Abstract
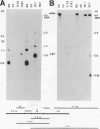
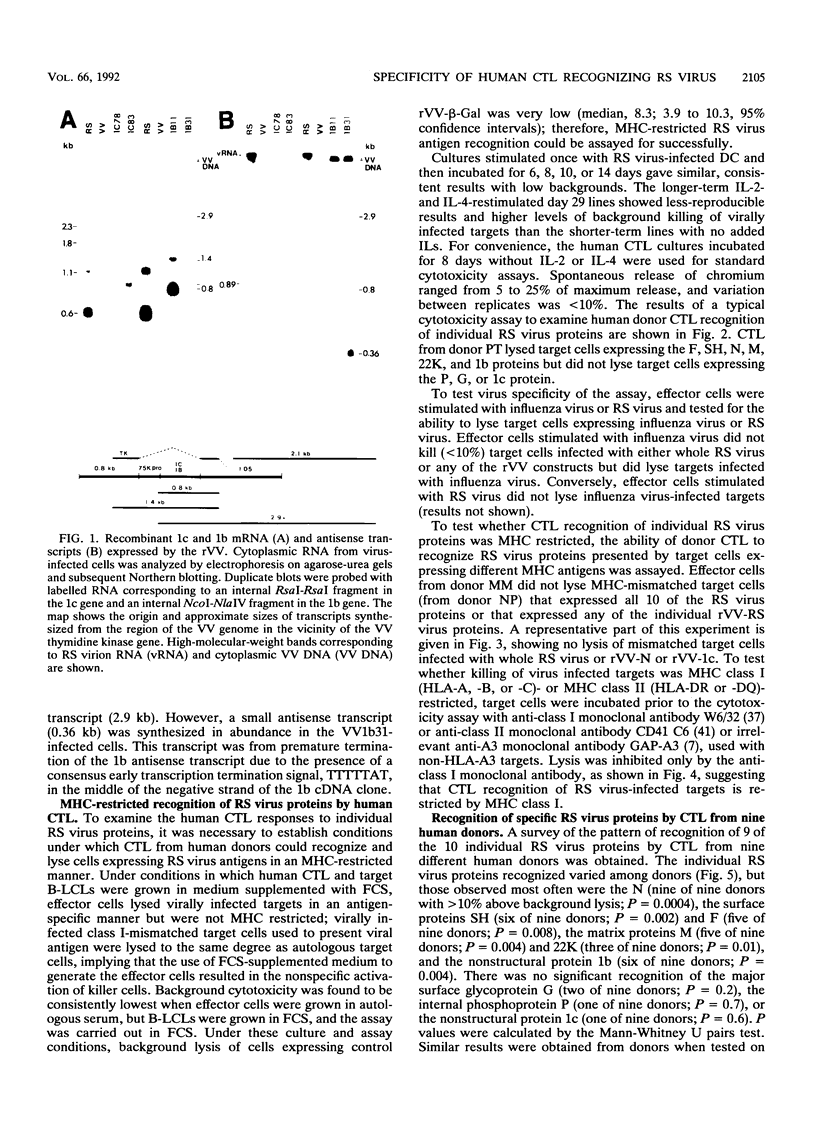
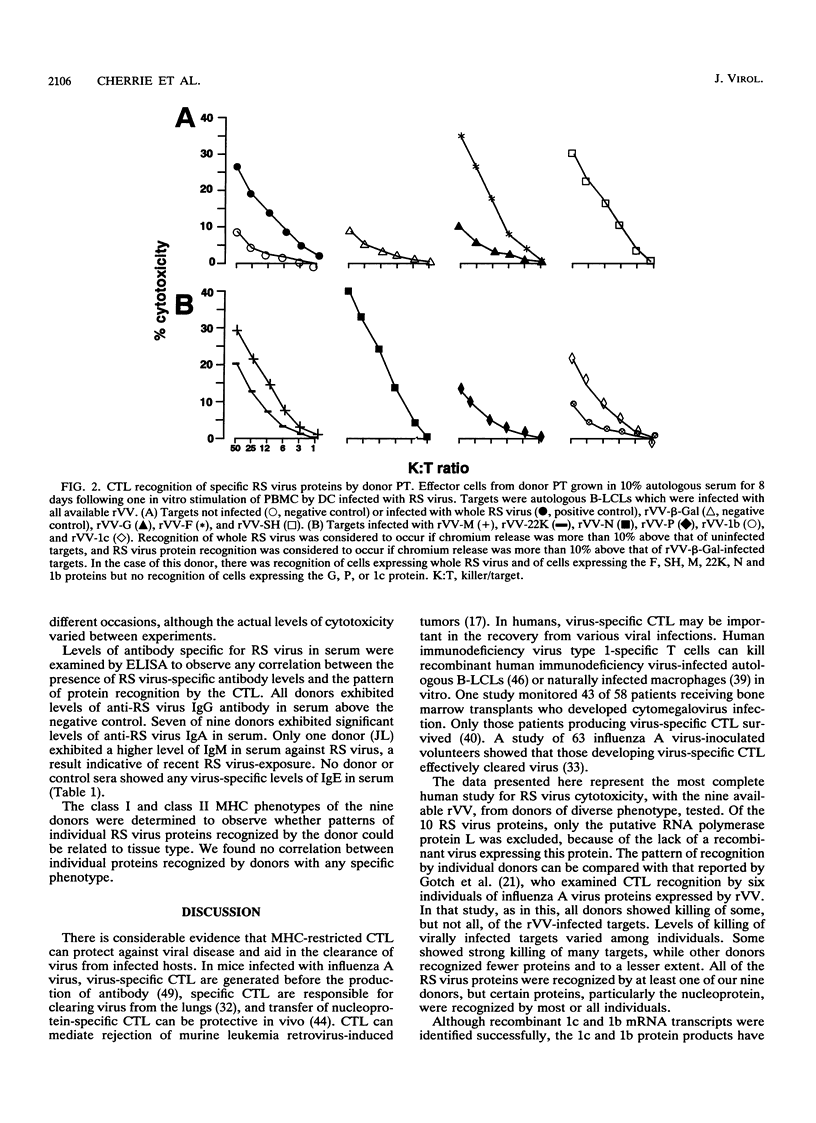
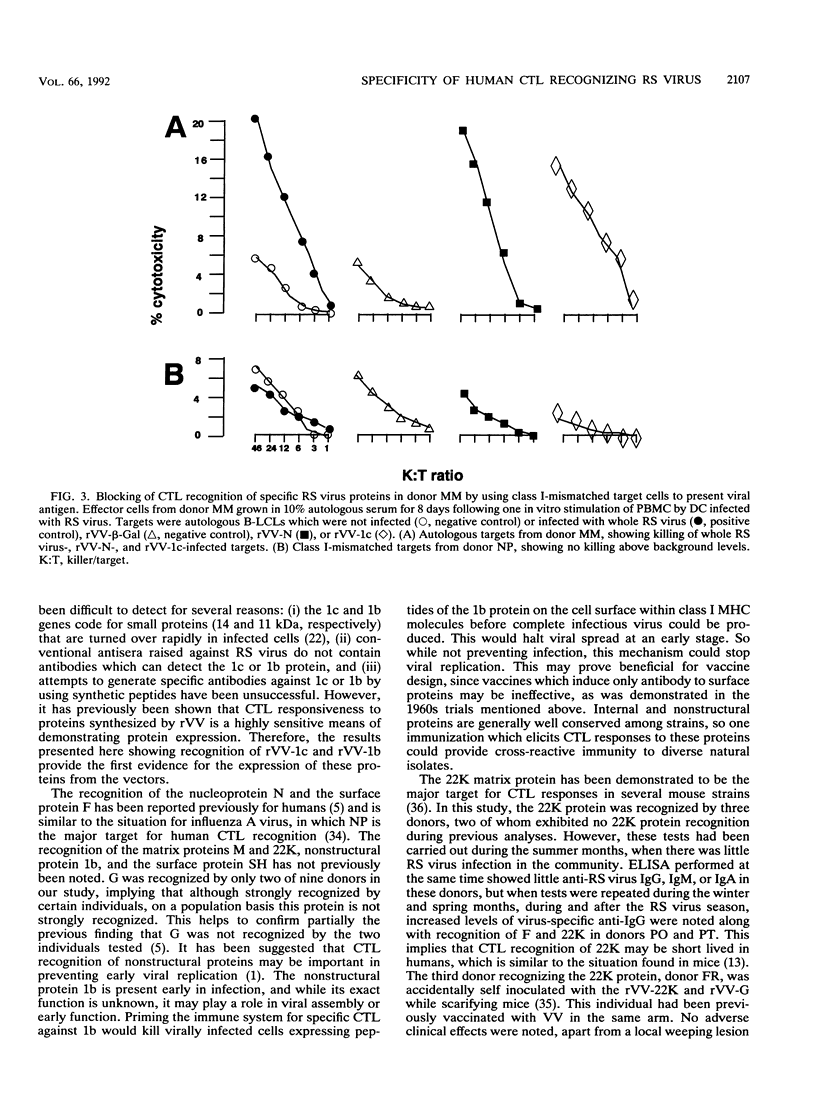
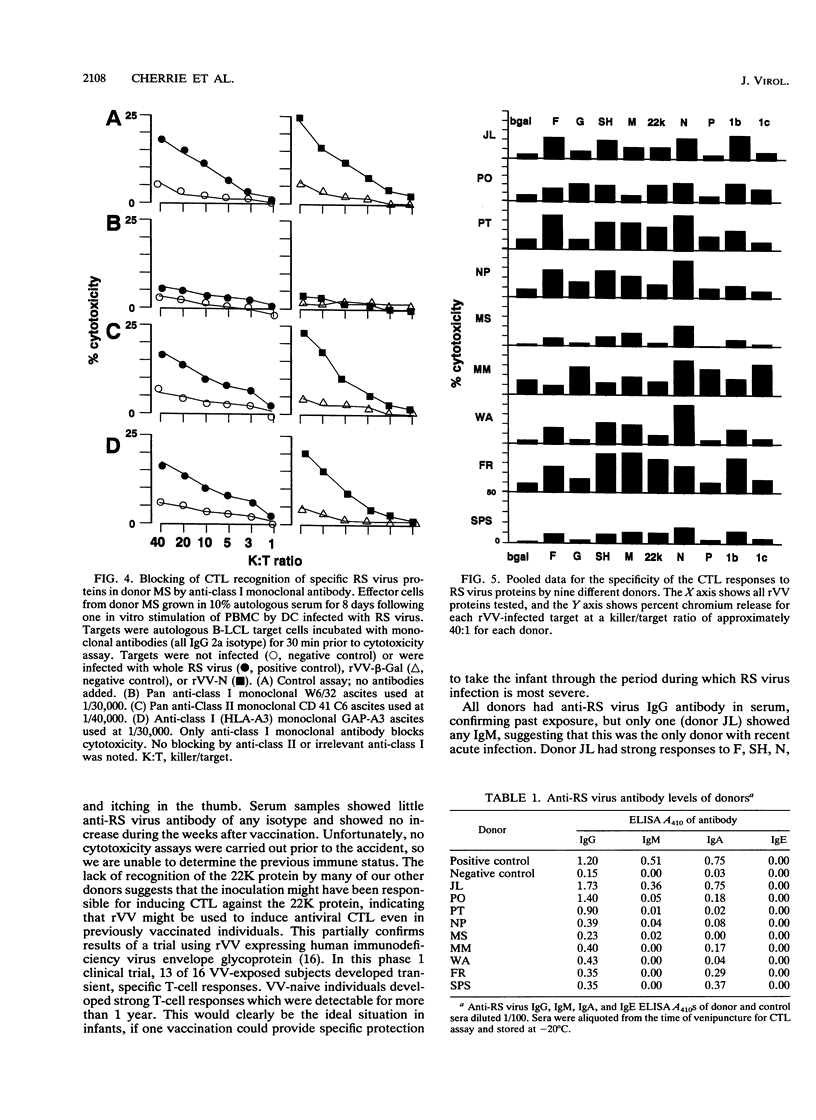
We examined the human cytotoxic T-cell repertoire of nine adults to 9 of the 10 proteins of respiratory syncytial (RS) virus. Peripheral blood mononuclear cells from normal adults were stimulated with RS virus in vitro. The resulting polyclonal cultures were tested for lysis of B-lymphoblastoid cell lines infected with recombinant vaccinia viruses expressing each of nine individual RS virus proteins. The use of peripheral blood dendritic cells to present antigen gave more easily reproducible results over a shorter culture period than conventional methods. The six RS virus proteins most strongly recognized were the nucleoprotein N (nine of nine donors with greater than 10% above background lysis; P = 0.0004), the surface proteins SH (six of nine donors; P = 0.002) and F (five of nine donors; P = 0.008), the matrix proteins M (five of nine donors; P = 0.004) and 22K (three of nine donors; P = 0.01) and the nonstructural protein 1b (six of nine donors; P = 0.004). There was no significant recognition of the major surface glycoprotein G (two of nine donors), the internal phosphoprotein P (one of nine donors), or the nonstructural protein 1c (one of nine donors). Recognition was major histocompatibility complex class I restricted, but no association between major histocompatibility complex phenotype and protein specificity of T cells was seen. Recognition of F and 22K appeared to be associated with recent infection