Abstract
Presynaptic N-type voltage-gated Ca2+ channels (Cav2.2) form part of an extensive macromolecular complex in the presynaptic terminal. Regulation of Cav2.2 is achieved via protein-protein interactions within the terminal and can directly impact transmitter release which is dependent on Ca2+ influx via these Cav2.2. We recently identified a novel Cav2.2 interacting partner—the collapsin response mediator protein (CRMP).1 CRMPs are a family of five proteins implicated in signal transduction of neurite outgrowth and axonal guidance. We showed that CRMP-2, a wellstudied member of this family, interacted with Cav2.2 via direct binding to cytoplasmic loops of Cav2.2. Depolarization enhanced the interaction. Further studies revealed that CRMP-2 facilitated an increase in Cav2.2 current density by inserting more Cav2.2 at the cell surface. As a consequence of CRMP-2-mediated increase in Ca2+ influx, release of the excitatory neurotransmitter glutamate was also increased. CRMP-2 localized to synapses where, surprisingly, its overexpression increased synapse size. We hypothesize that the CRMP-2-calcium channel interaction represents a novel mechanism for modulation of Ca2+ influx into nerve terminals and, hence, of synaptic strength. In this addendum, we further discuss the significance of this study and the possible implications to the field.
Key words: axonal outgrowth, CRMP-2, growth cone, presynaptic calcium channels, surface trafficking, Cav2.2, synaptic transmission
Ca2+ entry into neurons through presynaptic Ca2+ channels couples membrane depolarization to integration of synaptic signals in soma and dendrites and to exocytosis of neurotransmitters within the nerve terminal.2 Identification and analyses of protein-protein interactions within the nerve terminal have demonstrated a functional coupling between presynaptic Ca2+ channels and the transmitter release machinery.2–6 New to this crowded transmitter release ‘neighborhood’ is a family of proteins, the collapsin response mediator proteins (CRMPs), which we recently discovered in a screen for proteins interacting with presynaptic N-type Ca2+ channels (Cav2.2).1
CRMP-2, the most well-studied member of this family of five phosphoproteins, has been implicated as an intracellular signal transducer in nervous system development, including induction of the growth of axons,7–9 and mediation of semaphorin 3A signaling.8 Overexpression of CRMP-2 induces multiple axon formation and elongation of the primary axon in hippocampal neurons.10 CRMP-2 mediates axonal differentiation, presumably by binding to the tubulin-heterodimer and promoting microtubule assembly10 and inducing tubulin’s GTPase activity.11,12 CRMP-2 is phosphorylated by Rho-kinase,13 and a sequential phosphorylation of CRMP2 by cyclin-dependent kinase 5 (Cdk5) and then glycogen synthase kinase-3β (GSK-3β) has also been implicated in semaphorin3A signaling.14 It has been hypothesized that CRMP proteins may also serve as adaptors/scaffold molecules.7
We have now shown that CRMP-2 is part of the Cav2.2 proteome.1 Immunocytochemistry and reciprocal co-immunoprecipitation have revealed a strong colocalization between CRMP-2 and Cav2.2 within hippocampal neurons. Traditional in vitro binding experiments and isothermal titration calorimetric analyses (Khanna M, Brittain JM and Khanna R, unpublished data) mapped the site interaction to two domains within the cytoplasmic loops of Cav2.2. The CRMP- 2-Cav2.2 interaction is dynamic as KClinduced depolarization led to an increase in the interaction. Functionally, these interactions led to an increased cell-surface expression of Cav2.2 and subsequent increase in Cav2.2 current density in hippocampal neurons. The CRMP-2-Cav2.2 interaction also resulted in an increase in the release of the excitatory neurotransmitter glutamate presumably through the increase in Ca2+ influx as toxin block of Cav2.2 eliminated this increase. As summarized in Figure 1, these results suggest CRMP-2 is a novel regulator of Ca2+ channel function and of transmitter release.1
Figure 1.
CRMP-2 signaling cascade: a novel role for CRMPs in Ca2+ channel regulation and transmitter release. Extracellular signals, such as extracellular matrix, growth factors and guidance cues (semaphorin 3A) activate Neuropilin-1/Plexin A receptors on membranes.7 A battery of kinases, including RhoK, Cdk5 and GSK-3β phosphorylate CRMPs. Phosphorylated CRMPs have a reduced affinity to tubulin and other interacting molecules and lose their positive effect on axon elongation, thereby causing growth arrest and growth cone collapse. In contrast, non-phosphorylated CRMPs bind strongly to tubulin heterodimers to promote microtubule assembly and Numb-mediated endocytosis30 thereby promoting axon elongation and branching.7 In addition to these classically defined roles for CRMPs, our results suggest that CRMPs (assuming both phosphorylated and non-phosphorylated forms) bind to cytoplasmic loops of the Ca2+ channel and increase their insertion into the membrane, resulting in an increased current density.1 This increase culminates into an increase in the release of the excitatory transmitter glutamate.1 Interestingly, CRMP-2 overexpression increases synapse size not number.1 This suggests that CRMP-2 regulation of transmitters may occur via a direct effect on CaV2.2 or through an effect on changes in synaptic vesicle machinery and release probabilities. Increased synaptic transmission is likely to contribute to synaptic plasticity.
