Abstract
Appropriate control of apoptosis during T lymphocyte differentiation is critical for destruction of T cells bearing potentially autoreactive or useless immuno-receptors, and for survival of those T cells bearing antigen receptors that may recognize foreign proteins. Despite the well-established importance of thymocyte survival, the exact signals regulating thymocyte apoptosis have not been fully elucidated. Here, we show that thymocytes lacking the endoplasmic reticulum (ER) protein CAML failed to undergo normal T cell development and exhibited dramatically increased rates of apoptosis. In vitro, CAML-deficient thymocytes accumulated high levels of reactive oxygen species (ROS) and underwent abnormally accelerated death in response to several cytotoxic stimuli, including treatment with etoposide, cytokine deprivation or Fas ligation. Although neither p53 deletion nor loss of Fas rescued the survival and continued development of CAML-deficient thymocytes, removal of the pro-apoptotic BH3-only Bcl-2 family member Bim significantly restored their survival. This work reveals CAML to be a critically important regulator of ROS- and Bim-dependent thymocyte death.
Keywords and Abbreviations: CAML: Calcium modulating cyclophilin ligand, ROS: reactive oxygen species, Bim: Bcl-2 interacting mediator of cell death
Introduction
Cell survival during T cell development is a carefully controlled process. Early in development, signals through the pre-T cell receptor (pre-TCR, composed of TCRβ and pTα) promote proliferation, survival and differentiation of immature (CD4−8−) progenitors. Further rearrangement of the α chain of the TCR completes the assembly of the T cell antigen receptor, and thymocytes are then subjected to stringent selection based on its specificity. Cells bearing a TCR with low affinity for self-peptide bound to a self-MHC molecule receive a survival signal and undergo continued differentiation, a process termed positive selection. Thymocytes with receptors that have high affinity for self-peptide MHC complexes undergo apoptosis in a process called negative selection, while those cells that are unable to bind any ligand undergo death by neglect (1). The developing thymocyte has a window of opportunity during which to sample a variety of ligand/MHC complexes and receive a positively selecting signal before it undergoes programmed cell death, but the signals maintaining thymocyte viability and the pathways that control cell survival or death in response to these interactions are not well understood.
Mammalian cells, including thymocytes, have two distinct, albeit ultimately converging, pathways of apoptosis, the ‘extrinsic’ (‘death receptor’-controlled) pathway and the ‘intrinsic’ (also called ‘mitochondrial’ or ‘Bcl-2-regulated) pathway (2). The ‘extrinsic’ pathway is activated by certain members of the TNF family of ligands that bind to so-called ‘death receptors’ (members of the TNF receptor family with an intracellular ‘death domain’) on the cell surface, triggering a cascade of intracellular events that result in cell death (2). Conversely, the ‘intrinsic’ apoptotic pathway is regulated by the balance between pro- and anti-apoptotic members of the Bcl-2 family. The pro-survival Bcl-2 family members – Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1 – share with each other up to 4 regions of homology (BH: Bcl-2 Homology regions) and are essential for cell survival functioning in a cell type-specific manner. There are two pro-apoptotic subgroups within the Bcl-2 family. Bak, Bax and possibly also Bok have 3 BH domains and share surprising structural similarity with their pro-survival relatives but are essential (in a redundant manner) for downstream events in apoptosis, including mitochondrial outer membrane permeabilization (MOMP) and consequent activation of the caspase cascade. BH3-only proteins, on the other hand, share with each other and the remainder of the Bcl-2 family only the BH3 domain and they are critical for initiation of apoptosis signaling, functioning in a cell death stimulus- and cell type-specific manner (2).
Bcl-2 interacting mediator of cell death (Bim) is a BH3-only Bcl-2 family member that is transcriptionally and/or post-translationally induced in response to diverse apoptotic stimuli, such as cytokine deprivation or deregulated calcium flux, in a broad range of hematopoeitic, epithelial and neuronal cell types (3–5). Experiments with gene-targeted mice showed that Bim is required for the death of thymocytes in response to growth factor withdrawal, calcium flux, taxol treatment, as well as strong T cell receptor ligation (6, 7). Bim is also critical for the apoptosis of peripheral T cells during the contraction phase of an acute immune response (8–10). As a potent mediator of cell death, Bim is highly regulated both transcriptionally and post-translationally. Bim is found in three major isoforms that have differing levels of pro-apoptotic activity (4) and are each regulated by various post-translational mechanisms, including phosphorylation (11), proteosomal degradation (12) and sequestration to the microtubular dynein motor complex (13). While much is known about Bim regulation, questions still remain as to which signaling pathways directly inhibit or promote Bim activity.
CAML is a transmembrane protein that localizes to the endoplasmic reticulum and small cytoplasmic vesicles (14, 15). It is ubiquitously expressed and evolutionarily highly conserved in all vertebrates. The exact cellular function of CAML is still unknown, but it has been implicated in diverse cell signaling processes, including calcium signaling (14), protein trafficking (16) and chromosome segregation (17). A CAML conditional knockout mouse in which CAML was deleted relatively late during thymic T cell development has been described (18). In that study, using CAMLfl/fl mice expressing the jmlckcre transgene, it was observed that CAML-deficient cells experienced increased T cell receptor stimulation, but that the overall processes of positive and negative selection were intact (19). While previous work suggested that CAML-deficient cells were more susceptible to apoptosis in response to T cell receptor stimulation compared to control cells, there was no direct evidence that CAML-deficient CD4+CD8+ (double positive; DP) thymocytes exhibit abnormally high rates of apoptosis. Furthermore, it was not examined how CAML may interact with apoptotic signaling pathways.
The current study represents the first description of mice in which CAML is deleted during the DN2/DN3 stage of development, considerably earlier than the timing of excision reported for the camlfl/fl mice expressing the jmlckcre transgene. In our study, we observed severe reductions in thymocyte subsets, beginning at the double negative (DN) 4 stage of development, which was due to increased apoptosis and did not result from a defect in cell proliferation. The loss of cellularity was dependent upon the presence of Bim, but did not require T cell receptor ligands (peptide/MHC complexes), p53 or Fas signaling.