indicated by increased levels of anti-RS virus immunoglobulin G antibody in serum measured by enzyme-linked immunosorbent assay. Since cytotoxic T-cell recognition of RS virus proteins has been demonstrated to be important in the clearance of virus from infected hosts, the N, M, SH, 1b, F, and 22K proteins should be considered potential vaccine components.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., McMichael A. J. Specific human cytotoxic T cells recognize B-cell lines persistently infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9183–9187. doi: 10.1073/pnas.83.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Beasley R., Coleman E. D., Hermon Y., Holst P. E., O'Donnell T. V., Tobias M. Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax. 1988 Sep;43(9):679–683. doi: 10.1136/thx.43.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J. Microplaque immunoperoxidase detection of infectious respiratory syncytial virus in the lungs of infected mice. J Virol Methods. 1987 Jul;16(4):293–301. doi: 10.1016/0166-0934(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Stott E. J., Taylor G., Askonas B. A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987 Sep;62(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Identification of a tenth mRNA of respiratory syncytial virus and assignment of polypeptides to the 10 viral genes. J Virol. 1984 Feb;49(2):572–578. doi: 10.1128/jvi.49.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Lahaye T., Sorenson G. D., Glasebrook A. L., Horvath C., Brunner K. T. Functional activity in vivo of effector T cell populations. II. Anti-tumor activity exhibited by syngeneic anti-MoMULV-specific cytolytic T cell clones. J Immunol. 1984 Sep;133(3):1664–1670. [PubMed] [Google Scholar]