While our findings extend the biological functions of CRMP-2 past axon dynamics to include regulation of channel function and modulation of downstream transmitter release, they also raise important points for further discussion including the unexplored role(s) of related CRMPs in Ca2+ channel regulation, the nature of the oligomeric state of CRMPs to which Cav2.2 binds, whether posttranslational modifications like phosphorylation can regulate the interaction and downstream effects, and the role CRMP-2-Cav2.2 interaction may play at the pre- synapse (i.e., growth cone). We consider these points in the following sections:
Do other CRMPs (i.e., besides CRMP-2) influence Ca2+ channel function?
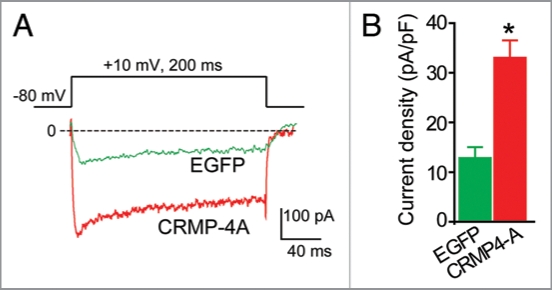
The five members of the CRMP family share ∼65% sequence homology. High resolution crystal structures of CRMP-1 and -2 have been solved, and homology modeling of CRMP-3 and -4 reveals a very high degree of structural conservation. Based on the high sequence similarity between CRMP-2 and -4, their similar pattern of expression during development, and the reported interaction between CRMP-4 and proteins involved in synaptic vesicle recycling,15 we hypothesized that CRMP-4 may regulate Ca2+ channels. To test this hypothesis, we expressed CRMP-4a—a developmentally unrestricted variant of CRMP-4—in hippocampal neurons and measured Ca2+ currents using wholecell voltage clamp electrophysiology. As shown in Figure 2, the Ca2+ current density in CRMP-4b expressing neurons was significantly higher than neurons expressing enhanced green fluorescent protein (EGFP). On average, the peak Ca2+ current density increased by about 2.5-fold, from 12.28 ± 2.0 (n = 8) in control neurons to 33 ± 3.5 (n = 8) pA/pF in CRMP-4a expressing neurons (holding potential of 0 mV, p < 0.05, ANOVA). This augmentation in current density is even higher than the 60% increase in current we reported for CRMP-2. Based on the CRMP-4 data (Fig. 2) and our report, it is tempting to speculate that all CRMPs act to regulate Ca2+ channel density. Whether this regulation results from altered channel biogenesis, trafficking, or stabilization remains to be investigated.
Figure 2.
CRMP-4 enhances Ca2+ current density in hippocampal neurons. (A) Exemplar current traces obtained from a cell transfected with EGFP and CRMP-4-EGFP evoked by 200-ms steps to +10 mV applied from a holding potential of -80 mV, as shown in the voltage protocol above the traces. Bath solutions contained 1 µM TT X, 10 mM TE A and 1 µM Nifidepine to block Na+, K+ and L-type voltage-gated Ca2+ channels, respectively. (B) Peak current density (pA/pF) measured at +10 mV for EGFP (n = 8) or CRMP-4a-EGFP (n = 8) transfected neurons. *p < 0.05 versus EGFP (One-way ANOVA).
Oligomerization status of CRMP-2’s interaction with Cav2.2.
Structural and biochemical studies have demonstrated that CRMPs exist as tetramers.16 Tetramer formation is stabilized by the presence of divalent cations, whereas in the presence of cation chelating agents almost half of CRMP-2 exists in a monomeric state.17 While we have not directly tested whether Cav2.2 binds to a tetrameric or monomeric form of CRMP-2, the latter is a more likely possibility given the presence of chelators in our binding assay buffers. If, however, CRMP tetramers bind Cav2.2, then the subunit composition of the tetramer will be an important determinant of its effect on Cav2.2. The composition of CRMP tetramers will vary due to the differential spatial and developmental patterns of CRMPs expression within the nervous system.18 Thus, CRMPs, by virtue of their ability to heteromultimerize in multiple combinations,16 may help in ‘fine tuning’ the effects on Ca2+ channel density, thereby adding another level of complexity in transmitter release regulation. This oligomeric assembly of CRMPs may also serve as the building blocks upon which presynaptic signaling complexes are erected.
