Abstract
The study of channel modulation by regulatory subunits has attracted considerable attention. Evidence indicates a pivotal role for accessory proteins in the channelosome. For instance, these regulatory subunits are necessary to recapitulate in vivo ion currents and to further understand the physiological role of ion channels. KCNEs are a family of regulatory subunits that interact with a wide range of channels. We have described for the first time a molecular interaction between KCNE4 and the voltage-dependent potassium channel KV1.3. The association of KCNE4, which alters the biophysical properties, trafficking and membrane localization of KV1.3, functions as an endogenous dominantnegative mechanism. Since both proteins are expressed in the immune system, KV1.3/KCNE4 channels may contribute to the fine-tuning of the immune response. Therefore, our results point to KCNE4 as a novel target for immunomodulation. KCNE4 is not the only KCNE which is expressed in leukocytes. All KCNEs (KCNE1-5) are present, and some members demonstrate modulation during proliferation and cancer. In summary, regulatory KCNE subunits are expressed in the immune system. In addition, several voltage-dependent K+ channels, which could interact with KCNEs, are also detected. Therefore, KCNE subunits may play a yet undiscovered role in the physiology of the immune system.
Key words: voltage-dependent potassium channels, KCNE4, KV1.3, leukocytes, regulatory subunits
KCNEs are a group of regulatory subunits composed of 5 members (KCNE1-5). KCNE peptides are small single spanning membrane proteins (<20 KDa) which modulate a large number of voltage-dependent K+ channels.1 The most well characterized interaction occurs with KCNQ1 (KV7.1) channels.2–4 KV7.1/KCNE1 channels recapitulate the cardiac Iks current.5 Several KCNE1 mutations, which trigger severe cardiac channelopathies, demonstrate the pivotal function of KCNE1 on cardiovascular physiology.6–8 The implication of that the remaining KCNE peptides may contribute to the modulation of Iks is now under intense investigation.9–13 Besides KV7.1, KCNE members associate with other K+ channels. Thus, KV11.1 in association with KCNE2 conducts the cardiac Ikr current.14
Although many KCNE subunits share tissue expression with Shaker K+ channels (KV1) channels, KCNE interactions with KV1 channels have attracted little research.1 Early in 1992, KV1.3 and KCNE1 were simultaneously cloned in human Jurkat T-cells.15 Ten years later, Grunnet et al. (2003) described that KCNE4 modulates KV1.1 and KV1.3 channels and their heteromeric forms. However, while KV1.1 and KV1.3 biophysics were affected, KV1.1 membrane surface targeting was not altered.16 Later, Melman et al. (2004) described that KCNE1 co-immunoprecipitated with KV1.5, but no further research was undertaken.17 Recently, Abbot et al. (2008) demonstrated that kcne2−/− mice exhibit altered cardiac Ikslow, identified an interaction between KV1.5 and KCNE2 in murine ventricles and suggested a functional role for KCNE2 in promoting KV1.5 surface expression.18
In this context, our recent contribution unequivocally demonstrates molecular interactions between KCNE4 and KV1.3, which impairs the trafficking and localization of these channels.19 KCNE4 acts as an endogenous dominant-negative regulatory subunit. Further inhibitory mechanisms by KCNE4 have been observed. Grunnet et al. (2002) describe that KCNE4 abrogates KV7.1 currents.20 Later, George and coworkers (2008) postulate that KCNE4 could form part of a KV7.1/KCNE1 heterocomplex, responsible for downregulating the Iks current.10 Furthermore, KCNE4 also inhibits the calcium-activated K+ channel (KCa1.1)21 and oligomeric KV4.2 + KChIP2 channels.22
Although limited, the immunitary system has a defined repertoire of K+ channel genes. Leukocytes express, KV1.3, KV1.5 and several KVβ regulatory subunits.23–25 We have demonstrated that KV1.3 and KV1.5 form heteromeric complexes to finetune the immunitary response.23,26 The evidence that KCNE4 is also implicated,19 the presence of KCNE1 in lymphocytes,15 and its putative association to KV1.5,17 suggest an unidentified role for KCNE peptides in immunitary system physiology.