Results
Loss of CAML early in T-cell development leads to a severe reduction in thymocyte numbers due to abnormally increased apoptosis
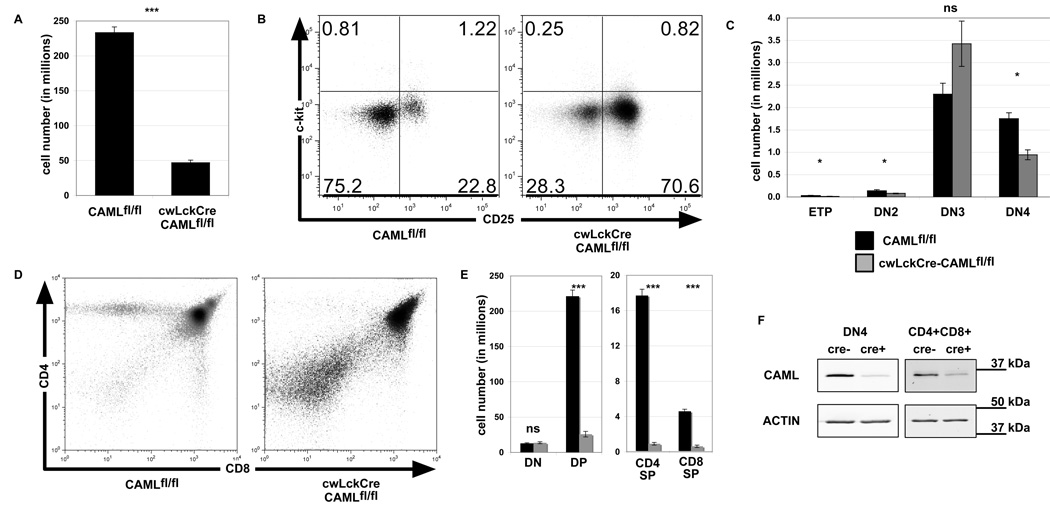
To determine the role of CAML in early T cell development, we crossed mice bearing loxP-flanked Caml alleles (CAMLfl/fl) to cwLckCre mice, which express the Cre recombinase prior to the DN3 stage of thymocyte development (20). The cwLckCre-CAMLfl/fl mice showed a severe depletion of total thymocytes (Figure 1A) in comparison to littermate control CAMLfl/fl mice. To investigate whether early T cell development was impaired by CAML deletion, we examined lineage marker (Mac-1, GR-1, CD8α, TCRβ, TCRγδ, CD3ε, B220, CD19, Ter119, and NK1.1) negative thymocytes for expression of c-kit and CD25 (Figure 1B). Quantitation of the earliest subsets of T cells: (ETPs: lin−, c-kit+, CD25−), DN2 (lin−, c-kit+, CD25+) and DN3 (lin−, c-kit−, and CD25+) indicated that the numbers of these cells were similar between cwLckCre-CAMLfl/fl and control CAMLfl/fl mice. However, the numbers of DN4 cells (lin−, c-kit−, CD25−) were reduced by ~50% in cwLckCre-CAMLfl/fl compared to CAMLfl/fl mice (Figure 1C). By staining thymocytes for expression of CD4 and CD8, we found that loss of CAML caused an 80–90% reduction in the percentages and total numbers of double-positive (CD4+CD8+, DP), CD4 single positive (CD4+CD8−, SP) as well as CD8 SP (CD4−CD8+) subsets compared to littermate controls (Figure 1D–E).
Figure 1. cwLckCre-CAMLfl/fl mice exhibit a defect in thymocyte development, beginning at the DN4 stage.
(A) Thymocytes from three-six week old cwLckCre-CAMLfl/fl mice or littermate controls were tweezed into single cell suspensions and counted. n=50+ for each genotype. (B) Cells were stained for surface expression of lineage markers, CD44, and CD25. Lineage marker (Mac-1 (CD11b), GR-1 (Ly6G/C), CD8α, TCRβ, TCRγδ, CD3ε, B220, CD19, Ter119, and NK1.1) -negative cells were analyzed for c-kit and CD25 expression, as shown in the representative flow cytometry profiles. (C) Lineage-negative subsets were quantified. ETP (lin−, c-kit+, CD25−), DN2 (lin−, c-kit+, CD25+), DN3 (lin−, c-kit−, and CD25+), and DN4 (lin−, c-kit− and CD25−) n=5 for each genotype. (D) Total thymocytes were analyzed for expression of CD4 and CD8, as shown in these representative flow cytometry profiles. (E) CD4 and CD8 subsets were quantified. n=17 for each genotype. Error bars represent standard error of the mean. (F) Total thymocytes were sorted using Miltenyi MACS beads based on expression of CD4, CD8, CD44, and CD25. CD4−CD8−CD44−CD25− (DN4) and CD4+CD8+ cells were isolated and lysed with 1% Triton lysis buffer. Western blots were probed for CAML and actin (loading control) levels.
In comparison to jmlckcre-CAMLfl/fl mice (19), the cwLckCre-CAMLfl/fl mice contained strikingly fewer thymocytes overall and within all subsets from the DN4 to the SP stages of development. For example, CD4+CD8+ DP cells in jmlckcre-CAMLfl/fl thymi were reduced 2.2-fold compared to age-matched littermate CAMLfl/fl controls whereas in cwLckCre-CAMLfl/fl CD4+CD8+ DP cells were reduced 8.6-fold compared to CAMLfl/fl age-matched littermate controls. In the single positive populations, jmlckcre-CAMLfl/fl thymi had 7.9-fold fewer CD4+CD8− SP cells compared to control littermates while the cwLckCre-CAMLfl/fl CD4+CD8− SP cells had 19.4-fold fewer cells compared CAMLfl/fl thymi. Finally, in the CD4−CD8+ SP population, jmlckcre-CAMLfl/fl thymi had 5.2-fold fewer cells when compared to CAMLfl/fl age-matched littermate controls while cwLckCre-CAMLfl/fl CD4-CD8+ SP cells had 7.3-fold fewer cells compared to CAMLfl/fl age-matched littermate controls.
To ensure the efficiency of deletion of the floxed Caml alleles in cwLckCre-CAMLfl/fl cells, we used magnetic sorting to enrich for CD4−CD8−CD44−CD25− (DN4) or CD4+CD8+ cells. Western blotting revealed that cwLckCre-CAMLfl/fl cells had a >90% reduction in CAML expression compared to CAMLfl/fl control cells (Fig 1F).
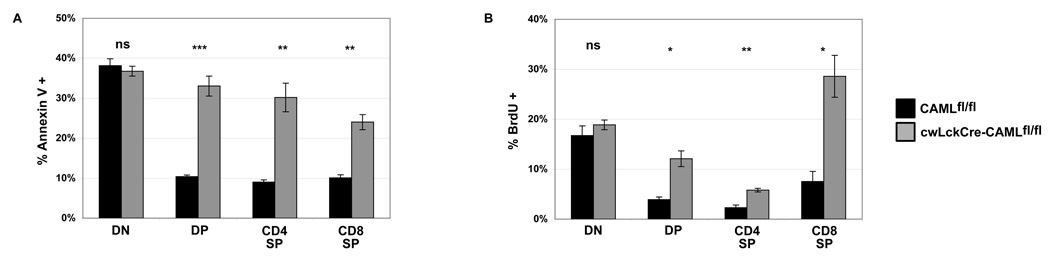
We next examined whether the loss of thymocytes in cwLckCre-CAMLfl/fl mice was due to increased cell death or decreased proliferation. We examined the number of apoptotic cells by staining with Annexin V and propidium iodide. A larger percentage of cells in each major thymocyte subset stained positive for Annexin V and propidium iodide in cwLckCre-CAMLfl/fl cells compared to CAMLfl/fl littermates (Figure 2A). As CAML has been implicated as a regulator of the mitotic spindle checkpoint and CAML-deficient cells were reported to exhibit defects in cellular division (17), it was important to determine whether CAML is essential for mitosis in thymocytes. To determine whether CAML loss was accompanied by defective proliferation, we injected mice with 2-bromodeoxyuridine (BrdU) and then removed thymi after 90 min. Thymocytes were surface stained with antibodies to CD4 and CD8 to define the four major thymocyte subsets and intra-cellularly with an antibody to BrdU to measure the rate of incorporation of this thymidine analog into dividing cells. Cells of all thymocyte subsets from cwLckCre-CAMLfl/fl showed increased proliferation compared to control CAMLfl/fl littermates (Figure 2B). Thus, we conclude that loss of CAML early in T cell development decreases the ability of pre-T cells to survive, resulting in reduced thymic numbers beginning at the DN4 stage of development (and possibly a compensatory increase in cell cycling). Additionally, the increase in proliferation in DN and DP subsets indicates that cwLckCre-CAMLfl/fl cells have intact pre-TCR signaling and that lack of a signal through the pre-TCR cannot account for the loss of the DP cwLckCre-CAMLfl/fl cells.
Figure 2. Abnormally increased apoptosis and increased proliferation are evident in cwLckCre-CAMLfl/fl thymocytes.
