, Hayashimoto M, Kojima Y, Onai K, Morishita M, et al. Identification and characterization of the Na+/H+ antiporter Nhas3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J Biol Chem. 2009;284:16513–16521. doi: 10.1074/jbc.M109.001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spetea C, Thuswaldner S. Update in nucleotidedependent processes in plant chloroplasts. In: Schoefs B, editor. Plant Cell Compartments—Selected Topics. Kerala: Research Signpost; 2008. pp. 105–149. [Google Scholar]
- 40.Reiser J, Linka N, Lemke L, Jeblick W, Neuhaus HE. Molecular physiological analysis of the two plastidic ATP/ADP transporters from Arabidopsis. Plant Physiol. 2004;136:3524–3536. doi: 10.1104/pp.104.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laloi M. Plant mitochondrial carriers: an overview. Cell Mol Life Sci. 1999;56:918–944. doi: 10.1007/s000180050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picault N, Hodges M, Palmieri L, Palmieri F. The growing family of mitochondrial carriers in Arabidopsis. Trends Plant Sci. 2004;9:138–146. doi: 10.1016/j.tplants.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Spetea C, Hundal T, Lundin B, Heddad M, Adamska I, Andersson B. Multiple evidence for nucleotide metabolism in the chloroplast thylakoid lumen. Proc Natl Acad Sci USA. 2004;101:1409–1414. doi: 10.1073/pnas.0308164100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thuswaldner S, Lagerstedt JO, Rojas-Stütz M, Bouhidel K, Der C, Leborgne-Castel N, et al. Identification, expression and functional analyses of a thylakoid ATP/ADP carrier from Arabidopsis. J Biol Chem. 2007;282:8848–8859. doi: 10.1074/jbc.M609130200. [DOI] [PubMed] [Google Scholar]
- 45.Ruban AV. Plants in light. Commun Integr Biol. 2009;2:50–55. doi: 10.4161/cib.2.1.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pesaresi P, Hertle A, Pribil M, Schneider A, Kleine T, Leister D. Optimizing photosynthesis under fluctuating light: The role of the Arabidopsis STN7 kinase. Plant Signal Behav. 2010;5:20–24. doi: 10.4161/psb.5.1.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- 48.Vass I, Cser K, Cheregi O. Molecular mechanisms of light stress of photosynthesis. Ann N Y Acad Sci. 2007;1113:114–122. doi: 10.1196/annals.1391.017. [DOI] [PubMed] [Google Scholar]
- 49.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta. 2007;1767:414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Ohad I, Kyle DJ, Arntzen CJ. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984;99:481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aro EM, Virgin I, Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 52.Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, et al. Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot. 2005;56:347–356. doi: 10.1093/jxb/eri041. [DOI] [PubMed] [Google Scholar]
- 53.Spetea C, Hundal T, Lohmann F, Andersson B. GTP bound to chloroplast thylakoid membranes is required for light-induced, multienzyme degradation of the photosystem II D1 protein. Proc Natl Acad Sci USA. 1999;96:6547–6552. doi: 10.1073/pnas.96.11.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z, Andersson B. The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell. 2000;12:419–431. doi: 10.1105/tpc.12.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haussühl K, Andersson B, Adamska I. A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II. EMBO J. 2001;20:713–722. doi: 10.1093/emboj/20.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva P, Thompson E, Bailey S, Kruse O, Mullineaux CW, Robinson C, et al. FtsH is involved in the early stages of repair of photosystem II in Synechocystis sp PCC 6803. Plant Cell. 2003;15:2152–2164. doi: 10.1105/tpc.012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapri-Pardes E, Naveh L, Adam Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell. 2007;19:1039–1047. doi: 10.1105/tpc.106.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tikkanen M, Nurmi M, Kangasjärvi S, Aro EM. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim Biophys Acta. 2008;1777:1432–1437. doi: 10.1016/j.bbabio.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Lundin B, Thuswaldner S, Shutova T, Eshaghi S, Samuelsson G, Barber J, et al. Subsequent events to GTP binding by the plant PsbO protein: structural changes, GTP hydrolysis and dissociation from the photosystem II complex. Biochim Biophys Acta. 2007;1767:500–508. doi: 10.1016/j.bbabio.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Lundin B, Nurmi M, Rojas-Stuetz M, Aro EM, Adamska I, Spetea C. Towards understanding the functional difference between the two PsbO isoforms in Arabidopsis thaliana-insights from phenotypic analyses of psbo knockout mutants. Photosynth Res. 2008;98:405–414. doi: 10.1007/s11120-008-9325-y. [DOI] [PubMed] [Google Scholar]