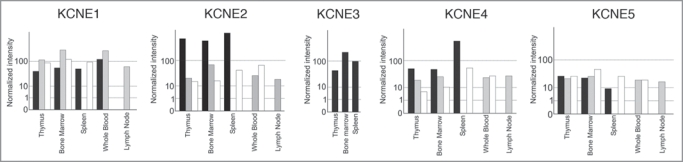
In fact, our recent study is not the first to describe the presence of the KCNEs in the immunitary system. KCNE1 was cloned in T-lymphocytes.15 Grunnet et al. (2003) found KCNE4 mRNA in lymphocytes. 16 In addition, KCNEs mRNA expression has also been investigated in thymus.10,27 Here we recapitulate the information available about the expression of KCNE1-5 in different tissue arrays, all derived from the immune system. To that end, data from tissues and tumors, cell lines and cancer cell lines are shown in Figures 1, 2 and 3, respectively. All KCNE1-5 members have been detected in myeloid and lymphoid lineages. It is noteworthy that while KCNE1 expression increases in some cancers, KCNE2 and KCNE4 decrease. Similarly, KCNE1 induction has also been detected in germinal tumors.28 We have previously described the importance of KV1.3 during activation and proliferation of macrophages.25,26 Although both processes augment KV1.3, the channel seems to play a dual role. Prolonged signaling during activation triggers apoptosis. 29,30 Additionally, highly proliferative cells exhibit lower KCNE4 expression than their counterparts do (Fig. 1). During an insult, KV1.3 is activated and sustained activation triggers cell death. The persistence of activated macrophages during inflammation leads to disease. In this scenario, KCNE4 would exert an inhibitory effect not needed in proliferation. Accordingly, it is worth noting that during proliferation, most cells become resistant to apoptosis.
Figure 1.
Human KCNE expression in healthy and cancer tissues. Affymetrix GeneChips HG-U95A-E (GeneNote, http://bioinfo2.weizmann.ac.il/cgi-bin/genenote/home_page.pl) and HG-U133A (GNF, (http://biogps.gnf.org) normalized as described in GeneCard (http://www.genecards.org). Black columns: healthy tissue (GeneNote); Grey columns: healthy tissues (GNF); White columns: cancer samples (GNF).
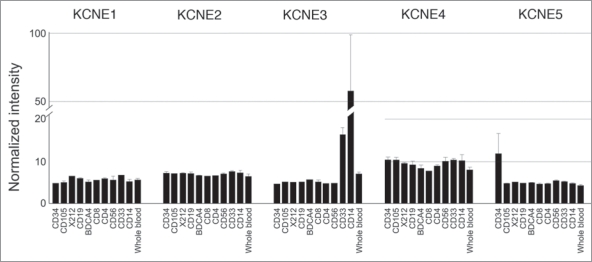
Figure 2.
KCNE expression in human leukocytic cell lines. Array data from GNF BioGPS (http://biogps.gnf.org) normalized according to Su et al.31 Legend: CD34, bone marrow CD34+ progenitors; CD105, endothelial; X212, B lymphoblasts; CD19, B cells; BDCA4, dendritic cells; CD8, CD8+ Tcells; CD4, CD4+ T-cells; CD56, NK cells; CD33, myeloid; CD14, monocytes.
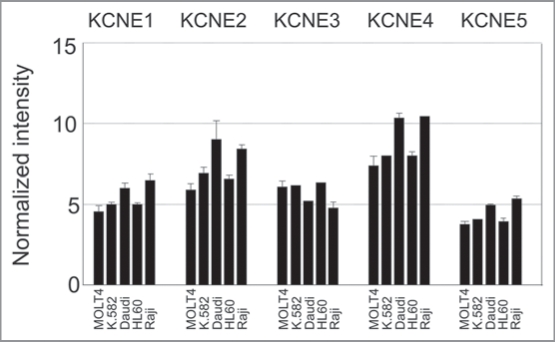
Figure 3.
KCNE expression in different human leukemia and lymphoma cell lines. Array data from GNF BioGPS (http://biogps.gnf.org) normalized according to Su et al.31 Legend: MOLT4, Lymphoblastic Leukemia; K.582, Chronic Myelogenous Leukemia; Daudi, Burkitt’s Lymphoma; HL60, Promyelocytic Leukemia; Raji, Burkitt’s Lymphoma.
Evidence demonstrates that KCNE subunits are present in the immune system and may interact with KV1 channels. Therefore, we suggest that KCNE subunits may play a yet undiscovered role in the immune system via associations with leukocyte K+ channels. These new interactions situate KCNE, specifically KCNE4, as novel targets for immunomodulation. Further research should be undertaken to shed some light on this new issue.
