Abstract
Background
Given growing evidence that respiratory dysregulation is a central feature of panic disorder (PD) interventions for panic that specifically target respiratory functions could prove clinically useful and scientifically informative. We tested the effectiveness of a new, brief, capnometry-assisted breathing therapy (BRT) on clinical and respiratory measures in PD.
Methods
Thirty-seven participants with PD with or without agoraphobia were randomly assigned to BRT or to a delayed-treatment control group. Clinical status, respiration rate, and end-tidal pCO2 were assessed at baseline, posttreatment, 2-month and 12-month follow-up. Respiratory measures were also assessed during homework exercises using a portable capnometer as a feedback device.
Results
Significant improvements (in PD severity, agoraphobic avoidance, anxiety sensitivity, disability, and respiratory measures) were seen in treated but not untreated patients, with moderate to large effect sizes. Improvements were maintained at follow-up. Treatment compliance was high for session attendance and homework exercises; dropouts were few.
Conclusions
The data provide preliminary evidence that raising end-tidal pCO2 by means of capnometry feedback is therapeutically beneficial for panic patients. Replication and extension will be needed to verify this new treatment’s efficacy and determine its mechanisms.
Keywords: panic, anxiety, breathing, therapy, pCO2, biofeedback
1. Introduction
Panic disorder (PD) is a common psychiatric disorder with a lifetime prevalence of approximately 3% (Kessler et al., 1994), associated with high levels of social, occupational, physical disability (Klerman et al., 1991; Keller et al., 1994), and considerable economic costs (Leon et al., 1995). Numerous outcome studies have established the efficacy of pharmacological (Mavissakalian & Michelson, 1986) and psychological treatments (Barlow et al., 2000). While these treatments are fairly successful, not all patients respond or achieve full recovery. For instance, in the landmark study of Barlow et al., (Barlow et al., 2000) panic-free status at 12-month follow-up in cognitive-behavioral therapy (CBT) responders was 41% and in imipramine responders, 19.7%. Twenty-seven percent of those assigned to CBT and 39% of those assigned to imipramine did not complete treatment for various reasons, among which was lack of efficacy.
1.1 Targeting Respiration in Panic Disorder Treatment
A current thrust of biological psychiatry is elucidation of brain mechanisms underlying mental disorders in the hope that this will lead us to more effective and better targeted pharmacological interventions. There has been relatively little effort, however, to apply biological theories and discoveries to the design of new, non-pharmacological treatments. Dysregulated breathing in panic may be an intriguing target for such efforts. In this context, breathing training deserves special empirical scrutiny (Marks & Dar, 2000; Meuret et al., 2003). The rationale for conventional breathing training is to eliminate persistent or acute decreases in arterial pCO2, the defining characteristic of hyperventilation, and thereby to prevent anxious states (Ley, 1985). Surprisingly, although the expressed goal of breathing training is to correct hyperventilation, pCO2 has never been used as an outcome measure (Meuret et al., 2003; Meuret et al., 2005), with the exception of one uncontrolled study (Salkovskis et al., 1986). That such training will result in higher levels of pCO2 cannot be taken for granted, since the usual instruction to breathe slowly can actually lead to decreases in pCO2 (Meuret et al., 2003; Ley, 1991), probably because of deeper individual breaths (higher tidal volumes) stimulated by feelings of suffocation.
Occurrence of such feelings have given rise to the hypothesis that faulty respiratory control mechanisms are important to the basic pathophysiology of PD. Klein’s suffocation alarm hypothesis (Klein, 1993), for example, suggests that both panic attacks and the consistent respiratory abnormalities seen in panic patients may be due to hypersensitive, medullary carbon dioxide (CO2) detectors. Among the cross sectional findings supporting this hypothesis are increased variability in tidal volume and minute ventilation (Abelson et al., 2001; Wilhelm et al., 2001; Gorman et al., 1988; Caldirola et al., 2004), exaggerated lactate responses to room air hyperventilation (Dager et al., 1995), and hypersensivity to inhaled carbon dioxide (Gorman et al., 2004) in PD patients compared to controls. Regarding baseline levels of pCO2 studies are more mixed, with some showing lower levels in PD patients than healthy controls during baseline periods (Papp et al., 1997; Wilhelm et al., 2001; Hegel and Ferguson, 1997) and others no differences in comparison to control or other anxious groups (e.g. Holt and Andrews, 1998; Woods et al., 1986). In addition, successful treatment of PD, whether by anti-panic medication (Perna et al., 2002) or by CBT (Gorman et al., 2004), reduces anxiogenic sensitivity to carbon dioxide. If respiratory dysregulation is a central and perhaps etiologic feature of panic disorder, then interventions specifically targeting respiratory dysregulation may be effective in treating it. Testing such interventions could lead to new, innovative treatments, and could also provide insight into the role of respiration in the pathophysiology of panic.
