Abstract
Many photosynthetic organisms exhibit light-dependent regulation of growth and development, including photoregulation of pigmentation, physiology, and form. We recently demonstrated that the photoregulation of cellular and filament morphology in Fremyella diplosiphon is under control of a photosensory photoreceptor and differentially impacted by photosynthetic pigment accumulation. Biliprotein photoreceptor RcaE controls the light-dependent regulation of pigmentation and of cell and filament morphology in F. diplosiphon, primarily in response to green and red light as a part of a light acclimation process known as complementary chromatic adaptation (CCA). Our recent investigations into the regulation of CCA underscored the largely independent regulation of pigmentation and cell shape by RcaE. However, recent studies on the regulation of phycobiliprotein biosynthesis indicated that filament length may depend upon correct photoregulation of photosynthetic pigment levels. Taken together, these studies suggest that aspects of the regulation of morphology in F. diplosiphon are independent of the regulation of pigmentation, yet other features of morphology depend upon the accurate photoregulation of pigment levels.
Key words: cellular morphology, complementary chromatic adaptation, cyanobacteria, phycobiliprotein, photomorphogenesis, photoregulation
Introduction
Cyanobacteria and other photosynthetic organisms display light-dependent regulation of growth and development in a process known as photomorphogenesis. As these organisms display limited mobility and depend upon light for carbon fixation and the generation of reductant, the ability to exhibit developmental plasticity in order to maximize growth and development is critical to their productivity, and ultimately their survival. One well-studied photomorphogenic process that occurs in cyanobacteria is complementary chromatic adaptation (CCA). CCA has been studied most extensively in the filamentous, freshwater cyanobacterium Fremyella diplosiphon.1,2 During the process of CCA, which is maximally responsive to red and green wavelengths of light,3 cyanobacterial cells exhibit light-dependent changes in the phycobiliprotein (PBP) composition of the light-harvesting complexes4–6 and cellular and filament morphology.7,8
Reflections on the Biological Implications of the Photoregulation of Morphology in F. diplosiphon
Whereas light-dependent changes in PBP content have been correlated with the location of the organism in the water column and the associated predominant wavelengths of light,9 no hypothesis about the biological implications of the role of regulating cellular or filament morphology during CCA has been prevalent. Our working hypothesis regarding the organismal significance of the photoregulation of cellular morphology is that larger cells in green light (GL) may be correlated with a physiological need for a greater thylakoid membrane surface area for increasing the total levels of PBP under GL conditions, because GL-absorbing phycoerythrin (PE) is the only photosynthetic light-harvesting pigment active in GL, whereas in red light (RL) phycocyanin (PC), allophycocyanin, and chlorophyll a (chla) absorb light for photosynthesis.2 Additionally, in the natural environment cells growing in green-enriched light are deeper in the water column and are exposed to lower overall light intensities,9 perhaps also necessitating a greater PBP content and a larger thylakoid membrane surface, which would be present in elongated cells, for improved productivity. Furthermore, the longer filaments containing more cells that are characteristic of GL7,8 may also be an outcome of increased cellular interdependence at the lower light levels that are found in GL-enriched natural habitats. That is, cells may become more dependent upon each other for mutual production and distribution of sugars and reductant at lower overall intensities of light and in conditions under which a single pigment, i.e., PE, is responsible for light absorption.