Does CRMP phosphorylation affect interaction with and regulation of Ca2+ channels?
CRMP-2 is a multi-phosphorylated protein in neurons.13,19,20 CRMP-2 phosphorylation is regulated by several kinases, such as GSK-3β, Cdk5 and Rho kinase.7 Phosphorylation by GSK-3β and/or Rho kinase lowers the ability of CRMP-2 to interact with tubulin which leads to arrest of axonal growth and collapse of growth cones.13,19,20 While we have not directly tested whether Cav2.2 binds to the phosphorylated or non-phosphorylated state of CRMP-2, it is likely that, like tubulin,10 Cav2.2 binds to the non-phosphorylated but active form of CRMP-2.21 Cav2.2 and tubulin binding to the active form of CRMP-2 in the growth cone are entirely consistent with the roles served by these proteins at these locations-Ca2+ influx/synaptic transmission and axon growth, respectively. A recent study demonstrated that the CRMP-2 priming kinase Cdk5 also phosphorylates the adaptor protein Ca2+/calmodulin-dependent serine protein kinase (CASK).22 CASK binds to voltage-gated Ca2+ channels;23–25 it was recently shown that Cdk5 phosphorylation of CASK frees it to interact with presynaptic proteins including Cav2.2. As Cdk5-mediated phosphorylation of CASK increased Ca2+ currents via Cav2.2 channels,22 it is possible that Cdk5 affects Cav2.2 indirectly by modulating CRMP-2 phosphorylation and thus Cav2.2 activity. Besides these reports, the issues of phosphorylation-dependent effects of CRMPs on Ca2+ channel function are still largely unknown.
Possible roles of the CRMP-2-Cav2.2 interaction at the pre-synapse (i.e., the growth cone).
Our study points to a novel role of CRMP-2 in regulating trafficking of Ca2+ channels1,21 and surprisingly, to synapse size.1 The latter results suggest a putative involvement of CRMPs in synapse formation and maintenance. Whereas the role of intracellular Ca2+ in growth cone activity and behavior have been well established,26,27 the involvement of the CRMP-2-Cav2.2 interaction in these events is unknown. One could speculate that the CRMPs may be responsible for transporting Cav2.2 into growth cones especially during synaptogenesis. In support of this idea, we observed robust colocalization between CRMP-2 and Cav2.2 co-localize within growth cones in dorsal root ganglion neurons.21 In another study, it was reported that the auxiliary Cavβ subunit was absent from the periphery of growth cones and filopodia in immature neurons.28 This is surprising given that Cavβ subunits are considered to be an obligatory component of the Ca2+ channel complex and are believed to associate with Cavβ subunits very early during their biogenesis.29 Therefore, it is possible that CRMP-2, not Cavβ, is important for supplying nascent growth cones with a complement of highly motile Ca2+ channel α subunits. The CRMP-2-Cav2.2 complex is also likely to contribute to axon growth as well as to growth cone dynamics.
In summary, we have shown that the CRMP-2-Ca2+ channel complex may serve multiple purposes in neurotransmitter release: (1) to sustain Ca2+ influx through functional regulation of Ca2+ channels; (2) to target the N-type Ca2+ channel to immature synapses during synaptogenesis; (3) to provide a scaffold for the Ca2+ channel macromolecular complex; and (4) to recruit synaptic vesicles to Ca2+ channels. Thus, our results identify CRMP-2 as a novel ‘neuromodulator’ of Ca2+ channels and of synaptic strength.1 It will be interesting to examine if dysregulation of CRMP-2 expression and the CRMP-2-Cav2.2 interaction may contribute to diseases of synaptic function or ‘synaptopathies’.
Acknowledgements
CRMP4a cDNA was a gift of Dr. Alyson Fournier (Montreal Neurological Institute, Montreal, Canada). We thank Drs. Gerry Oxford and May Khanna and Lisa D. King and Brian W. Jarecki for critically reading the manuscript. This work was supported by grants from the Indiana State Department of Health—Spinal Cord and Brain Injury Fund [Grant 4786219 to R.K.] and The Indiana University Biomedical Committee—Research Support Funds [Grant 2286501 to R.K.].
