Abstract
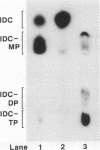
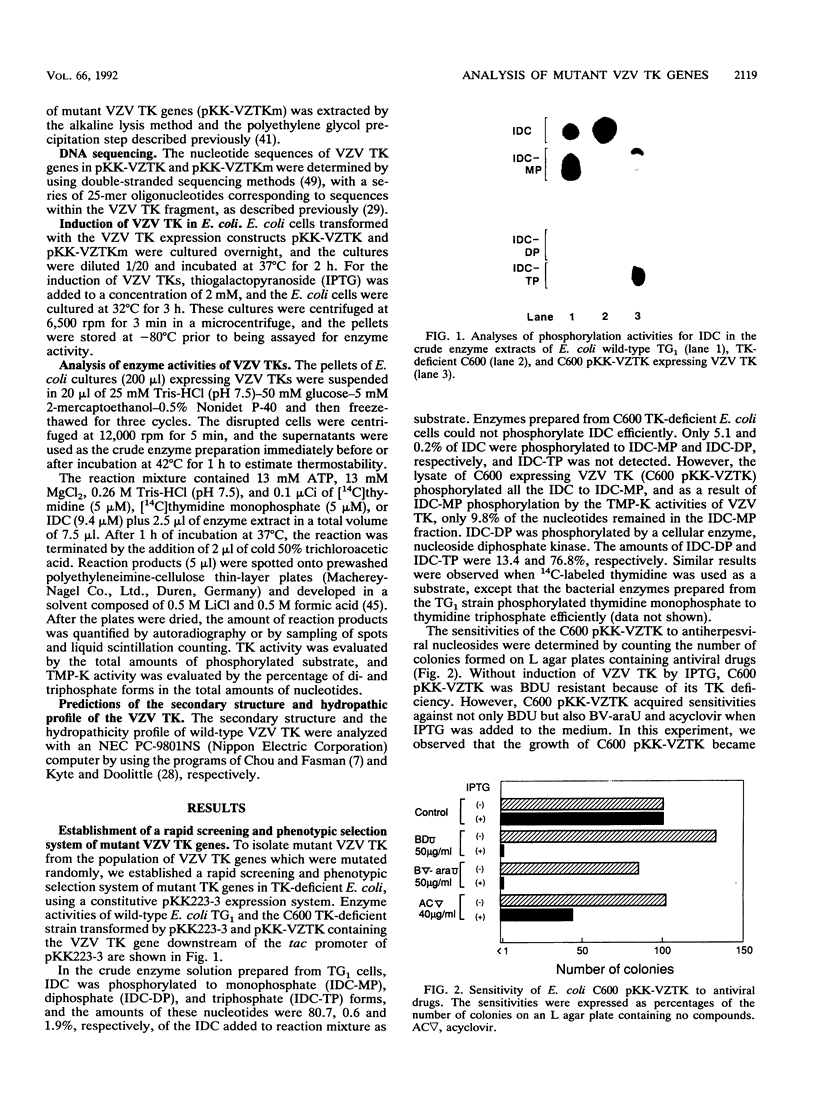
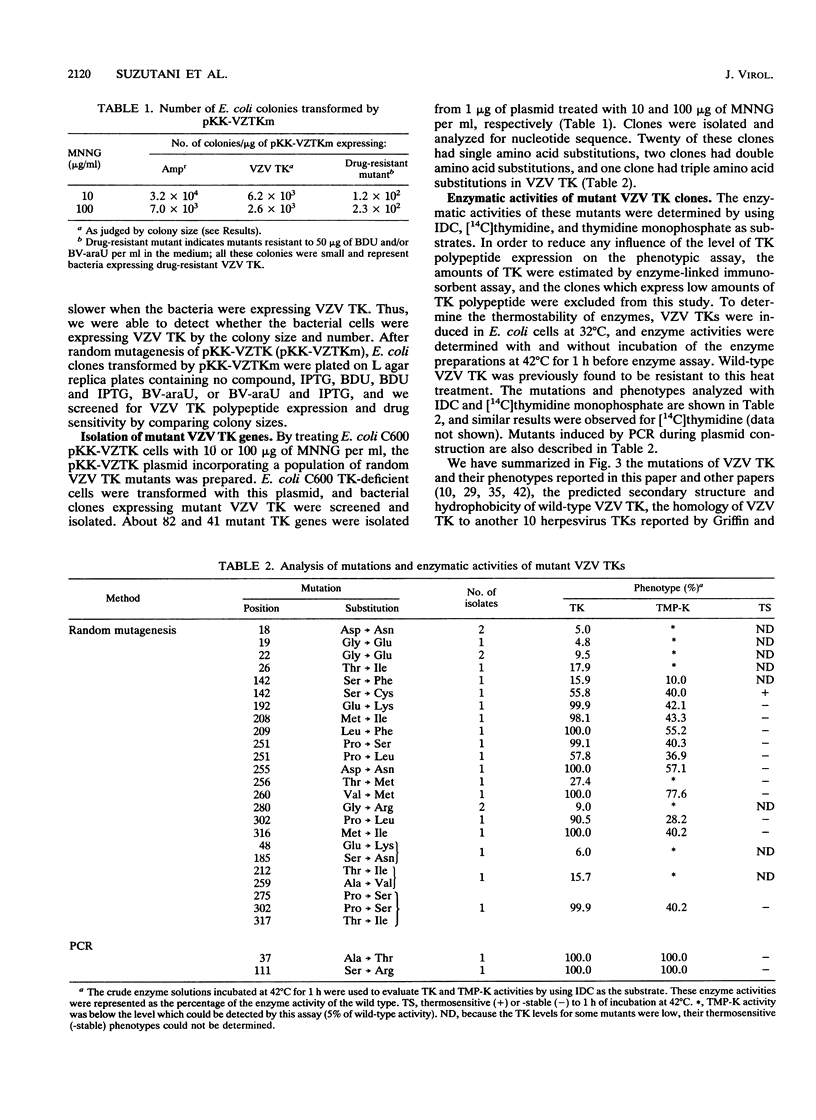
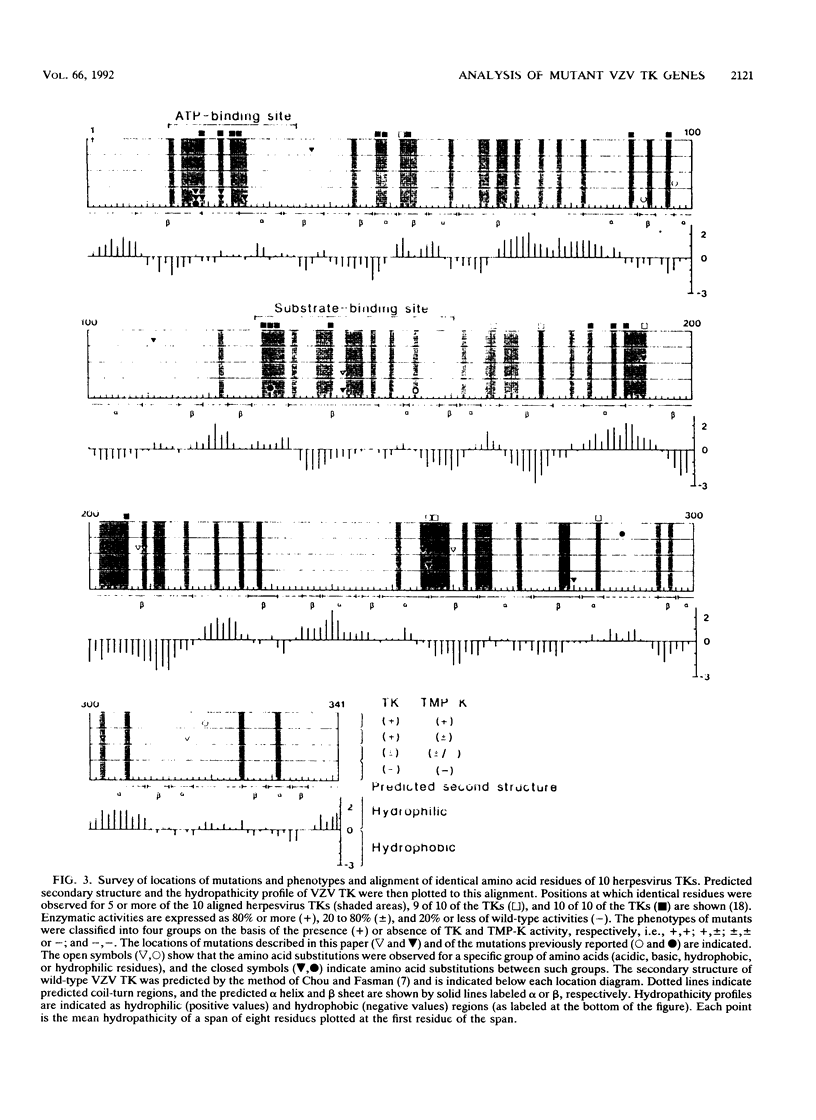
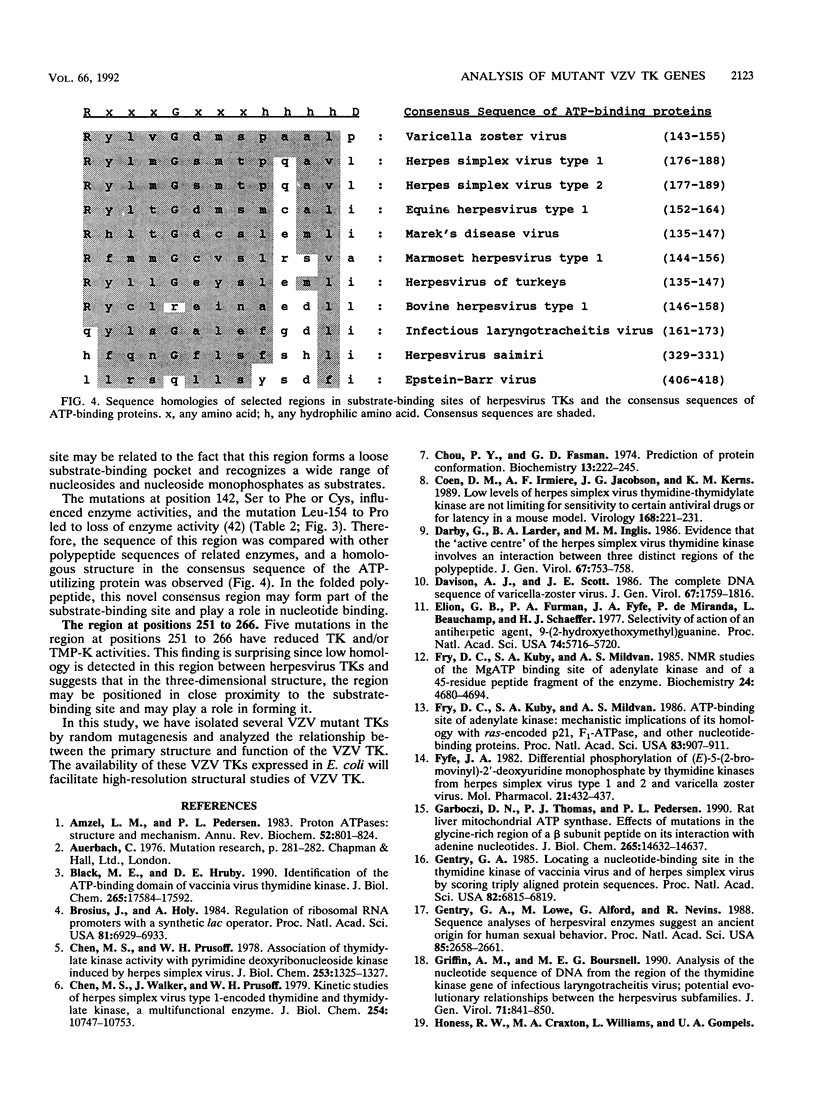
To understand the relationship between the primary structure and function of varicella-zoster virus thymidine kinase (VZV TK; EC 2.7.1.21), we established rapid screening and phenotypic selection of mutant VZV TK genes in TK-deficient Escherichia coli C600 by using a constitutive pKK223-3 expression plasmid. In this screening system, mutant TK genes generated by random mutagenesis were identified by the sensitivity of E. coli-expressing VZV TKs to 5-bromo-2'-deoxyuridine and 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl) uracil. Twenty-four mutant clones with amino acid substitutions were isolated, and their nucleotide sequence and enzymatic activities were determined. Of the 24 clones, 20 had single amino acid substitutions, 2 clones had double amino acid substitutions, and 1 clone had triple amino acid substitutions. In 17 cases of single amino acid substitution, six mutations led to lost enzyme activity, and four of these six mutations centered in the ATP-binding site. The other 11 mutations resulted in reduction of both TK and thymidylate kinase activities or only thymidylate kinase activity and were located in scattered positions in the VZV TK gene, although 5 mutations showed a tendency to cluster in the region between positions 251 and 260.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Pedersen P. L. Proton atpases: structure and mechanism. Annu Rev Biochem. 1983;52:801–824. doi: 10.1146/annurev.bi.52.070183.004101. [DOI] [PubMed] [Google Scholar]