Photoregulation of Pigmentation is Largely Independent of Cellular Morphology Regulation in F. diplosiphon
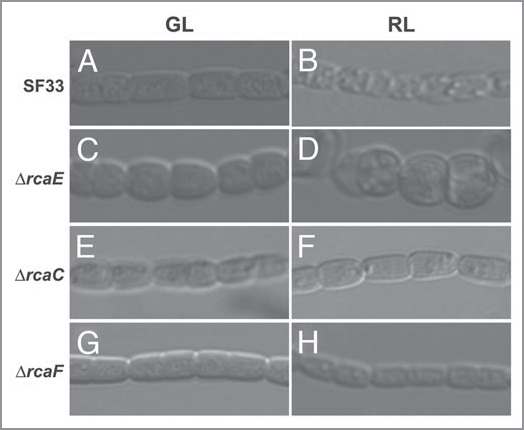
F. diplosiphon exhibits cells that are longer and more rod shaped under GL growth conditions than the smaller, rounded cells observed under RL.7,8 In recent studies, we demonstrated that this light-dependent regulation of cellular morphology is photoreversible, i.e., cells grown in GL and switched to RL exhibit cell shortening that is reversed when cells are again transitioned back to GL.8 Detailed comparisons of the timing of the photoreversible, light-dependent changes in morphology with light-dependent changes in the accumulation of photosynthetic pigments demonstrate that the observed changes in cellular morphology precede changes in PBP accumulation. 8 The regulation of both morphology and pigmentation were shown to be under control of the biliprotein photoreceptor RcaE.8 More recent studies indicated that severe depletion of PBP levels in a ΔcpcF mutant did not result in major changes in cell shape; only marginal differences in cell shape were identified for the ΔcpcF mutant relative to its parental SF33 strain under RL conditions.10 Therefore, results from independent studies indicate that the photoregulation of pigmentation is largely independent of the photoregulation of cellular morphology in F. diplosiphon. Thus, though both pigmentation and morphology are controlled by the photoreceptor RcaE, we can anticipate that distinct RcaE-dependent effectors are involved in the photo-control of pigmentation and morphology. It is currently accepted that the phosphorylation of two response regulators (RRs), i.e., RcaF and RcaC, operating downstream of RcaE, is required for the RL-dependent regulation of PC accumulation11 (see Fig. 1). Elevated levels of RcaC in RL also contribute to the accurate photoregulation of PC levels.12 Mutants in rcaF and rcaC are red in color under RL due to constitutive PE accumulation and an inability to accumulate PC.11 Analyses of the roles of these RRs in the light-dependent control of cellular morphology indicated that RcaF and RcaC also are required for the induction of the RL-dependent cell shape. That is, ΔrcaF and ΔrcaC mutants appear fixed in the GL-dependent cell shape even under RL growth8 (see Fig. 2). Thus, although the regulation of cellular morphology clearly requires RcaE under GL based on the phenotype of the ΔrcaE mutant8 (Fig. 2), RcaC and RcaF do not appear to be required for the GL-dependent regulation of cell shape. Thus, under GL the regulation of cell shape may be largely independent of known factors acting downstream of RcaE (see Fig. 1). Distinct effectors required for GL-dependent regulation of cellular morphology are under investigation.
Figure 1.
Model for photoregulation of pigmentation and cell and filament morphology in F. diplosiphon. Under green light (GL), RcaE works through response regulators RcaF and RcaC to induce accumulation of phycoerythrin (PE) pigment. In GL, RcaE is required for correct regulation of cellular and filament morphology and appears to work independently of RcaC and RcaF in the regulation of cell shape. Under red light (RL), RcaE works through RcaF and RcaC to regulate both pigmentation, i.e., accumulation of phycocyanin (PC), and cellular and filament morphology. However, the RcaE-dependent regulation of pigmentation and cell shape in RL occur independently of each other. Thus, the pathways of regulation likely branch downstream of RcaC. Notably, increased accumulation and phosphorylation of RcaC (RcaC-P) are correlated with RL-dependent PC accumulation.
Figure 2.
Cellular morphology of F. diplosiphon strains grown in green and red light. Representative slices from a Z-series of Differential Interference Contrast (DIC) images of filaments grown under green light (GL) or red light (RL): SF33 (A and B), ΔrcaE (C and D), ΔrcaC (E and F), and ΔrcaF (G and H) with 40× oil objective.
Photoregulation of Pigmentation Impacts the Regulation of Filament Length in F. diplosiphon
The recent isolation and analysis of a PC lyase mutant, i.e., ΔcpcF, indicated that an inability to covalently attach a bilin to PC apoprotein results in a feedback regulatory response that renders F. diplosiphon severely deficient in all PBPs, not just PC, under both RL and GL conditions.10 Of the photosynthetic pigments measured, only chla is maintained at wild-type (WT) levels.10 Notably, in the ΔcpcF mutant, although cell shape is not significantly different in GL and is only marginally reduced in length under RL, the filaments of the mutant are significantly longer than WT filaments under both light conditions.10 Thus, although light-dependent differences in cellular morphology are minor, significant changes in filament length are associated with a severe reduction in PBP levels.10 This observation suggests that the regulation of pigmentation and filament morphology may be linked, even indirectly. We propose that the severely reduced levels of photosynthetic PBPs are correlated with reduced overall photosynthetic efficiency that may necessitate greater cooperativity and sharing of photosynthates between cells.
