Abstract
Sexually deceptive orchids provide no reward to their pollinators. Instead, they mimic the sex pheromone of receptive insect females to attract males which pollinate the flowers in mating attempts. Nearly all species of the Mediterranean orchid genus Ophrys are sexually deceptive and pollinated by solitary bees and wasps. Due to the use of a highly specific olfactory communication channel most Ophrys species have, in contrast to food deceptive or rewarding orchids, an inconspicuous greenish perianth and a dark brownish labellum. However, some species possess a bright pink or white perianth, and the functional significant of such color signals in the orchid-pollinator communication system is unknown. We recently showed that the pink perianth of Ophrys heldreichii increases the performance of its bee pollinator, males of the long-horned bee Eucera (Tetralonia) berlandi, to detect the flower at short-range. At great distances (>30 cm) from the flower, male search behavior was found to be olfactory guided and unaffected by the spectral property of the perianth, i.e., chromatic and green receptor-specific contrast. However, in the near vicinity of the flower (<30 cm), where spatial vision is sufficient to detect the flower, search time only correlated with the green receptor-specific contrast between the perianth and the background.
Key words: visual perception, colour vision, pollination, signal evolution, Apidae, orchids
The mimicry of the sex pheromone of a particular bee or wasp allows Ophrys to selectively attract its “private” pollinator and thus maximise pollen transfer. In contrast, a “non-private” color signal may impose additional costs in terms of pollen lost by accidentally attracting non-specific flower visitors and is thus difficult to explain in evolutionary terms of selective benefit. Here, we present evidence that the presence of an unspecific color signal in Ophrys is related to the visual performance and mate search behavior of the corresponding pollinator. We also suggest that the appearance of a color signal has been independently evolved, at least, twice within the genus.
The orchid family is one of the largest of flowering plants with up to 30,000 species, of these about 1/3 are pollinated by deception.1 One of the most remarkable strategies to achieve pollination without providing any reward to the pollinator is sexual deception. Sexually deceptive orchids attract their pollinators by imitating the sex pheromone of receptive females. The males become attracted to the female decoy and attempt to copulate with the labellum of the orchid. During this “pseudocopulation” the pollinaria become attached to the male’s body and are transferred during further visits to other flowers of the same species.2,3 Since the sex pheromone components produced by the flower attract only males of the target species, pollen transfer is highly efficient and pollen lost low.
One of the largest taxa of sexually deceptive orchids is the Mediterranean genus Ophrys with almost 300 species, of which nearly all are pollinated by sexual deception, mainly by males of solitary bees and wasps.4–6 The dark brownish labellum of the orchids approximately matches the size of the female’s body and provides the substrate on which the males copulate. Since the males are predominantly attracted by olfaction, the perianth is usually inconspicuously green and, at first glance, indistinguishable from the leaves or the stem due to its coloration6 (Fig. 1A). Interestingly, about one quarter of the species possess a bright pink or white perianth, but the functional significance of this color signal in the flower-pollinator communication is unknown. Moreover, a color signal may increase the risk of pollen lost by attracting non-specific pollinators and thus, it can be assumed that selection should favour individuals having an inconspicuous colouration.
Figure 1.
Two types of perianths in the sexually deceptive orchid genus Ophrys. O. cephalonica (A) possesses a green perianth which appears similar in colour to the leaves and stem. O. heldreichii (B), in contrast, shows a conspicuous pink perianth. Males of Eucera (Tetralonia) berlandi (C) have enlarged eyes and antennae compared to their females and are the pollinators of O. heldreichii.
Recently, we investigated the role of the colored perianth in the flower-pollinator communication system of Ophrys heldreichii and its pollinator, the long-horned bee Eucera (Tetralonia) berlandi (Fig. 1B and C).7,8 The approach flights of males towards intact orchid flowers, towards flowers in which we removed the original perianth, and in which we substituted the perianth with an artificial one of a particular color were filmed in the field and subsequently analyzed frame by frame. A bee-specific color model9 was applied to calculate the color contrast between the flower and the background. In addition to color information, bees also use an achromatic visual channel which relies on information from green-sensitive photoreceptors for detection of flowers at small angular sizes.10 At distances greater than 30 cm, male search time correlated only with wind speed and not with the spectral properties of the perianth. This indicates that the males use olfaction to navigate. However, at close range (<30 cm), where the flower subtends an angle of at least 5°, search time correlated only with the green receptor-specific contrast between perianth and background. The data thus suggest that males deploy different sensory modalities during the course of an approach. Olfaction is used to orientate from a distance; however, in the near vicinity, where spatial vision is sufficient to detect the flower, males’ approach is only visually guided.
Our results indicate that flowers which possess a colored perianth receive on average more visits due to an increased detectability which finally may lead to a higher pollination rate. Further, they also suggest that in a mixed population comprising plants which possess colored and green perianths, the proportion of individuals with colored perianths will spread over time due to a higher reproductive success.