1.2. Aims of the Study
We have developed a new treatment approach based on the idea that if PD patients could learn to control their breathing to efficiently raise their pCO2 levels, they might reduce the risk hyperventilation-induced panic, while at the same time possibly desensitizing a hypersensitive suffocation alarm; both of these outcomes might translate into meaningful clinical improvement. We devised a capnometry-assisted breathing training therapy (BRT) that uses immediate feedback to teach patients how to raise their pCO2 over a series of training and practice sessions. Initial case studies supported the potential usefulness of this approach (Meuret et al., 2001; Meuret et al., 2004). Compared to earlier attempts to reduce hyperventilation in PD by breathing retraining, which had shown mixed success (for review see Meuret et al., 2003), our biofeedback technique has the advantage of targeting the essential features of the respiratory physiology, pCO2 and respiration rate, directly. Here, we present a preliminary test of the efficacy of this treatment in the short-term, with a long-term uncontrolled follow-up, examining impact on both clinical outcomes and respiratory parameters.
2. Methods and Material
2.1 Recruitment and Patients
Participants included 37 patients with a principal DSM-IV diagnosis of panic disorder with (n=31) or without agoraphobia (n=6) who were randomly assigned to BRT (n=20) or a wait-list control group (n=17). Participants were recruited from the community via advertisements that were posted locally. Participants who appeared eligible based on an initial telephone screen were invited for diagnostic interview (First et al., 1994) conducted by a trained clinical psychologist and confirmed by a psychiatrist. The diagnostic interview was followed by the clinician administered Panic Disorder Severity Scale (PDSS, Shear et al., 1997), which was repeated at posttreatment and follow-up by two independent raters for each patient (κ= .65–.90).
The majority of the sample was female (n=24), married (n=22), white (n=32), employed (n=27), and well educated (mean: 17 years, range: 12–25). The mean (SD) age was 41 years (8.9). Other ethnic origins included Hispanic (n=1), African American (n=1), Asian (n=3). The duration of PD averaged 9 years (range 0.5–32). Agoraphobic avoidance was reported by 83.7% (29.7% mild, 32.4% moderate, 10.8% severe and 10.8% extreme, as measured by item 4 of the PDSS). Eighteen participants had at least one secondary current DSM-IV Axis I diagnosis - 13 had another anxiety disorder, 1 an additional mood disorder and 4 both an anxiety and mood disorder. Fewer than half (n=12) were receiving a stable dose of psychotropic medications (benzodiazepines (n=6), antidepressants (n=4), beta-blockers (n=1), and other anxiolytics (n=1)). Based on DSM-IV panic symptoms reported during the initial structured diagnostic interview we classified 60% of the patients as belonging to a respiratory subgroup (Briggs et al., 1993). The majority of patients’ (n=26) initial values of pCO2 were in a hypocapnic range (pCO2<35 mmHg; Oakes, 1996). No group differences were observed on any of the above variables.
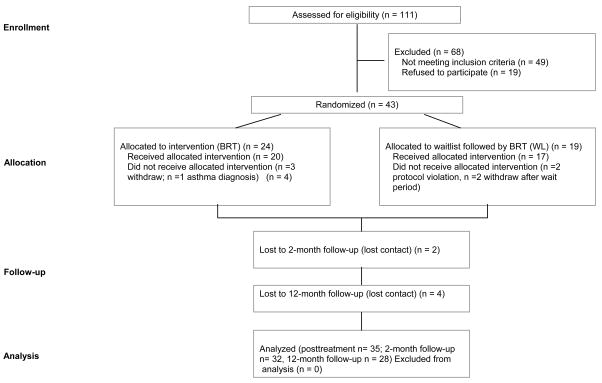
The study criteria were as followed: (i) age 18 to 60; (ii) if on psychotropic medications, on stable doses for at least 3 months prior to treatment with an agreement not to change dosage at least until after the 2-month follow-up; (ii) no evidence of organic mental disorder, suicidality, schizophrenia, alcohol or drug dependence, cardiovascular disease, pulmonary disease, epilepsy, or pregnancy; and (iv) no additional psychological treatment until after the 2-month follow-up. Of 43 people who were eligible, 20 completed the BRT protocol and 17 participated in the control group. An overview of the recruitment procedures is provided in Figure 1.