(A) Freshly isolated thymocytes were stained with anti-CD4 plus anti-CD8 antibodies plus Annexin V-Cy5 and propidium iodide to determine the percentage of apoptotic cells in each of the four major thymocyte subsets. Data from one representative of three independent experiments is shown. n=5 of each genotype. (B) Mice were injected intraperitoneally with 15 µg BrdU. After 90 min, thymocytes were isolated and stained for surface expression of CD4 and CD8 and intra-cellularly for uptake of BrdU. n=3 of each genotype. Error bars represent standard error of the mean. * p<.05 ** p<.005 *** p<.001
CAML is critical for thymocyte survival after exposure to a specific subset of apoptotic stimuli
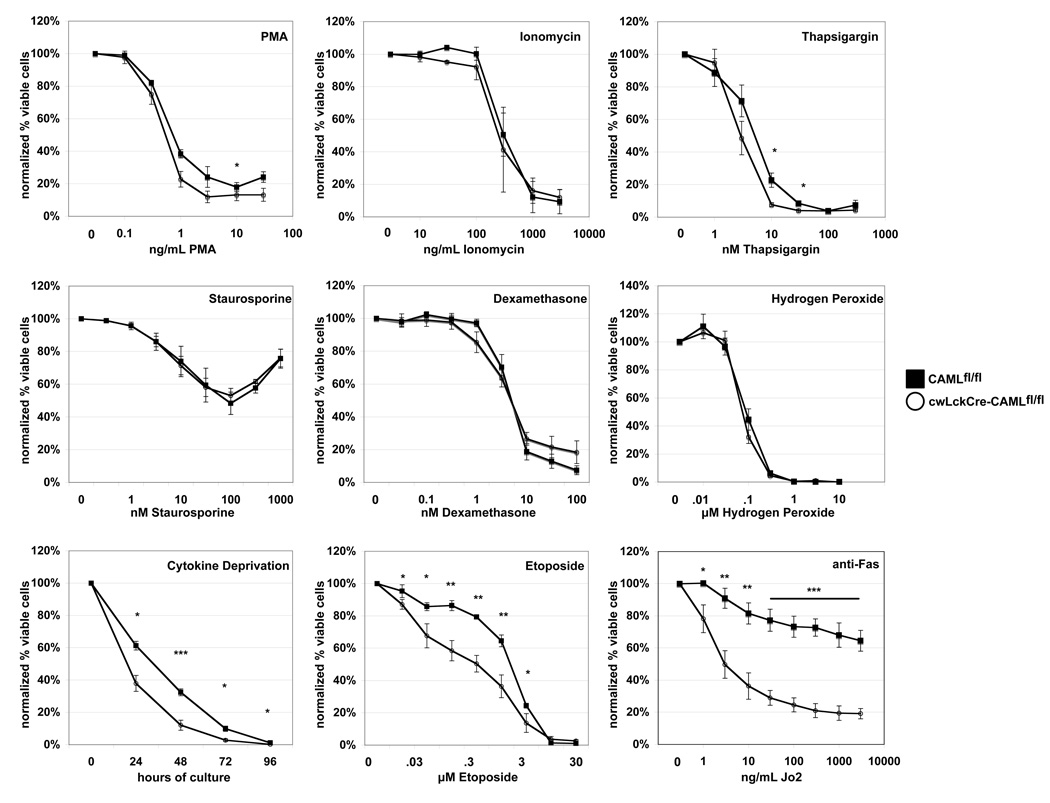
To further investigate the differences in survival between control and CAML-deficient thymocytes, we subjected cwLckCre-CAMLfl/fl and control CAMLfl/fl cells to a variety of apoptotic stimuli. We observed that in response to treatment with phorbol ester, ionomycin, thapsigargin, staurosporine, dexamethasone, or hydrogen peroxide, cell death was comparable between control and CAML-deficient cells (Figure 3). In contrast, cwLckCre-CAMLfl/fl thymocytes were significantly more sensitive than control cells to certain other stimuli, including cytokine deprivation, treatment with etoposide (even 100-fold lower concentrations) or stimulation of the death receptor Fas (Figure 3).
Figure 3. cwLckCre-CAMLfl/fl CD4+CD8+ thymocytes show abnormally increased susceptibility to a subset of apoptotic stimuli.
Total thymocytes (1 × 106) were cultured for 14 h with graded concentrations of the indicated reagents. Cells were stained with anti-CD4 plus anti-CD8 antibodies, Annexin V-Cy5 and propidium iodide to quantify cell survival. The average of three to five independent experiments is shown. Error bars represent standard error of the mean. Cytokine deprivation: thymocytes (1 × 106) were cultured for four days in regular medium in the absence of additional exogenous cytokines. Cell survival was examined daily as described above. One representative of three independent experiments is shown. Error bars represent standard error of the mean. * p<.05 **p<.005 ***p<.001
P53 is not required for the enhanced apoptosis caused by CAML-deficiency
Cell death following treatment with the topo-II inhibitor etoposide is dependent upon p53 and its transcriptional induction of the pro-apoptotic BH3-only protein Puma (21). To investigate whether the enhanced apoptosis in CAML-deficient thymocytes might be due to abnormally increased activation of p53, we crossed cwLckCre-CAMLfl/fl mice to p53−/− animals. However, cwLckCre-CAMLfl/fl;p53−/− mice showed no increase in thymocyte numbers compared to cwLckCre-CAMLfl/fl controls (Figure S1), indicating that p53 is not required for CAML-deficiency induced thymocyte death in vivo.