- Black M. E., Hruby D. E. Identification of the ATP-binding domain of vaccinia virus thymidine kinase. J Biol Chem. 1990 Oct 15;265(29):17584–17592. [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Chen M. S., Walker J., Prusoff W. H. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J Biol Chem. 1979 Nov 10;254(21):10747–10753. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. NMR studies of the MgATP binding site of adenylate kinase and of a 45-residue peptide fragment of the enzyme. Biochemistry. 1985 Aug 13;24(17):4680–4694. doi: 10.1021/bi00338a030. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Garboczi D. N., Thomas P. J., Pedersen P. L. Rat liver mitochondrial ATP synthase. Effects of mutations in the glycine-rich region of a beta subunit peptide on its interaction with adenine nucleotides. J Biol Chem. 1990 Aug 25;265(24):14632–14637. [PubMed] [Google Scholar]
- Gentry G. A. Locating a nucleotide-binding site in the thymidine kinase of vaccinia virus and of herpes simplex virus by scoring triply aligned protein sequences. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6815–6819. doi: 10.1073/pnas.82.20.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Lowe M., Alford G., Nevins R. Sequence analyses of herpesviral enzymes suggest an ancient origin for human sexual behavior. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2658–2661. doi: 10.1073/pnas.85.8.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Griffin A. M., Boursnell M. E. Analysis of the nucleotide sequence of DNA from the region of the thymidine kinase gene of infectious laryngotracheitis virus; potential evolutionary relationships between the herpesvirus subfamilies. J Gen Virol. 1990 Apr;71(Pt 4):841–850. doi: 10.1099/0022-1317-71-4-841. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Craxton M. A., Williams L., Gompels U. A. A comparative analysis of the sequence of the thymidine kinase gene of a gammaherpesvirus, herpesvirus saimiri. J Gen Virol. 1989 Nov;70(Pt 11):3003–3013. doi: 10.1099/0022-1317-70-11-3003. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Karkaria C. E., Chen C. M., Rosen B. P. Mutagenesis of a nucleotide-binding site of an anion-translocating ATPase. J Biol Chem. 1990 May 15;265(14):7832–7836. [PubMed] [Google Scholar]
