Abstract
Many cells and organisms go through polarized growth phases during their life. Cell polarization is achieved by local accumulation of signaling molecules which guide the cytoskeleton and vesicular trafficking to specific parts of the cell and thus ensure polarity establishment and maintenance. Polarization of signaling molecules is also fundamental for the lifestyle of filamentous fungi such as Aspergillus niger and essential for their morphogenesis, development and survival under environmental stress conditions. Considerable advances in our understanding on the protagonists and processes mediating polarized growth in filamentous fungi have been made over the past years. However, how the interplay of different signaling pathways is coordinated has yet to be determined. We found that the A. niger RmsA protein is central for the polarization of actin at the hyphal tip but also of vital importance for the metabolism, viability and stress resistance of A. niger. This suggests that RmsA could occupy an important position in the global network of pathways that balance growth, morphogenesis and survival of A. niger.
Key words: Aspergillus niger, morphogenesis, polar growth, TOR signaling, RmsA, Sin1, Avo1, environmental stress, metabolism
Aspergillus niger is a eukaryotic microorganism belonging to the group of filamentous fungi, which are naturally capable of secreting large amounts of proteins and metabolites. Therefore, A. niger and other filamentous fungi are exploited in biotechnology as cell factories for the commercial production of chemicals, pharmaceuticals and enzymes.1 Growth of filamentous fungi is in general characterised by the formation of highly polarised hyphae which send out branches from apical or lateral regions thereby forming a highly dense cellular meshwork, the mycelium. Long-distance transport of RNA, proteins, vesicles and organelles along cytoskeletal tracks ensures that new cell material is exclusively added to the growth zone at the hyphal tip. Thus, transport along microtubules and actin is essential to maintain the morphology of filamentous fungi and has also been shown to be a prerequisite for polarised protein secretion.2–5
Recently, we demonstrated that the RmsA protein of A. niger plays a key role in the maintenance of hyphal polarity.6 We obtained evidence that RmsA is a functional equivalent of the Avo1p/Sin1 protein, which as a component of the highly conserved protein complex TORC2 is essential for actin polarisation and hence determination of cell polarity in Saccharomyces cerevisiae, Dictyostelium and mammals.7 By using the strain ramosa-1, which carries a temperature-sensitive allele of RmsA (RmsAY447N), we could show that disturbed RmsA function causes actin depolarisation and thereby loss of hyphal polarity. A. niger cells, however, are very dynamic in counteracting this event—if the integrity of the polar axis is disturbed, two new polarity axis become established resulting in apically branched tips. The transcriptomic fingerprint of apically branched hyphae suggested that the stage for actin repolarisation and formation of two new branches is set by increased activity of different signaling pathways such as TORC2 signaling, phospholipid signaling, cell wall integrity signaling and calcium signaling.6
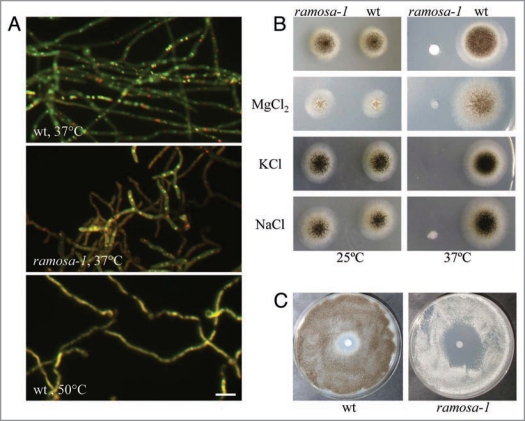
Surprisingly, the transcriptomic fingerprint obtained uncovered that inactivation of RmsA also results in considerable changes in the transcription of genes predicted to function in ion homeostasis, energy metabolism and protein fate,6 which led us to speculate that the function of RmsA is not only important for actin polarisation but also to ensure redox balance and metabolic integrity of A. niger. Supportive for this assumption is the observation that prolonged cultivation of ramosa-1 at restrictive temperature slows down its growth rate, prevents asexual development and that deletion of the rmsA gene is lethal for A. niger.6,8 To further substantiate the hypothesis that RmsA is crucial for the metabolism of A. niger, we cultivated ramosa-1 and the respective wild type strain T312 at restrictive temperature (37°C) and subjected it to staining with the vital dye FUN-1. Living and metabolically active cells metabolise FUN-1 to red fluorescent intravacuolar structures known as CIVS. CIVS formation is thus a reflection of the metabolic activity of a cell and depends on intact cellular ATP synthesis and on functional vesicle cycling between the prevacuolar compartment and the Golgi.9,10 As shown in Figure 1A, loss of RmsA activity results in hyphae with reduced CIVS formation, suggesting that RmsA is of vital importance for the energy status of A. niger hyphae. As, moreover, exposure of ramosa-1 to different salts (NaCl, KCl, MgCl2, LiCl), oxidative agents (H2O2) and cell wall stress inducing compounds (Calcofluor white) results in a hypersensitive phenotype (Fig. 1B and C and data not shown), indicates that RmsA also safeguards A. niger against environmental stress.
Figure 1.
Phenotypic analyses of ramosa-1 and the wild type strain T312. (A) Spores of both strains were allowed to germinate for 17 h at 25°C, after which the temperature was set to 37°C for 4 h. Hyphae were stained with 2 µM FUN-1 and subjected to microscopy using FITC and dsRed filters. FUN-1 stains nucleic acids yielding to a diffuse green cytoplasmic fluorescence. Only metabolic active cells transport FUN-1 to the vacuole and convert it to red fluorescent CIVS.9 A control staining is shown in the lower panel where the wt strain was heat-inactivated for 4 h at 50°C. Bar, 10 µm. (B) 104 spores of both strains were point-inoculated on minimal agar plates containing 500 mM of different salts. Control plates did not contain any additional supplements (upper). Plates were incubated at 25°C for 4 days or at 37°C for 3 days. (C) 5 × 105 spores of both strains were evenly spread on minimal agar plates. A Whatman paper disc was wetted with 5 µl of a 30% H2O2 solution and the plates were incubated at 37°C for 3 days.