Fas is not required for the enhanced apoptosis caused by CAML-deficiency
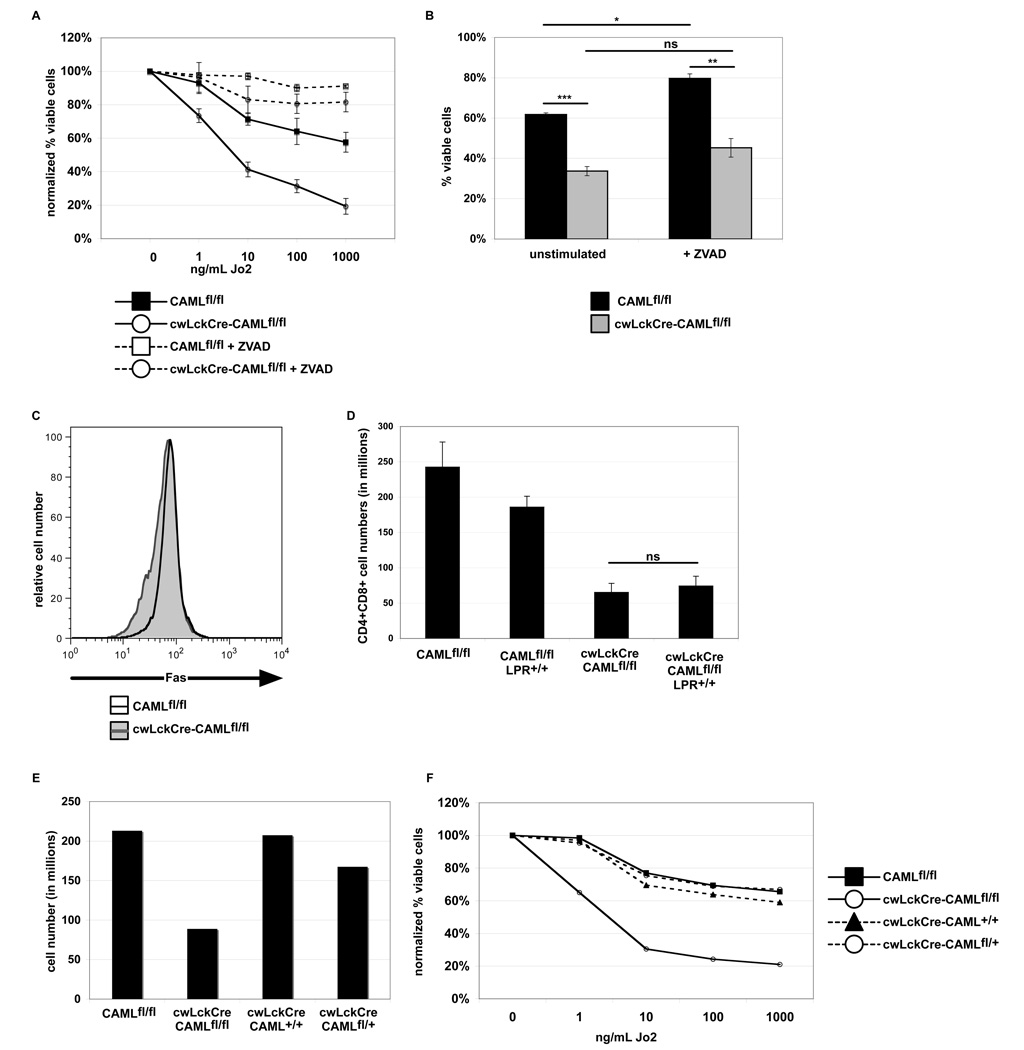
Since CAML-deficient thymocytes exhibited abnormally increased sensitivity to Fas-induced apoptosis, we explored whether Fas is critical for the abnormal thymocyte reduction caused by loss of CAML. Fas-mediated apoptosis was dependent upon caspase activity, as treatment with ZVAD rescued both cwLckCre-CAMLfl/fl and CAMLfl/fl cells from this death (Figure 4A–B). Next, we analyzed Fas surface expression on cwLckCre-CAMLfl/fl and CAMLfl/fl littermates and found that the levels were comparable (Figure 4C). The increased sensitivity to Fas stimulation was unexpected, since evidence suggests that Fas does not play a role in the developmentally programmed death of thymocytes (22). Thus, we considered two possible scenarios: one in which the Fas receptor had been inappropriately activated in the absence of CAML, and another in which the Fas-sensitivity of cwLckCre- CAMLfl/fl cells reflected a deficiency of a central survival pathway within the cells. To distinguish between these two possibilities, cwLckCre-CAMLfl/fl mice were crossed to Fas deficient Faslpr/lpr mice (23). Interestingly, loss of Fas did not cause a rescue of thymic cellularity in cwLckCre-CAMLfl/fl;Faslpr/lpr mice (Figure 4D, compare 3rd and 4th columns). This supports the notion that Fas does not mediate the abnormally enhanced in vivo death of cwLckCre-CAMLfl/fl thymocytes, but instead suggests that these cells have a more general aberration in survival that renders them more sensitive to Fas stimulation in vitro plus other death stimuli.
Figure 4. Fas is not essential for the abnormally increased apoptosis of cwLckCre-CAMLfl/fl thymocytes.
(A) Caspase inhibition protects cwLckCre-CAMLfl/fl thymocytes against Fas-induced apoptosis. Thymocytes (1 × 106) were cultured for 14 h with graded concentrations of anti-mouse Fas antibody with or without 50 µM ZVAD. Cells were stained with anti-CD4 plus anti-CD8 antibodies, Annexin V-Cy5 and propidium iodide to quantify cell survival. The average of three independent experiments is shown. (B) Non-normalized viability of thymocytes after an overnight incubation with or without 50 µM ZVAD. Cell survival was quantified as described above. One representative of three independent experiments is shown. (C) Thymocytes from cwLckCre-CAMLfl/fl mice express normal levels of Fas. Freshly isolated thymocytes from cwLckCre-CAMLfl/fl mice or littermate controls were stained for surface expression of CD4, CD8, and Fas. The histogram shows Fas surface expression on CD4+CD8+ cells. One representative of three independent experiments is shown. (D) Numbers of CD4+CD8+ thymocytes in cwLckCre-CAMLfl/fl and cwLckCre-CAMLfl/fl lpr/lpr mice. Error bars represent standard error of the mean. (E) Deletion of CAML, and not cre expression, is responsible for the decreased thymic cellularity and increased Fas susceptibility. Thymi from three to six week old mice CAMLfl/fl cwLckCre-CAMLfl/fl cwLckCre-CAML+/+ cwLckCre-CAMLfl/+ were tweezed into single cell suspensions and counted. (F) Thymocytes (1 × 106) were cultured for 14 h with graded concentrations of anti-mouse Fas antibody (Jo2). Cells were stained with anti-CD4 plus anti-CD8 antibodies, Annexin V-Cy5 and propidium iodide to quantify cell survival. The average of two independent experiments is shown.
Others have used cwLckCre mice for multiple analyses and have not reported nonspecific toxicity due to thymic Cre expression. Nonetheless, to rule out a possible cytotoxic effect (24) of Cre in our mice, we also analyzed cwLckCre-CAML+/+ mice for overall thymic numbers and sensitivity to Fas-mediated apoptosis. As seen in Figure 4E–F, CAML loss, and not the cre transgene, was responsible for the loss of thymic cellularity and the abnormally increased sensitivity to Fas-mediated apoptosis.
Loss of Bim partially rescues thymocyte survival in CAML-deficient mice
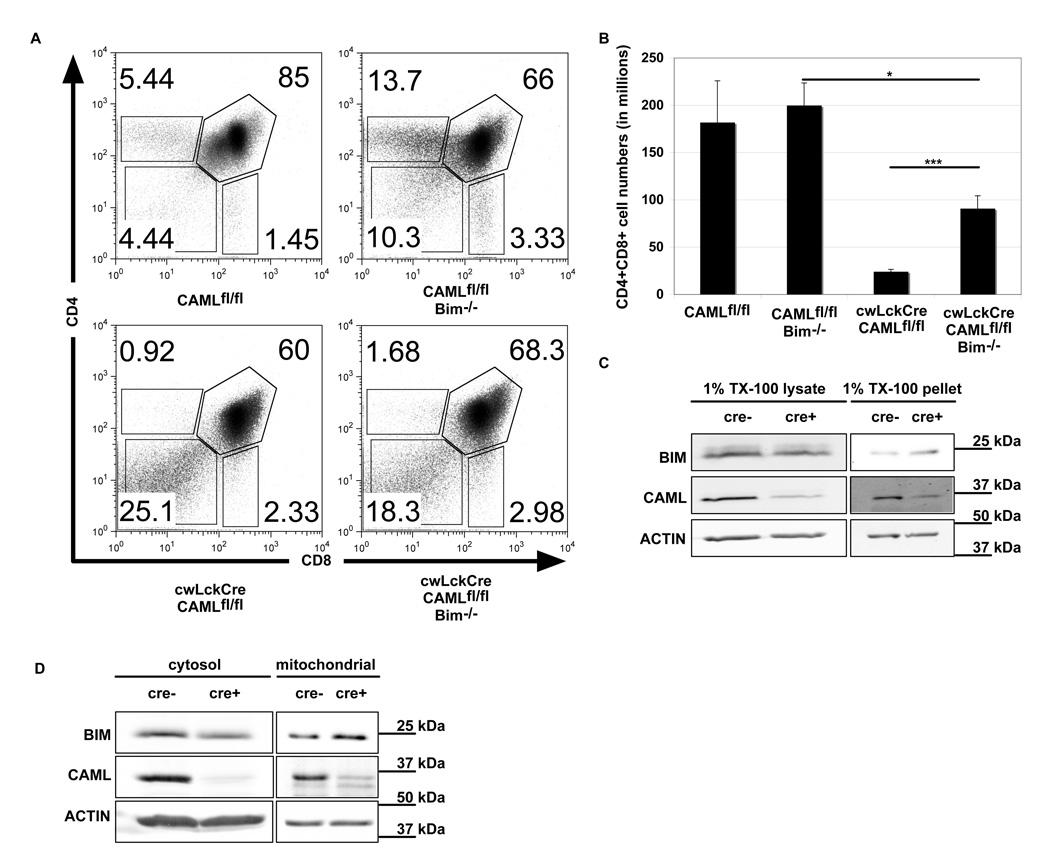
The pro-apoptotic BH3-only protein Bim has been shown to be essential for the initiation of several pathways that induce apoptosis in thymocytes (7), including cytokine withdrawal and calcium flux. We therefore crossed bim−/− mice with the conditional CAML knockout mice to test whether Bim is required for the reduction in cell numbers observed in cwLckCre-CAMLfl/fl thymi. Interestingly, cwLckCre-CAMLfl/fl;bim−/− thymi showed a significant increase in the number of DP thymocytes. While cwLckCre-CAMLfl/fl mice had only 13% of the numbers of double positive cells compared to CAMLfl/fl animals, cwLckCre-CAMLfl/fl;bim−/− mice had 46% of the numbers of these thymocytes compared to CAMLfl/fl;bim−/− littermate controls (Figure 5A–B), indicating that Bim must contribute to the enhanced death of CAML-deficient thymocytes.