- Freke A., Stott E. J., Roome A. P., Caul E. O. The detection of respiratory syncytial virus in nasopharyngeal aspirates: assessment, formulation, and evaluation of monoclonal antibodies as a diagnostic reagent. J Med Virol. 1986 Feb;18(2):181–191. doi: 10.1002/jmv.1890180210. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Gotch F., McMichael A., Smith G., Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987 Feb 1;165(2):408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Collins P. L., Wertz G. W. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 1985 Mar;2(2):157–173. doi: 10.1016/0168-1702(85)90246-1. [DOI] [PubMed] [Google Scholar]
- Isaacs D., Bangham C. R., McMichael A. J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987 Oct 3;2(8562):769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Leikin S. L., Arrobio J., Brandt C. D., Chanock R. M., Parrott R. H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976 Jan;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- King A. M., Stott E. J., Langer S. J., Young K. K., Ball L. A., Wertz G. W. Recombinant vaccinia viruses carrying the N gene of human respiratory syncytial virus: studies of gene expression in cell culture and immune response in mice. J Virol. 1987 Sep;61(9):2885–2890. doi: 10.1128/jvi.61.9.2885-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. D., Taylor P. M., Askonas B. A. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology. 1989 Jul;67(3):375–381. [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J., Alwan W. H., Cherrie A. H., Record F. M. Accidental infection of laboratory worker with recombinant vaccinia virus. Lancet. 1991 Aug 17;338(8764):459–459. doi: 10.1016/0140-6736(91)91093-a. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Pemberton R. M., Cannon M. J., Openshaw P. J., Ball L. A., Wertz G. W., Askonas B. A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987 Aug;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Sachs J. A., Fernandez N., Kurpisz M., Okoye R., Ogilvie J., Awad J., Labeta M., Festenstein H. Serological biochemical and functional characterisation of three different HLA-DR monoclonal antibodies derived from C57BL6 mice. Tissue Antigens. 1986 Oct;28(4):199–207. doi: 10.1111/j.1399-0039.1986.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Satake M., Venkatesan S. Nucleotide sequence of the gene encoding respiratory syncytial virus matrix protein. J Virol. 1984 Apr;50(1):92–99. doi: 10.1128/jvi.50.1.92-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Toms G. L., Webb M. S., Milner P. D., Milner A. D., Routledge E. G., Scott R., Stokes G. M., Swarbrick A., Taylor C. E. IgG and IgM antibodies to viral glycoproteins in respiratory syncytial virus infections of graded severity. Arch Dis Child. 1989 Dec;64(12):1661–1665. doi: 10.1136/adc.64.12.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Kaul A., Ogra P. L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979 Mar;94(3):370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Stott E. J., Young K. K., Anderson K., Ball L. A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987 Feb;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]