In summary, these studies highlight multiple cellular roles of RmsA. Not only spatial growth and thereby morphogenesis of A. niger is dependent on RmsA, also temporal growth, viability and survival of A. niger require an intact RmsA protein. But how does RmsA achieve so many functions? In all organisms studied so far, the homologous Avo1p/Sin1 proteins have been identified as a stabilising component of the TORC2 complex. However, the function of TORC2 is not conserved, e.g., in the S. cerevisiae, TORC2 is crucial for actin polarisation but not involved in sexual reproduction; in the fission yeast Schizzosaccharomyces pombe, TORC2 is required for sexual development but dispensable for proper actin polarisation.7 In mammalian cells, TORC2 seems to exert its most complex function—it is important for many processes including actin polarisation, salt balance, metabolism and survival.7 In addition to a role in TORC2 signaling, Avo1p/Sin1 proteins have been reported to interact with other proteins, e.g., with stress-activated protein kinases in S. pombe and humans,11,12 with S. cerevisiae and human Ras GTPases,13,14 with the apoptosis-related human transcription factor ATF-2,11 and with the human antiapoptotic RNA binding protein PCBP2,15 suggesting that Avo1p/Sin1 proteins orchestrate organism-specifically many cellular processes. With our recent genetic, transcriptomic and phenotypic studies on ramosa-1 and the RmsA protein, we got only a first glimpse of the role(s) of RmsA for A. niger. Future studies will disclose on how RmsA is embedded in different signaling pathways and participates in driving growth, morphology and development of A. niger.
Acknowledgements
This project was carried out within the research programme of the Kluyver Centre for Genomics of Industrial Fermentation which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/10983
References
- 1.Meyer V. Genetic engineering of filamentous fungi—progress, obstacles and future trends. Biotechnol Adv. 2008;26:177–185. doi: 10.1016/j.biotechadv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Torralba S, Raudaskoski M, Pedregosa AM, Laborda F. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology. 1998;144:45–53. doi: 10.1099/00221287-144-1-45. [DOI] [PubMed] [Google Scholar]
- 3.Torralba S, Pedregosa AM, De Lucas JR, Diaz MS, Monistrol IF, Laborda F. Effect of the microtubule inhibitor methyl benzimidazol-2-yl carbamate (MBC) on production and secretion of enzymes in Aspergillus nidulans. Mycol Res. 1996;100:1375–1382. [Google Scholar]
- 4.Harris SD. Branching of fungal hyphae: regulation, mechanisms and comparison with other branching systems. Mycologia. 2008;100:823–832. doi: 10.3852/08-177. [DOI] [PubMed] [Google Scholar]
- 5.Harris SD. Cell polarity in filamentous fungi: shaping the mold. Int Rev Cytol. 2006;251:41–77. doi: 10.1016/S0074-7696(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 6.Meyer V, Arentshorst M, Flitter SJ, Nitsche BM, Kwon MJ, Reynaga-Pena CG, et al. Reconstruction of signalling networks regulating fungal morphogenesis by transcriptomics. Eukaryot Cell. 2009 doi: 10.1128/EC.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 8.Reynaga-Pena CG, Bartnicki-Garcia S. Apical branching in a temperature sensitive mutant of Aspergillus niger. Fungal Genet Biol. 1997;22:153–167. doi: 10.1006/fgbi.1997.1003. [DOI] [PubMed] [Google Scholar]
- 9.Millard PJ, Roth BL, Thi HP, Yue ST, Haugland RP. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol. 1997;63:2897–2905. doi: 10.1128/aem.63.7.2897-2905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essary BD, Marshall PA. Assessment of FUN-1 vital dye staining: Yeast with a block in the vacuolar sorting pathway have impaired ability to form CIVS when stained with FUN-1 fluorescent dye. J Microbiol Methods. 2009;78:208–212. doi: 10.1016/j.mimet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Makino C, Sano Y, Shinagawa T, Millar JB, Ishii S. Sin1 binds to both ATF-2 and p38 and enhances ATF-2-dependent transcription in an SAPK signaling pathway. Genes Cells. 2006;11:1239–1251. doi: 10.1111/j.1365-2443.2006.01016.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson MG, Pino TS, Tournier S, Buck V, Martin H, Christiansen J, et al. Sin1: an evolutionarily conserved component of the eukaryotic SAPK pathway. EMBO J. 1999;18:4210–4221. doi: 10.1093/emboj/18.15.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colicelli J, Nicolette C, Birchmeier C, Rodgers L, Riggs M, Wigler M. Expression of three mammalian cDNAs that interfere with RAS function in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:2913–2917. doi: 10.1073/pnas.88.7.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder WA, Buck M, Cloonan N, Hancock JF, Suhrbier A, Sculley T, Bushell G. Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell Signal. 2007;19:1279–1289. doi: 10.1016/j.cellsig.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh D, Srivastava GP, Xu D, Schulz LC, Roberts RM. A link between SIN1 (MAPKAP1) and poly(rC) binding protein 2 (PCBP2) in counteracting environmental stress. Proc Natl Acad Sci USA. 2008;105:11673–11678. doi: 10.1073/pnas.0803182105. [DOI] [PMC free article] [PubMed] [Google Scholar]