Figure 5. Bim deficiency significantly rescues intra-thymic T cell development in cwLckCre-CAMLfl/fl.
(A) CD4/CD8 thymic profiles of freshly isolated thymocytes, comparing cwLckCre-CAMLfl/fl and cwLckCre-CAMLfl/fl Bim−/− mice. (B) Quantification of thymocyte subsets defined by CD4 and CD8 expression (CD4−8−, CD4+8+, CD4+8−, CD4−8+). Error bars represent standard error of the mean. Total numbers of thymocytes are as follows: Bim+/+ CAMLfl/fl: 220 × 106 cells, Bim−/− CAMLfl/fl: 312 × 106 cells, Bim+/+ cwLckCre-CAMLflfl: 40 × 106 cells and Bim−/− cwLckCre-CAMLfl/fl: 136 × 106 cells. (C) CD4+8+ cells were isolated and lysed in 1% TX-100 lysis buffer. Lysates were centrifuged at 17,000 g for 10 minutes and pellets were suspended in Laemmli Sample buffer. Western blots show Bim levels in the supernatant and pellet fractions. (D) Mitochondria were isolated from CD4+CD8+ thymocytes and examined for levels of Bim.
Given that Bim appeared responsible for a significant portion of thymocyte death elicited by loss of CAML, we examined whether Bim expression is abnormal in CAML-deficient cells. We first evaluated total cellular levels of Bim in CD4+CD8+ double positive cells and found it to be comparable or slightly diminished in the absence of CAML when compared to control cells (Fig. 5C). In addition to total cellular levels, Bim is also regulated by intracellular localization (13), including subcellular localization to the mitochondrial outer membrane, and by association with pro-survival Bcl-2 family members (25). We found that in CAML-deficient cells, Bim was reproducibly increased in the pellet fraction of cells lysed with 1% TritonX-100 (TX-100) and modestly increased in isolated mitochondria (Fig. 5D), indicating that loss of CAML causes mislocalization of Bim.
T cell receptor interaction with peptide/MHC complexes is not required for the enhanced apoptosis of CAML-deficient thymocytes
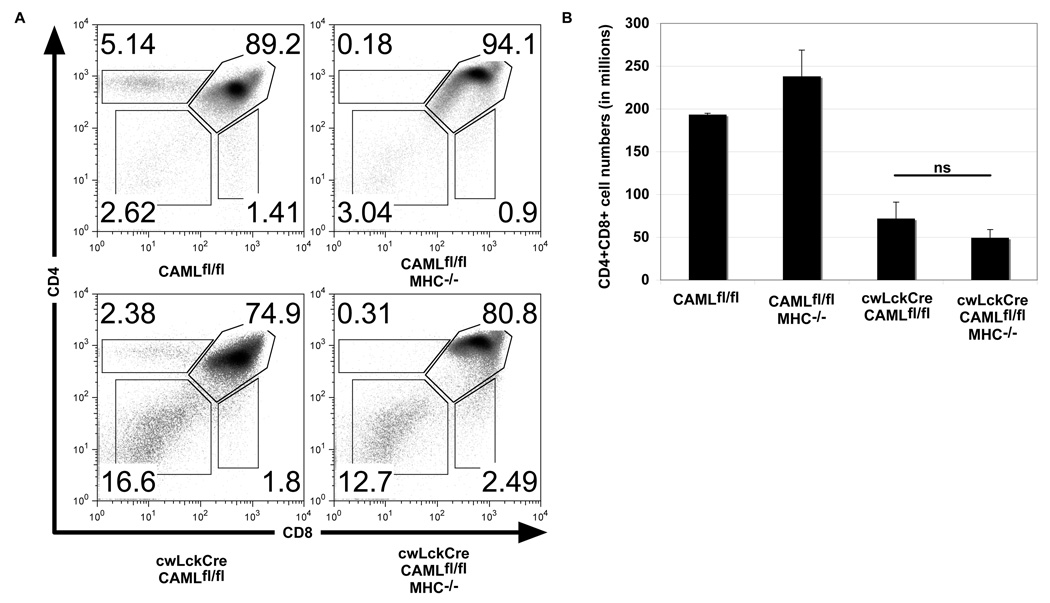
Bim is essential for thymocyte apoptosis caused by strong TCR stimulation and critical for negative selection of autoreactive thymocytes. Since we previously reported that jmLckCre-CAMLfl/fl T lymphocytes exhibit abnormally increased T cell receptor stimulation (19), it appeared likely that augmented TCR signaling might be responsible for their abnormally increased apoptosis. Others have demonstrated the role of TCR signaling in DP thymocyte killing in MINK-deficient mice by removing class I and II major histocompatibility antigens (26). In similar fashion, we examined whether MHC/T cell receptor interaction was required for the enhanced death of cwLckCre-CAMLfl/fl thymocytes by crossing cwLckCre-CAMLfl/fl mice with MHC class II- (Ab0) (27) and MHC class I-deficient (β2 microglobulin−/−) mice (28). In contrast to thymocytes lacking MINK in which MHC deficiency rescued normal double positive development, loss of MHC did not rescue the survival of DP thymocytes in cwLckCre-CAMLfl/fl mice (Figure 6A–B). This indicates that T cell receptor/MHC interactions are not required for the abnormally increased death of CAML-deficient pre-T cells.
Figure 6. T cell receptor ligand (MHC) deficiency does not rescue the defect in thymocyte development in cwLckCre-CAMLfl/fl mice.
(A) Representative flow cytometry profiles of anti-CD4 plus anti-CD8 antibody stained, freshly isolated thymocytes from CAMLfl/fl mice with or without the cwLckCre transgene that contained or lacked β2-microglobulin and class II major histocompatibility (MHC) proteins (β2-microglobulin−/− and class II MHC−/− mice are denoted MHC−). (B) Total numbers of CD4+CD8+ thymocytes. Error bars represent standard error of the mean.