- Karlström A. R., Gronowitz J. S. An optimized thymidylate kinase assay, based on enzymatically synthesized 5-[125I]iododeoxyuridine monophosphate and its application to an immunological study of herpes simplex virus thymidine-thymidylate kinases. Anal Biochem. 1987 May 1;162(2):500–510. doi: 10.1016/0003-2697(87)90426-x. [DOI] [PubMed] [Google Scholar]
- Kironde F. A., Parsonage D., Senior A. E. Random mutagenesis of the gene for the beta-subunit of F1-ATPase from Escherichia coli. Biochem J. 1989 Apr 15;259(2):421–426. doi: 10.1042/bj2590421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Dubbs D. R. Distinctive properties of thymidine kinase isozymes induced by human and avian hepresviruses. Int J Cancer. 1974 Nov 15;14(5):598–610. doi: 10.1002/ijc.2910140506. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D., Jorgensen G. Gel electrophoresis and isoelectric focusing of mitochondrial and viral-induced thymidine kinases. Int J Cancer. 1974 Feb 15;13(2):203–218. doi: 10.1002/ijc.2910130208. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase. Microbiol Sci. 1985 Dec;2(12):369–375. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lacey S. F., Suzutani T., Powell K. L., Purifoy D. J., Honess R. W. Analysis of mutations in the thymidine kinase genes of drug-resistant varicella-zoster virus populations using the polymerase chain reaction. J Gen Virol. 1991 Mar;72(Pt 3):623–630. doi: 10.1099/0022-1317-72-3-623. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Summers W. C. Site-directed mutagenesis of a nucleotide-binding domain in HSV-1 thymidine kinase: effects on catalytic activity. Virology. 1988 Apr;163(2):638–642. doi: 10.1016/0042-6822(88)90308-x. [DOI] [PubMed] [Google Scholar]
- Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986 Mar;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec-Baron A., Baron G., Sampérez S., Jouan P. Mise en évidence d'une forme inédite de la thymidine kinase dans les cancers du sein. C R Acad Sci III. 1989;309(17):669–673. [PubMed] [Google Scholar]
- Martignetti J. A., Barrell B. G. Sequence of the HindIII T fragment of human cytomegalovirus, which encodes a DNA helicase. J Gen Virol. 1991 May;72(Pt 5):1113–1121. doi: 10.1099/0022-1317-72-5-1113. [DOI] [PubMed] [Google Scholar]
- Mori H., Shiraki K., Kato T., Hayakawa Y., Yamanishi K., Takahashi M. Molecular analysis of the thymidine kinase gene of thymidine kinase-deficient mutants of varicella-zoster virus. Intervirology. 1988;29(6):301–310. doi: 10.1159/000150060. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Kit S. Nucleotide sequence of the marmoset herpesvirus thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide. Virology. 1984 Jun;135(2):316–330. doi: 10.1016/0042-6822(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Parsonage D., Wilke-Mounts S., Senior A. E. E. coli F1-ATPase: site-directed mutagenesis of the beta-subunit. FEBS Lett. 1988 May 9;232(1):111–114. doi: 10.1016/0014-5793(88)80397-1. [DOI] [PubMed] [Google Scholar]
- Robertson G. R., Whalley J. M. Evolution of the herpes thymidine kinase: identification and comparison of the equine herpesvirus 1 thymidine kinase gene reveals similarity to a cell-encoded thymidylate kinase. Nucleic Acids Res. 1988 Dec 9;16(23):11303–11317. doi: 10.1093/nar/16.23.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T. Strains of varicella-zoster virus resistant to 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Antimicrob Agents Chemother. 