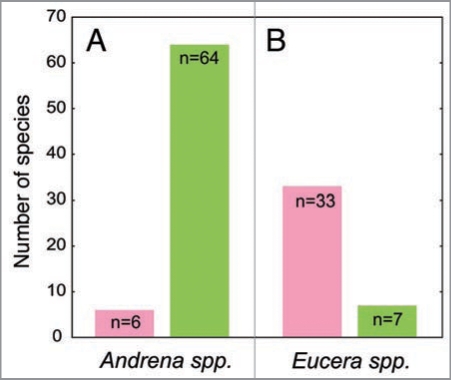
However, if this depicted scenario is true, why should not at least all bee-pollinated Ophrys species possess a colored perianth? We hypothesise that a color signal occurs only in such species where the advantage of an increased detectability outweighs the cost of potential pollen lost due to the attraction of unspecific visitors. E. berlandi belongs to the Eucerini, a group of bees which shows a distinct sensory sexual dimorphism. Males have enlarged eyes and antennae, and during mate search they rely heavily on vision.11 In a search of the literature, we discovered that 83% (33 out of 40) of all Ophrys species, in which the known pollinator belongs to the Eucerini, possess a colored perianth (Fig. 2). In contrast, only 9% (6 out of 70) of the species that are pollinated by Andrena males show a color floral signal (Fig. 2). Males from the latter genus constitute the largest group of Ophrys pollinators; in contrast to Eucerini, they usually lack a distinct sexual dimorphism of the sensory system, and vision seems to play only a minor role compared to olfaction during mate search and detection.11
Figure 2.
Frequency of Ophrys species which possess a green (green column) and a pink or white (pink column) perianth and are pollinated by Andrena (A) and Eucerini (B) males.
To obtain additional evidence for our hypothesis, future research in two directions is necessary. First, a reliable phylogeny needs to be established, e.g., one based on recently suggested nuclear genes which provide sufficient variation among Ophrys species.12 Then, the presence/absence of a colored perianth can be plotted as a character onto the phylogenetic tree and the origin(s) of the color signal within the genus inferred. Evidence already indicates that it is evolved independently in different groups. For instance, Ophrys heldreichii and O. tenthredinifera possess both a pink perianth and are pollinated by Eucerini males (E. berlandi and E. nigrilabris, respectively).13 However, the first species belongs to the O. holoserica-oestrifera group, whereas the latter is more related to the O. bombyliflora group, which are only very distantly related to each other but more closely related to species which possess a green perianth.14
Second, the relative increase of reproductive success caused by the color signal should be quantified for both flower types, those possessing a colored and those a green perianth. This can be performed by manipulating the perianth in the field and counting fruit production at the end of the flowering season.15
In sum, the interaction between Ophrys flowers and their pollinators is a promising system to help us better understand the evolution of color signals in entomophilous plants and also allow to test current theoretical models on the evolution of floral deception, for instance the sensory trap and the sensory exploitation models.16
References
- 1.van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, Florida: University of Miami Press,; 1966. [Google Scholar]
- 2.Alcock J. Enthusiasm for orchids: Sex and deception in plant evolution. Oxford: Oxford University Press,; 2005. [Google Scholar]
- 3.Schiestl FP. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- 4.Kullenberg B. Studies in Ophrys pollination. Zool Bidrag Uppsala. 1961;34:1–340. [Google Scholar]
- 5.Paulus HF, Gack C. Pollinators as prepollinating isolation factors: Evolution and speciation in Ophrys (Orchidaceae) Isr J Bot. 1990;39:43–79. [Google Scholar]
- 6.Delforge P. Orchids of Europe, North Africa and Middle East. Oregon: Timber Press,; 2006. [Google Scholar]
- 7.Spaethe J, Moser W, Paulus HF. Increase of pollinator attraction by means of a visual signal in the sexually deceptive orchid, Ophrys heldreichii (Orchidaceae) Plant Syst Evol. 2007;264:31–40. [Google Scholar]
- 8.Streinzer M, Paulus HF, Spaethe J. Floral color signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. J Exp Biol. 2009;212:1365–1370. doi: 10.1242/jeb.027482. [DOI] [PubMed] [Google Scholar]
- 9.Chittka L. The color hexagon: a chromaticity diagram based on photoreceptor excitation as a generalized representation of color opponency. J Comp Physiol A. 1992;170:533–543. [Google Scholar]
- 10.Dyer AG, Spaethe J, Prack S. Comparative psychophysics of bumblebee and honeybee color discrimination and object detection. J Comp Physiol A. 2008;194:617–627. doi: 10.1007/s00359-008-0335-1. [DOI] [PubMed] [Google Scholar]
- 11.Michener CD. The bees of the world. Baltimore and London: Johns Hopkins University Press,; 2000. [Google Scholar]
- 12.Schlüter PM, Kohl G, Stuessy TF, Paulus HF. A screen of low-copy nuclear genes reveals the LFY gene as phylogenetically informative in closely related species of orchids (Ophrys) Taxon. 2007;56:493–504. [Google Scholar]
- 13.Paulus HF. Wie Insekten-Mänchen von Orchideenblüten getäuscht werden-Bestäubungstricks und Evolution in der mediterranen Ragwurzgattung Ophrys. Denisia. 2007;20:255–294. [Google Scholar]
- 14.Devey DS, Bateman RM, Fay MF, Hawkins JA. Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Ann Bot. 2008;101:385–402. doi: 10.1093/aob/mcm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandersson R, Ägren J. Population size, pollinator visitation and fruit production in the deceptive orchid Calypso bulbosa. Oecologia. 1996;107:533–540. doi: 10.1007/BF00333945. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer HM, Ruxton GD. Deception in plants: mimicry or perceptual exploitation? Trends Ecol Evol. 2009;24:675–685. doi: 10.1016/j.tree.2009.06.006. [DOI] [PubMed] [Google Scholar]