Abnormally elevated levels of Reactive Oxygen Species (ROS) in cwLckCre-CAMLfl/fl mice
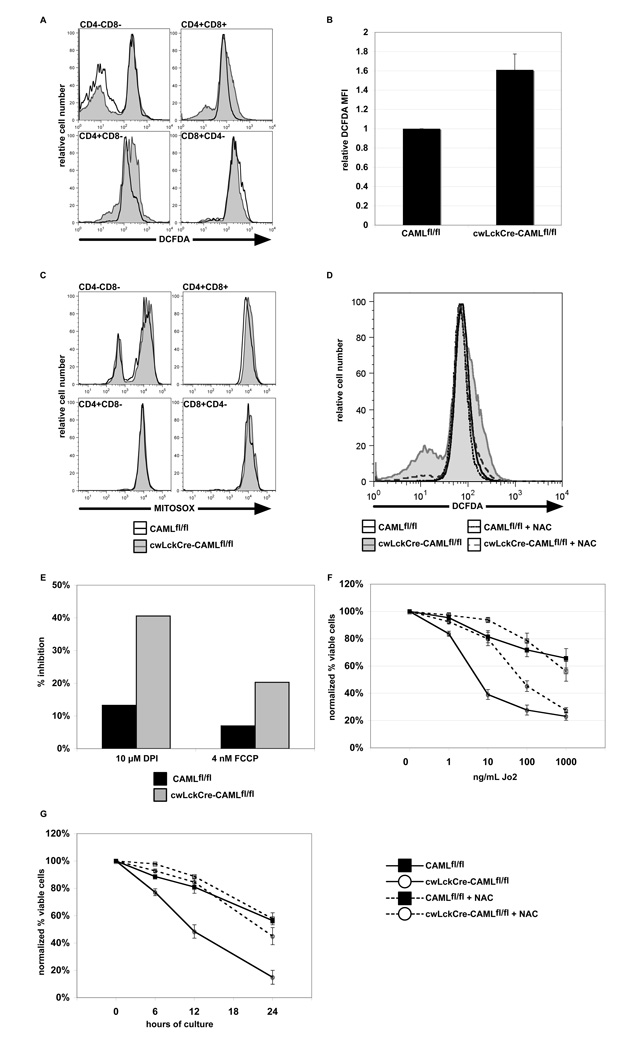
ROS have been reported to induce Bim-dependent death in T cells (29). Since loss of Bim increases thymocyte numbers in the absence of CAML, we compared ROS levels between CAML-deficient and control thymocytes by intracellular staining with DCFDA. cwLckCre-CAMLfl/fl CD4+CD8+ thymocytes were reproducibly found to have elevated ROS levels (Figure 7A–B) in comparison to cells from littermate control animals. ROS in cwLckCre-CAMLfl/fl cells appeared to be of mitochondrial origin, as verified by staining with the mitochondrial-localizing ROS dye Mitosox (30) (Figure 7C).
Figure 7. cwLckCre-CAMLfl/fl thymocytes contain abnormally increased levels of reactive oxygen species.
(A) Freshly isolated thymocytes from cwLckCre-CAMLfl/fl mice and littermate controls were stained with anti-CD4 plus anti-CD8 antibodies and the reactive oxygen species (ROS)-specific dye 6-carboxy-2',7'-dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) (DCFDA). Histograms depicting ROS levels in each CD4/CD8 subset are shown. One representative of three independent experiments is shown. (B) Quantitation of the ROS staining in the CD4+CD8+ thymic sub-population. The average of three independent experiments is shown. (C) Freshly isolated thymocytes from cwLckCre-CAMLfl/fl mice and littermate controls were stained with anti-CD4 plus anti-CD8 antibodies and the mitochondrial ROS-specific dye, Mitosox. Levels of mitochondrial ROS found in CD4−CD8− and CD4+CD8+ thymic sub-populations are shown. One representative of three independent experiments is shown. (D) Mice of the indicated genotypes were fed NAC in their water at 10 mg/mL for two weeks. Thymocytes were removed and examined for levels of ROS via DCFDA staining. (E) Thymocytes were treated for 2 h with DPI plus FCCP and were then stained with DCFDA to determine percent inhibition of ROS levels. One representative experiment of two independent experiments is shown. (F) Antioxidant treatment reduces Fas-mediated apoptosis in cwLckCre-CAMLfl/fl thymocytes. Thymocytes (1 × 106) from mice of the indicated genotypes were cultured for 14 h with anti-mouse Fas antibody with or without N-acetyl cysteine, a potent antioxidant. Cells were stained with anti-CD4 plus anti-CD8 antibodies, Annexin V-Cy5 and propidium iodide to quantify cell survival. The average of three independent experiments is shown. Error bars represent standard error of the mean. DN double negative, DP double positive, SP single positive thymocytes. (G) Anti-oxidant treatment reduces cytokine deprivation induced apoptosis of cwLckCre-CAMLfl/fl thymocytes. Experimental set-up and analysis were the same as for (F) except neither serum nor anti-Fas antibody was added. One representative experiment of two is shown.
To ensure that DCFDA staining was indeed labeling reactive oxygen species, we administered the antioxidant N-acetyl-cysteine (NAC) to mice to reduce ROS, removed thymi and then stained the cells with DCFDA. As expected, treatment of mice with NAC reduced the high ROS levels found in thymocytes from CAML-deficient mice (Figure 7D). Additionally, we treated cells in vitro with inhibitors of ROS generation: diphenyleneiodonium chloride (DPI), an inhibitor of NADPH oxidase, and carbonyl cyanide-p-trifluoromethoxyphenyl-hydrazone (FCCP), a mild decoupling agent that reduces mitochondrial ROS when used at low concentrations (31). We found that both reagents reduced ROS levels to a greater extent in the cwLckCre-CAMLfl/fl cells than within CAMLfl/fl cells (7E).
Abnormally increased ROS levels could be a cause of death or simply a downstream consequence of programmed cell death. To distinguish between these possibilities, we treated cwLckCre-CAMLfl/fl and CAMLfl/fl cells with NAC during stimulation of cells with anti-Fas antibody. Although addition of NAC had little effect on normal thymocytes, NAC-treated cwLckCre-CAMLfl/fl cells were significantly protected from Fas-induced apoptosis (Figure 7F), indicating that ROS are required for the abnormally increased sensitivity of CAML-deficient thymocytes to Fas-mediated death. To determine whether NAC could also diminish the sensitivity of cwLckCre-CAMLfl/fl cells to other apoptotic stimuli, we added NAC to cells immediately after isolation from the thymus and cultured them for 6, 12, and 24 h in simple medium (to mimic cytokine deprivation). Remarkably, NAC was able to augment the viability of cwLckCre-CAMLfl/fl thymocytes cultured without cytokines at each time point (Figure 7G).
Discussion
Here, we establish an essential role for CAML in maintaining the survival of developing thymocytes. CAML does not protect thymocytes indiscriminately against all cytotoxic insults; rather, it protects them from Fas stimulation, etoposide treatment and cytokine deprivation but not from treatment with phorbol ester, ionomycin, thapsigargin, staurosporine, dexamethasone or hydrogen peroxide. Our data suggest that CAML does not prevent TCR or p53-mediated death, but instead protects cells from Bim-induced death, most likely by blocking the production of reactive oxygen species. Although previous studies suggested a connection between CAML and cell survival, the role of CAML in this process has been a mystery. CAML interacts with K7 (32), a viral protein important for maintaining cell viability during infection, and E3-6.7K, a TACI viral homologue (33) that was shown to increase survival of cells exposed to extrinsic apoptotic stimuli (34).