Other Light-Dependent Cellular Morphology Responses in Cyanobacteria
Other light-dependent cellular responses have been observed in cyanobacteria that appear to be correlated with increased survivability or adaptation. For example, reversible compression of the spiral structure has been observed in response to photosynthetic active radiation (PAR, 400–700 nm) supplemented with ultraviolet radiation (UVR, 280–400 nm), i.e., PAR + UVR, in Arthrospira platensis.13 The tightening of spirals was shown to be correlated with a reduction in photoinhibition in response to exposure to damage-inducing levels of light.13 These results together with recent results obtained from studies of F. diplosiphon suggest that photoregulation of cyanobacterial cellular and filament morphologies are linked with organismal adaptation to environmentally important light variations. We thus anticipate that continued studies in the photoregulation of pigmentation and morphology in cyanobacterial systems will lead to additional insights into the physiologically relevant photoadaptations that occur in these systems.
Acknowledgements
Research on light sensing and photomorphogenesis in cyanobacteria in the corresponding author’s laboratory is supported by the National Science Foundation (Grant MCB-0643516 to B.L.M.) and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (DE-FG02-91ER20021 to B.L.M.). The authors thank Bagmi Pattanaik for critical assessment of the manuscript.
Abbreviations
- CCA
complementary chromatic adaptation
- chla
chlorophyll a
- GL
green light
- PBP
phycobiliprotein
- PC
phycocyanin
- PE
phycoerythrin
- RL
red light
- RR
response regulator
Addendum to: Bordowitz JR, Montgomery BL. Photoregulation of cellular morphology during complementary chromatic adaptation requires sensor-kinase-class protein RcaE in Fremyella diplosiphon. J Bacteriol. 2008;190:4069–4074. doi: 10.1128/JB.00018-08. [PubMed—indexed for MEDLINE] and Whitaker MJ, Bordowitz JR, Montgomery BL. CpcF-dependent regulation of pigmentation and development in Fremyella diplosiphon. Biochem Biophys Res Commun. 2009;389:602–606.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/ article/10367
References
- 1.Kehoe DM, Gutu A. Responding to color: The regulation of complementary chromatic adaptation. Annu Rev Plant Biol. 2006;57:127–150. doi: 10.1146/annurev.arplant.57.032905.105215. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery BL. Shedding new light on the regulation of complementary chromatic adaptation. Cent Eur J Biol. 2008;3:351–358. [Google Scholar]
- 3.Scheibe J. Photoreversible pigment: occurrence in a blue-green alga. Science. 1972;176:1037–1039. doi: 10.1126/science.176.4038.1037. [DOI] [PubMed] [Google Scholar]
- 4.Gantt E. Phycobilisomes. Annu Rev Plant Physiol. 1981;32:327–347. [Google Scholar]
- 5.Glazer AN. Phycobiliproteins. Methods Enzymol. 1988;167:291–303. doi: 10.1016/0076-6879(88)67034-0. [DOI] [PubMed] [Google Scholar]
- 6.Tandeau de Marsac N, Cohen-Bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci USA. 1977;74:1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett A, Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973;58:419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordowitz JR, Montgomery BL. Photoregulation of cellular morphology during complementary chromatic adaptation requires sensor-kinase-class protein RcaE in Fremyella diplosiphon. J Bacteriol. 2008;190:4069–4074. doi: 10.1128/JB.00018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postius C, Neuschaefer-Rube O, Haid V, Böger P. N2-fixation and complementary chromatic adaptation in non-heterocystous cyanobacteria from Lake Constance. FEMS Microbiol Ecol. 2001;37:117–125. [Google Scholar]
- 10.Whitaker MJ, Bordowitz JR, Montgomery BL. CpcF-dependent regulation of pigmentation and development in Fremyella diplosiphon. Biochem Biophys Res Commun. 2009;389:602–606. doi: 10.1016/j.bbrc.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Kehoe DM, Grossman AR. New classes of mutants in complementary chromatic adaptation provide evidence for a novel four-step phosphorelay system. J Bacteriol. 1997;179:3914–3921. doi: 10.1128/jb.179.12.3914-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Kehoe DM. Abundance changes of the response regulator RcaC require specific aspartate and histidine residues and are necessary for normal light color responsiveness. J Bacteriol. 2008;190:7241–7250. doi: 10.1128/JB.00762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Z, Gao K. Photoregulation of morphological structure and its physiological relevance in the cyanobacterium Arthrospira (Spirulina) platensis. Planta. 2009;230:329–337. doi: 10.1007/s00425-009-0947-x. [DOI] [PubMed] [Google Scholar]