- 61.Lundin B, Hansson M, Schoefs B, Vener AV, Spetea C. The Arabidopsis PsbO2 protein regulates dephosphorylation and turnover of the photosystem II reaction centre D1 protein. Plant J. 2007;49:528–539. doi: 10.1111/j.1365-313X.2006.02976.x. [DOI] [PubMed] [Google Scholar]
- 62.Allahverdiyeva Y, Mamedov F, Holmström M, Nurmi M, Lundin B, Styring S, et al. Comparison of the electron transport properties of the psbo1 and psbo2 mutants of Arabidopsis thaliana. Biochim Biophys Acta. 2009;1787:1230–1237. doi: 10.1016/j.bbabio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Gropp T, Brustovetsky N, Klingenberg M, Müller V, Fendler K, Bamberg E. Kinetics of electrogenic transport by the ADP/ATP carrier. Biophys J. 1999;177:714–726. doi: 10.1016/S0006-3495(99)76926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bacconi A, Virkki LV, Biber J, Murer H, Forster IC. Renouncing electroneutrality is not free of charge: switching on electrogenicity in a Na+-coupled phosphate cotransporter. Proc Natl Acad Sci USA. 2005;102:12606–12611. doi: 10.1073/pnas.0505882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygenevolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 66.Bartsevich VV, Pakrasi HB. Manganese transport in the cyanobacterium Synechocystis sp PCC 6803. J Biol Chem. 1996;271:26057–26061. doi: 10.1074/jbc.271.42.26057. [DOI] [PubMed] [Google Scholar]
- 67.Ettinger WF, Clear AM, Fanning KJ, Peck ML. Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol. 1999;119:1379–1386. doi: 10.1104/pp.119.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krause GH. Light-induced movement of magnesium ions in intact chloroplasts. Spectroscopic determination with Eriochrome Blue SE. Biochim Biophys Acta. 1977;460:500–510. doi: 10.1016/0005-2728(77)90088-3. [DOI] [PubMed] [Google Scholar]
- 69.Tester M, Blatt MR. Direct measurement of K channels in thylakoid membranes by incorporation of vesicles into planar lipid bilayers. Plant Physiol. 1989;91:249–252. doi: 10.1104/pp.91.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang Z, Mi F, Berkowitz GA. Molecular and physiological analysis of a thylakoid K+ channel protein. Plant Physiol. 1995;108:1725–1734. doi: 10.1104/pp.108.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pottosin II, Schönknecht G. Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J Membr Biol. 1996;152:223–233. doi: 10.1007/s002329900100. [DOI] [PubMed] [Google Scholar]
- 72.Segalla A, Szabo I, Costantini P, Giacometti GM. Study of the effect of ion channel modulators on photosynthetic oxygen evolution. J Chem Inf Model. 2005;245:1691–1700. doi: 10.1021/ci0501802. [DOI] [PubMed] [Google Scholar]
- 73.Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foyer CH, Lelandais M. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasma membranes of pea leaf mesophyll cells. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- 75.Peltier JB, Emanuelsson O, Kalume DE, Ytterberg J, Friso G, Rudella A, et al. Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell. 2002;14:211–236. doi: 10.1105/tpc.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schubert M, Petersson UA, Haas BJ, Funk C, Schröder WP, Kieselbach T. Proteome map of the chloroplast lumen of Arabidopsis thaliana. J Biol Chem. 2002;277:8354–8365. doi: 10.1074/jbc.M108575200. [DOI] [PubMed] [Google Scholar]
- 77.Shipman RL, Inoue K. Suborganellar localization of plastidic type I signal peptidase 1 depends on chloroplast development. FEBS Lett. 2009;583:938–942. doi: 10.1016/j.febslet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425:734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 79.Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell. 2008;20:648–657. doi: 10.1105/tpc.107.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villarejo A, Shutova T, Moskvin O, Forssén M, Klimov VV, Samuelsson G. A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J. 2008;21:1930–1938. doi: 10.1093/emboj/21.8.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shutova T, Kenneweg H, Buchta J, Nikitina J, Terentyev V, Chernyshov S, et al. The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J. 2008;27:782–791. doi: 10.1038/emboj.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3-transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2009;106:5990–5995. doi: 10.1073/pnas.0812885106. [DOI] [PMC free article] [PubMed] [Google Scholar]