What is the molecular mechanism by which CAML potentiates thymocyte survival? Several important steps of this process are revealed by this work. First, CAML-deficient cells have enhanced levels of ROS. ROS have been implicated in many types of cell death, including activation-induced apoptosis of T cells (35) and increasing the susceptibility of cells to extrinsic death stimuli (36). The decreased redox potential of CAML-deficient cells may render them more susceptible to Fas ligation and cytokine deprivation, as evidenced by the ability of the antioxidant NAC to reduce the susceptibility of CAML-deficient cells to Fas stimulation and cytokine deprivation. The ability of NAC to inhibit this enhanced death also provides evidence that the high ROS levels are not a consequence of cellular demolition but rather that the ROS generated in CAML-deficient cells contribute to increased apoptosis signaling.
Second, the death of thymocytes lacking CAML requires the pro-apoptotic BH3-only Bcl-2 family member Bim. Bim has been shown to be critical for negative selection of auto-reactive thymocytes and immature B cells, and for cytokine-withdrawal induced death of a broad range of cell types (6), and Bim is induced by TCR or BCR signaling (7, 37) and ROS (38). In contrast to our previous study of more mature single positive thymocytes that showed abnormally augmented TCR signaling as a consequence of CAML-deficiency (19), we did not find evidence for TCR signal dependence of the enhanced death of CAML-deficient thymocytes, although Bim is clearly required for the excess thymocyte death. Ca2+ signaling also regulates Bim (39), but we do not suspect that CAML-deficient cells have abnormal Ca2+ influx, because there was no defect in pro-T cell proliferation, which also requires Ca2+ signaling. Moreover, CAML-deficient thymocytes had a normal content of intracellular free Ca2+ (data not shown). We examined how Bim might be activated to promote the destruction of CAML-deficient cells and found that Bim was not increased at the protein level, nor did it show altered migration on SDS-PAGE, which would suggest a difference in phosphorylation. However, in the absence of CAML, Bim was localized more strongly to the pellet fraction of cells lysed with 1% Triton lysis buffer. Although we have not conclusively shown that CAML directly alters Bim localization, in light of CAML’s role in trafficking the EGFR (16), it is tempting to speculate that CAML may play a role in preventing Bim from moving to the mitochondria.
Precisely how CAML acts to block ROS induction and Bim activation is not yet clear. However, CAML has previously been shown to interact with several protein kinases, including EGFR (16), Lck (19) and Hck (unpublished data). Modulation of the activity or intracellular location of one or more such kinases may therefore constitute one of the critical functions of CAML. Indeed, in preliminary studies, we have identified a physical interaction between the serine-threonine kinase Raf-1 and CAML (Figure S2). Notably, Raf has been implicated in signaling pathways that inhibit apoptosis (40), and dominant negative mutants of Raf block thymocyte development (41). Moreover, Raf-1 was shown to regulate cellular levels of reactive oxygen species and is thought to thereby control cellular responses to cytokine-withdrawal (31). Importantly for this study, it has been shown that pharmacological blockade of Raf signaling kills certain tumor cells via Bim induction (42). Raf-1 has also been reported protect cells from external apoptotic stimuli, including Fas ligation (43), a stimulus to which CAML-deficient thymocytes are extremely sensitive. It is therefore tempting to speculate that CAML might regulate the survival function of Raf-1. Future studies will be required to determine whether the Raf-CAML interaction participates in the pro-survival role of CAML in this cell type.
Materials and methods
Mice
All experiments used 3–6 week old gender-matched littermate controls that were on a mixed C57BL/6x129/SvJ background. Dr. David Tran developed CAMLfl/fl mice (19), which were generated using 129/SvJ ES cells. cwlckcre (C57BL/6NTac-TgN(Lck-Cre) stock number: 004197) mice were purchased from Taconic. Bim-deficient (B6.129S1-Bcl2l11tm1.1Ast/J stock number: 004525) and LPR (B6.MRL-Faslpr/J stock number: 000482) mice (both backcrossed onto a C57BL/6 background for >20 generations) were purchased from Jackson Labs. p53-deficient mice were a gift from Dr. Jan VanDeurson. MHC-deficient (β2microglobulin−/− and class II MHC−/−) mice were a gift from Dr. Chella David. All animal experiments were performed according to IACUC guidelines and were approved by the IACUC.
Reagents
Ionomycin (407952) and thapsigargin (586005) were purchased from Calbiochem. PMA (13139-019) was purchased from Gibco. Etoposide (E1383), hydrogen peroxide (349887), Diphenyleneiodonium chloride (DPI D2926), carbonyl cyanide-p-trifluoromethoxyphenyl-hydrazone (FCCP C2920) and N-acetyl cysteine (NAC A9165) were purchased from Sigma. Jo2 (554254) hamster anti-mouse Fas antibody was purchased from BD Pharmingen.
Flow Cytometry
Labeled anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-c-kit (clone 2B8) and anti-CD25 (clone 3C7) antibodies, propidium iodide (#51-66211E) and Cy5-conjugated Annexin V (#559934) were obtained from BD Pharmingen. Cocktails of lineage marker-specific antibodies were a generous gift from Dr. Virginia Shapiro and consisted of FITC-labeled antibodies specific for Mac-1 (CD11b), GR-1 (Ly6G/C), CD8α, TCRβ, TCRγδ, CD3ε, B220, CD19, Ter119, and NK1.1. DCFDA (C2938) was purchased from Invitrogen. Mitosox (M36008) was purchased from Molecular Probes. Analyses were performed on BD FACS Calibur and Accuri C6 flow cytometers.
Cell Survival Assays
Thymi were removed from mice and tweezed into a single cell suspension. Two million thymocytes were cultured for 16 h in 24 well plates with the indicated reagents. Cells were then stained with anti-CD4 and anti-CD8 antibodies, Annexin V-Cy5 and PI to assess DP thymocyte viability.
Immunoprecipitation and Western Blotting
CD4+CD8+ double positive cells were isolated using Miltenyi MACS multisort kit. Cells were lysed for 5 min with TX-100 lysis buffer on ice (1% Triton X-100, 20 mM HEPES [pH 7.4], 5 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM Iodoacetamide, 100 µM Na2VO4, 1 mM PMSF, 10 µg/ml leupeptin, and 45 µg/ml aprotinin). Proteins were precipitated with 5 µg of antigen-specific antibodies: CAML (polyclonal rabbit serum raised against amino acids 1–189 of CAML), and Raf-1 (BD Pharmingen 610152), or isotype-matched control antibodies (BD Pharmingen). Western blots were probed for CAML (polyclonal rabbit serum raised against amino acids 1–189 of CAML), Raf-1 (BD Pharmingen 610152), actin (Millipore C4), and Bim (Cell Signaling 2819).
Mitochondrial Isolation
CD8+ thymocytes (96–98% pure for CD4+CD8+ double positive cells) were isolated using Miltenyi MACS CD8 isolation beads. Mitochondria isolation protocol was followed according to a protocol modified from (44). 60 × 106 cells were lysed in 10 mM Hepes-KOH, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.2 mM PMSF, 1 mM DTT, and 0.6% NP-40. After 45 minutes on ice, lysates were centrifuged 300g for 1 minute to remove the nuclear fraction. Supernatant was transferred to a new tube and centrifuged at 700g for 10 minutes and subsequently 17,000g for 40 minutes. A brown mitochondrial pellet was observed after this final centrifugation. The supernatant, a cytosolic fraction, was removed and the mitochondrial pellet was lysed in 10 uL of the lysis buffer described above. Lysates were loaded onto a 15% SDS-PAGE gel, transferred, and probed with Bim-specific (Cell Signaling 2819) and actin-specific (Millipore C4) antibodies.