Acknowledgements
The authors thank to all present and past members of the molecular physiology laboratory. Supported by the Ministerio de Ciencia e Innovación (MICINN), Spain (BFU2008-00431 and CSD2008-00005). L.S. is a fellow from MICINN.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/10602
References
- 1.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Haitin Y, Wiener R, Shaham D, Peretz A, Cohen EB, Shamgar L, et al. Intracellular domains interactions and gated motions of I(KS) potassium channel subunits. EMBO J. 2009;28:1994–2005. doi: 10.1038/emboj.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Zheng R, Melman YF, McDonald TV. Functional interactions between KCNE1 C-terminus and the KCNQ1 channel. PLoS One. 2009;4:5143. doi: 10.1371/journal.pone.0005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang C, Tian C, Sonnichsen FD, Smith JA, Meiler J, George AL, Jr, et al. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 6.Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, et al. KCNE1 mutations cause jervell and Lange-Nielsen syndrome. Nat Genet. 1997;17:267–268. doi: 10.1038/ng1197-267. [DOI] [PubMed] [Google Scholar]
- 7.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 8.Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000;36:1–12. doi: 10.1016/s0735-1097(00)00716-6. [DOI] [PubMed] [Google Scholar]
- 9.Lundquist AL, Manderfield LJ, Vanoye CG, Rogers CS, Donahue BS, Chang PA, et al. Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks) J Mol Cell Cardiol. 2005;38:277–287. doi: 10.1016/j.yjmcc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Manderfield LJ, George AL., Jr. KCNE4 can coassociate with the I(Ks) (KCNQ1-KCNE1) channel complex. Febs J. 2008;275:1336–1349. doi: 10.1111/j.1742-4658.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- 11.Tseng GN. The phenotype of a KCNQ 1 mutation depends on its KCNE partners: is the cardiac slow delayed rectifier (IKs) channel more than a KCNQ1/KCNE1 complex? Heart Rhythm. 2007;4:1542–1543. doi: 10.1016/j.hrthm.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin TJ, Kobertz WR. A derivatized scorpion toxin reveals the functional output of heteromeric KCNQ1-KCNE K+ channel complexes. ACS Chem Biol. 2007;2:469–473. doi: 10.1021/cb700089s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, et al. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc Res. 2005;67:529–538. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 15.Attali B, Romey G, Honore E, Schmid-Alliana A, Mattei MG, Lesage F, et al. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J Biol Chem. 1992;267:8650–8657. [PubMed] [Google Scholar]
- 16.Grunnet M, Rasmussen HB, Hay-Schmidt A, Rosenstierne M, Klaerke DA, Olesen SP, Jespersen T. KCNE4 is an inhibitory subunit to KV1.1 and KV1.3 potassium channels. Biophys J. 2003;85:1525–1537. doi: 10.1016/S0006-3495(03)74585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melman YF, Um SY, Krumerman A, Kagan A, McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–937. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f ) Faseb J. 2008;22:3648–3660. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sole L, Roura-Ferrer M, Perez-Verdaguer M, Oliveras A, Calvo M, Fernandez-Fernandez JM, Felipe A. KCNE4 suppresses KV1.3 currents by modulating trafficking, surface expression and channel gating. J Cell Sci. 2009;122:3738–3748. doi: 10.1242/jcs.056689. [DOI] [PubMed] [Google Scholar]
- 20.Grunnet M, Jespersen T, Rasmussen HB, Ljungstrom T, Jorgensen NK, Olesen SP, Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–130. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DI, Wanderling S, Biemesderfer D, Goldstein SA. MiRP3 acts as an accessory subunit with the BK potassium channel. Am J Physiol Renal Physiol. 2008;295:380–387. doi: 10.1152/ajprenal.00598.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varro A, et al. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res. 2006;71:695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, et al. Association of KV1.5 and KV1.3 contributes to the major voltagedependent K+ channel in macrophages. J Biol Chem. 2006;281:37675–37685. doi: 10.1074/jbc.M605617200. [DOI] [PubMed] [Google Scholar]
- 24.Vicente R, Escalada A, Soler C, Grande M, Celada A, Tamkun MM, et al. Pattern of KV beta subunit expression in macrophages depends upon proliferation and the mode of activation. J Immunol. 2005;174:4736–4744. doi: 10.4049/jimmunol.174.8.4736. [DOI] [PubMed] [Google Scholar]
- 25.Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, et al. Differential voltagedependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–46320. doi: 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- 26.Villalonga N, Escalada A, Vicente R, Sanchez-Tillo E, Celada A, Solsona C, Felipe A. KV1.3/KV1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem Biophys Res Commun. 2007;352:913–918. doi: 10.1016/j.bbrc.2006.11.120. [DOI] [PubMed] [Google Scholar]
- 27.Lundquist AL, Turner CL, Ballester LY, George AL., Jr Expression and transcriptional control of human KCNE genes. Genomics. 2006;87:119–128. doi: 10.1016/j.ygeno.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Tsevi I, Vicente R, Grande M, Lopez-Iglesias C, Figueras A, Capella G, et al. KCNQ1/KCNE1 channels during germ-cell differentiation in the rat: expression associated with testis pathologies. J Cell Physiol. 2005;202:400–410. doi: 10.1002/jcp.20132. [DOI] [PubMed] [Google Scholar]
- 29.Detre C, Kiss E, Varga Z, Ludanyi K, Paszty K, Enyedi A, et al. Death or survival: membrane ceramide controls the fate and activation of antigenspecific T-cells depending on signal strength and duration. Cell Signal. 2006;18:294–306. doi: 10.1016/j.cellsig.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Szabo I, Adams C, Gulbins E. Ion channels and membrane rafts in apoptosis. Pflugers Arch. 2004;448:304–312. doi: 10.1007/s00424-004-1259-4. [DOI] [PubMed] [Google Scholar]
- 31.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]