1984 Jun;25(6):742–746. doi: 10.1128/aac.25.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Inchauspe G., Biron K. K., Waters D. J., Straus S. E., Ostrove J. M. Molecular analysis of the pyrimidine deoxyribonucleoside kinase gene of wild-type and acyclovir-resistant strains of varicella-zoster virus. J Gen Virol. 1988 Oct;69(Pt 10):2585–2593. doi: 10.1099/0022-1317-69-10-2585. [DOI] [PubMed] [Google Scholar]
- Scott S. D., Ross N. L., Binns M. M. Nucleotide and predicted amino acid sequences of the Marek's disease virus and turkey herpesvirus thymidine kinase genes; comparison with thymidine kinase genes of other herpesviruses. J Gen Virol. 1989 Nov;70(Pt 11):3055–3065. doi: 10.1099/0022-1317-70-11-3055. [DOI] [PubMed] [Google Scholar]
- Smith G. L., de Carlos A., Chan Y. S. Vaccinia virus encodes a thymidylate kinase gene: sequence and transcriptional mapping. Nucleic Acids Res. 1989 Oct 11;17(19):7581–7590. doi: 10.1093/nar/17.19.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzutani T., Machida H., Sakuma T., Azuma M. Effects of various nucleosides on antiviral activity and metabolism of 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1988 Oct;32(10):1547–1551. doi: 10.1128/aac.32.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzutani T., Machida H., Sakuma T. Efficacies of antiherpesvirus nucleosides against two strains of herpes simplex virus type 1 in Vero and human embryo lung fibroblast cells. Antimicrob Agents Chemother. 1988 Jul;32(7):1046–1052. doi: 10.1128/aac.32.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Konno K., Mori S., Shigeta S., Kumagai M., Watanabe Y., Machida H. Mechanism of selective inhibition of varicella zoster virus replication by 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Mol Pharmacol. 1989 Aug;36(2):312–316. [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]