Supplementary Material
The numbers of CD4+CD8+ thymocytes in CAMLfl/fl mice +/− the cwLck-Cre transgene that were p53+/+ or p53−/− were quantified by flow cytometric analysis and cell counting. Error bars represent standard error of the mean.
(A) 30 million freshly isolated thymocytes from wt mice were lysed, and the lysate was incubated with a CAML-specific antibody or a control antibody. Immunoprecipitates were washed, captured proteins size-fractionated using SDS-PAGE electrophoresis, transferred onto membranes and immunoblotted with an antibody to Raf-1. One representative of three independent experiments is shown. (B) 30 million Jurkat T cells were lysed and lysates were incubated with a Raf-1-specific antibody or a control antibody. Lysis buffer alone was also incubated with an anti-Raf-1 antibody. Immunoprecipitates were washed, captured proteins size-fractionated using SDS PAGE electrophoresis, and immunoblotted with anti-Raf-1 or anti-CAML antibodies. One representative of three independent experiments is shown.
Acknowledgments
Reagents and mice were kindly provided by Drs Chella David, Virginia Shapiro, and Jan VanDeurson.
This work was supported by the National Institute of Heath (grant 2RO1AI074320) (R.J.B.), the Mayo Foundation (R.J.B.), Joseph Bloom Children’s Disease Research (R.J.B.), NIH training grant T32 AI07425-14 (C.E.) and the Australian NHMRC (program 461221, fellowship 461229).
Footnotes
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 3.Hsu SY, Lin P, Hsueh AJ. BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members. Mol Endocrinol. 1998 Sep;12(9):1432–1440. doi: 10.1210/mend.12.9.0166. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998 Jan 15;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, et al. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000 Aug;157(2):449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999 Nov 26;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 7.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002 Feb 21;415(6874):922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 8.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008 Feb;28(2):197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008 Feb;28(2):218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008 Feb;28(2):206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A, et al. Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol Cell Biol. 2001 Feb;21(3):854–864. doi: 10.1128/MCB.21.3.854-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003 Oct 2;22(43):6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 13.Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999 Mar;3(3):287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 14.Bram RJ, Crabtree GR. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature. 1994 Sep 22;371(6495):355–358. doi: 10.1038/371355a0. [DOI] [PubMed] [Google Scholar]
- 15.Holloway MP, Bram RJ. Co-localization of calcium-modulating cyclophilin ligand with intracellular calcium pools. J Biol Chem. 1998 Jun 26;273(26):16346–16350. doi: 10.1074/jbc.273.26.16346. [DOI] [PubMed] [Google Scholar]
- 16.Tran DD, Russell HR, Sutor SL, van Deursen J, Bram RJ. CAML is required for efficient EGF receptor recycling. Dev Cell. 2003 Aug;5(2):245–256. doi: 10.1016/s1534-5807(03)00207-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Malureanu L, Jeganathan KB, Tran DD, Lindquist LD, van Deursen JM, et al. CAML loss causes anaphase failure and chromosome missegregation. Cell Cycle. 2009 Mar 15;8(6):940–949. doi: 10.4161/cc.8.6.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran DD, Edgar CE, Heckman KL, Sutor SL, Huntoon CJ, van Deursen J, et al. CAML is a p56Lck-interacting protein that is required for thymocyte development. Immunity. 2005 Aug;23(2):139–152. doi: 10.1016/j.immuni.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001 Nov;15(5):763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 21.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003 Nov 7;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 22.Sidman CL, Marshall JD, Von Boehmer H. Transgenic T cell receptor interactions in the lymphoproliferative and autoimmune syndromes of lpr and gld mutant mice. Eur J Immunol. 1992 Feb;22(2):499–504. doi: 10.1002/eji.1830220231. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007 Jul;8(7):665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci U S A. 2004 May 18;101(20):7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty N, Paust S, Ikizawa K, Dan I, Li X, Cantor H. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nat Immunol. 2005 Jan;6(1):65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 27.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, et al. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 28.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 29.Sade H, Sarin A. Reactive oxygen species regulate quiescent T-cell apoptosis via the BH3-only proapoptotic protein BIM. Cell Death Differ. 2004 Apr;11(4):416–423. doi: 10.1038/sj.cdd.4401347. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2(9):2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuznetsov AV, Smigelskaite J, Doblander C, Janakiraman M, Hermann M, Wurm M, et al. Survival signaling by C-RAF: mitochondrial reactive oxygen species and Ca2+ are critical targets. Mol Cell Biol. 2008 Apr;28(7):2304–2313. doi: 10.1128/MCB.00683-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng P, Park J, Lee BS, Lee SH, Bram RJ, Jung JU. Kaposi's sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002 Nov;76(22):11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant JR, Moise AR, Jefferies WA. Identification of a novel immunosubversion mechanism mediated by a virologue of the B-lymphocyte receptor TACI. Clin Vaccine Immunol. 2007 Jul;14(7):907–917. doi: 10.1128/CVI.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedict CA, Norris PS, Prigozy TI, Bodmer JL, Mahr JA, Garnett CT, et al. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J Biol Chem. 2001 Feb 2;276(5):3270–3278. doi: 10.1074/jbc.M008218200. [DOI] [PubMed] [Google Scholar]
- 35.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999 Jun;10(6):735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 36.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005 Mar 11;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003 Oct 6;198(7):1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007 Feb 15;26(7):970–981. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- 39.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim [corrected] J Immunol. 2006 Feb 15;176(4):2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 40.Troppmair J, Rapp UR. Raf and the road to cell survival: a tale of bad spells, ring bearers and detours. Biochem Pharmacol. 2003 Oct 15;66(8):1341–1345. doi: 10.1016/s0006-2952(03)00483-0. [DOI] [PubMed] [Google Scholar]
- 41.O'Shea CC, Crompton T, Rosewell IR, Hayday AC, Owen MJ. Raf regulates positive selection. Eur J Immunol. 1996 Oct;26(10):2350–2355. doi: 10.1002/eji.1830261012. [DOI] [PubMed] [Google Scholar]
- 42.Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008 Nov;118(11):3651–3659. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piazzolla D, Meissl K, Kucerova L, Rubiolo C, Baccarini M. Raf-1 sets the threshold of Fas sensitivity by modulating Rok-alpha signaling. J Cell Biol. 2005 Dec 19;171(6):1013–1022. doi: 10.1083/jcb.200504137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008 May 12;205(5):1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The numbers of CD4+CD8+ thymocytes in CAMLfl/fl mice +/− the cwLck-Cre transgene that were p53+/+ or p53−/− were quantified by flow cytometric analysis and cell counting. Error bars represent standard error of the mean.
(A) 30 million freshly isolated thymocytes from wt mice were lysed, and the lysate was incubated with a CAML-specific antibody or a control antibody. Immunoprecipitates were washed, captured proteins size-fractionated using SDS-PAGE electrophoresis, transferred onto membranes and immunoblotted with an antibody to Raf-1. One representative of three independent experiments is shown. (B) 30 million Jurkat T cells were lysed and lysates were incubated with a Raf-1-specific antibody or a control antibody. Lysis buffer alone was also incubated with an anti-Raf-1 antibody. Immunoprecipitates were washed, captured proteins size-fractionated using SDS PAGE electrophoresis, and immunoblotted with anti-Raf-1 or anti-CAML antibodies. One representative of three independent experiments is shown.