Figure 1.
Flow diagram of study patients
This study was approved by the Institutional Review Boards at Stanford University and the VA Palo Alto Health Care System, where the research took place, and all subjects signed written informed consent before entering the study.
2.2. Treatment
The treatment was aimed towards voluntarily increasing self-monitored end-tidal pCO2 and reducing respiration rate (RR) by means of breathing exercises. Patients underwent a four-week BRT consisting of five weekly 1-hr treatment sessions (initial session + four treatment sessions).
The treatment had five major components: (a) educating patients about the role of breathing in the etiology and maintenance of PD, (b) directing their attention to potentially problematic respiratory patterns, particularly those observed during the extended physiological monitoring, (c) having them perform different breathing maneuvers with capnometer feedback to experience how changes in breathing affect physiology, symptoms, and mood, (d) teaching them ways to simultaneously control pCO2 level and RR (e) and having them practice breathing exercises daily. The weekly sessions were aimed at reviewing changes in pCO2 and RR along with changes in symptoms and emotions. Individual training exercises, to be performed twice-daily for 17-min, at home or elsewhere, consisted of three parts: (a) a baseline period (baseline), during which patients sat quietly with their eyes closed for 2-min, (b) a 10-min paced breathing period (paced) during which patients breathed in synchrony with tones while occasionally checking their pCO2 and RR on a feedback device, and (c) a 5-min breathing period without pacing tones during which patients were to maintain their previously paced RR and pCO2 level using the feedback device (transfer). The paced breathing was used as a guiding tool to gradually shape slower breathing across treatment weeks. Patients were instructed to gradually adjust their breathing patterns (RR, rhythm, and depth) to reach or maintain pCO2 in a normocapnic range (pCO2>35 mmHg; Oakes, 1996). In the first two weeks the emphasis was on stabilization of breathing patterns (RR and rhythm), while in the last two weeks the emphasis shifted to normalizing pCO2. For the minority of patients (n=11) with normocapnic pCO2 levels, treatment focused on regularity of breathing (regular rate, avoiding of sigh breaths).
Patients performed home-training exercises using a light, handheld, battery-operated capnometry device (Capnocount mini, Weinmann), which analyzes exhaled breath pumped into the device through a nasal cannula. The instrument displays breath-by-breath end-tidal pCO2 (in mmHg) and RR (in breaths/min), and records them with the time and date of the measurement, which served as verification of compliance and treatment progress in this study. The adherence of the portable mainstream capnometer technique to international accuracy standards been demonstrated recently (Biedler et al., 2003). In addition, patients were provided with a pocket-sized tape player and audiotapes with instructions and pacing tones for their exercises. The tones were set to correspond to a RR of 13 breaths per minute in the first week, and rates of 11, 9, and 6 breaths per minute in successive following weeks. The Capnocount unit, tape player, and pacing tapes were collected at the end of treatment. Unlike other studies (Clark et al., 1999), we did not conduct booster sessions or systematically maintain therapeutic contact after treatment had ended.
An assessment battery was administered at baseline (week 0), posttreatment (week 4), 2-month follow-up (week 12), and 12-month follow-up (week 53). It included the PDSS (Shear et al., 1997), a clinician-rated scale of PD severity and the Clinical Global Impression Scale (CGI; Guy, 1976) (both assessed by independent raters). Secondary measures were the Anxiety Sensitivity Index (ASI; Reiss et al., 1996), Sheehan Disability Scale (SDS; Sheehan et al., 1996), Mobility Inventory for Agoraphobia (MI-AAL; Chambless et al., 1984), and the Beck Depression Inventory (BDI; Beck et al., 1961). Week-by-week changes in end-tidal pCO2 (mmHg) and RR (breaths/min) were collected during the home exercises and stored by the capnometry device to allow assessment of treatment compliance and progress. In addition, we obtained respiratory data during two standardized, supervised periods of extended quiet sitting period (QS) and a voluntary hyperventilation test (VH) (Wilhelm et al., 2001). For the BRT group these tests were conducted before and after treatment and at follow-ups. For the WL group they were conducted before the waiting period, after the waiting period, end of treatment, and at follow-ups. During the QS, patients sit quietly for 8 min (no feedback provided). During the VH, patients breathe deeply following pacing tones at 18 breath/min for 3 min, while maintaining end-tidal pCO2 at 20 mmHg using continuous feedback, followed by 8 min of recovery (also sitting quietly). Means of raw pCO2 and RR data for the last 3-min of the quiet sitting (QS/RR, QS/pCO2) and for the last 3-min of VH recovery (VH/RR, VH/pCO2) were calculated, after exclusion of artifacts.