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/10620
References
- 1.Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated Ca2+ channels. J Biol Chem. 2009;284:31375–31390. doi: 10.1074/jbc.M109.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Davies JN, Zamponi GW. Old proteins, developing roles: The regulation of calcium channels by synaptic proteins. Channels. 2008;2:130–138. doi: 10.4161/chan.2.2.6214. [DOI] [PubMed] [Google Scholar]
- 4.Sheng ZH, Westenbroek RE, Catterall WA. Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery. J Bioenerg Biomembr. 1998;30:335–345. doi: 10.1023/a:1021985521748. [DOI] [PubMed] [Google Scholar]
- 5.Khanna R, Li Q, Bewersdorf J, Stanley EF. The presynaptic Cav2.2 channel-transmitter release site core complex. Eur J Neurosci. 2007;26:547–559. doi: 10.1111/j.1460-9568.2007.05680.x. [DOI] [PubMed] [Google Scholar]
- 6.Khanna R, Zougman A, Stanley EF. A proteomic screen for presynaptic terminal N-type calcium channel (Cav2.2) binding partners. J Biochem Mol Biol. 2007;40:302–314. doi: 10.5483/bmbrep.2007.40.3.302. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki H, Kato Y, Hamajima N, Nonaka M, Sasaki M, Eimoto T. Differential expression of dihydropyrimidinase-related protein genes in developing and adult enteric nervous system. Histochem Cell Biol. 2000;113:37–41. doi: 10.1007/s004180050005. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 10.Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 11.Chae YC, Lee S, Heo K, Ha SH, Jung Y, Kim JH, et al. Collapsin response mediator protein-2 regulates neurite formation by modulating tubulin GTPase activity. Cell Signal. 2009;21:1818–1826. doi: 10.1016/j.cellsig.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Majava V, Greffier A, Hayes RL, Kursula P, Wang KK. Collapsin response mediator protein-2 is a calmodulin-binding protein. Cell Mol Life Sci. 2009;66:526–536. doi: 10.1007/s00018-008-8362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, et al. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275:23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- 14.Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 2. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 15.Quinn CC, Chen E, Kinjo TG, Kelly G, Bell AW, Elliott RC, et al. TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J Neurosci. 2003;23:2815–2823. doi: 10.1523/JNEUROSCI.23-07-02815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LH, Strittmatter SM. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J Neurochem. 1997;69:2261–2269. doi: 10.1046/j.1471-4159.1997.69062261.x. [DOI] [PubMed] [Google Scholar]
- 17.Majava V, Loytynoja N, Chen WQ, Lubec G, Kursula P. Crystal and solution structure, stability and posttranslational modifications of collapsin response mediator protein 2. FEBS J. 2008;275:4583–4596. doi: 10.1111/j.1742-4658.2008.06601.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang LH, Strittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci. 1996;16:6197–6207. doi: 10.1523/JNEUROSCI.16-19-06197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Chi XX, Schmutzler BS, Brittain JM, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltagegated calcium (Cav2.2) channels and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci. 2009;122:4351–4362. doi: 10.1242/jcs.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels BA, Hsueh YP, Shu T, Liang H, Tseng HC, Hong CJ, et al. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron. 2007;56:823–837. doi: 10.1016/j.neuron.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maximov A, Sudhof TC, Bezprozvanny I. Association of neuronal calcium channels with modular adaptor proteins. J Biol Chem. 1999;274:24453–24456. doi: 10.1074/jbc.274.35.24453. [DOI] [PubMed] [Google Scholar]
- 24.Maximov A, Bezprozvanny I. Synaptic targeting of N-type calcium channels in hippocampal neurons. J Neurosci. 2002;22:6939–6952. doi: 10.1523/JNEUROSCI.22-16-06939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna R, Sun L, Li Q, Guo L, Stanley EF. Long splice variant N type calcium channels are clustered at presynaptic transmitter release sites without modular adaptor proteins. Neuroscience. 2006;138:1115–1125. doi: 10.1016/j.neuroscience.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer NC. Spontaneous activity: functions of calcium transients in neuronal differentiation. Perspect Dev Neurobiol. 1995;2:379–3786. [PubMed] [Google Scholar]
- 27.Spitzer NC. Activity-dependent neuronal differentiation prior to synapse formation: the functions of calcium transients. J Physiol Paris. 2002;96:73–80. doi: 10.1016/s0928-4257(01)00082-1. [DOI] [PubMed] [Google Scholar]
- 28.Spafford JD, Van Minnen J, Larsen P, Smit AB, Syed NI, Zamponi GW. Uncoupling of calcium channel alpha1 and beta subunits in developing neurons. J Biol Chem. 2004;279:41157–41167. doi: 10.1074/jbc.M403781200. [DOI] [PubMed] [Google Scholar]
- 29.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, et al. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]