2.3. Statistical analysis
Statistical analysis included MANCOVAs (post-treatment values with pre-treatment values as covariates) for three groups of outcome variables (i) anxiety-related psychopathology, (ii) basal respiration during QS, and (iii) respiratory activation during recovery from VH; significant findings were followed by ANCOVA for individual variables. To evaluate change in physiological measures from home exercises across treatment weeks (Week 1–4) we calculated two-way repeated measures MANOVAs (Period: baseline, paced, transfer; Week: 1–4) for RR and pCO2, followed by univariate two-way repeated measures ANOVAs. P levels were corrected for nonsphericity using the Greenhouse-Geisser ε when necessary. We tested for linear trends because we expected a monotonic decrease in RR and a monotonic increase in pCO2 across treatment weeks. The three significance levels for linear trends on the three levels of the period variable within ANOVAs for pCO2 and RR were Bonferroni-corrected. Tukey HSD post-hoc tests were used for comparison of individual means.
To evaluate the significance of overall change (pretreatment through 12-month follow-up) in clinical and respiratory measures, linear regression was used to calculate slopes for each subject, using fractions of time corresponding to the assessment times (pretreatment, posttreatment, 2FU, 12FU) (Kraemer & Thiemann, 1989). Thus, these time points were weighted 0, 4/53, 12/53, and 1 (53 weeks was the average measurement time for the 12-month FU). Significance of the difference of the slopes from zero was calculated using the Wilcoxon signed-rank test. It has been demonstrated that this method is superior to ANOVAs for handling missing data (Kraemer & Thiemann, 1989). We experienced some random data loss due to technical failures of the capnometry devices and/or ambulatory monitor. On average, 90% of the respiratory data (range: 77%–100%) was available for analysis. Using this method, we were able to retain all patients and variables for analysis, since at least two data points were available in every case.
Finally, Bivariate correlation analysis (Spearman, two-tailed) of these slopes (pretreatment through 12-month follow-up) was used to determine the association between psychological and physiological change during treatment.
3. Results
3.1. Therapy Compliance
Therapy session attendance was 100%. Of a total of 52 homework exercises over the course of four weeks, patients completed an average of 47.6 (91.3%). Attrition was very low, with no dropout during treatment, two (2.8%) at 2-month follow-up, and four (12.1%) at 12-month follow-up. Attrition was largely related to inability to schedule assessments for participants who had moved. During the maintenance period (between 2 and12-month FU) nine of the patients taking psychotropic medication had reduced their doses or discontinued medication altogether; four patients initiated alternative (n=3, relaxation, yoga, spiritual guidance) or psychological treatment (n=1, cognitive therapy).
3.2. Immediate Treatment Outcome: Breathing Training versus Wait-list Control
Group effects of MANCOVAs were significant for three groups of variables, psychopathology (F(5,24)=9.27, p<.001, η2=.66), respiration during QS (F(2,32)=9.95, p<.001, η2=.38) and VH recovery (F(2,29)=6.04, p<.006, η2=.29). Univariate ANCOVAs showed that for patients in the BRT group, means of questionnaire measures of psychopathology and RR decreased, and pCO2 increased, whereas for those in the WL group, measures did not change or changed slightly in the anti-therapeutic direction (Table 1) (Footnote 1). Effect size (Cohen, 1988) differences between BRT and WL at posttreatment were large (d>0.80) for both psychological and respiratory measures, except for QS/pCO2 (d=0.59) and VH/pCO2 (d=0.77), which were at moderate levels.
Table 1.
Means and standard deviations of pre- and post-treatment values, pre- and post-waiting-list values, and significance of ANCOVA group effects.
| Measures | BRT |
WL |
df | F ratios for group effects | da | ||
|---|---|---|---|---|---|---|---|
| Pre-TX | Post-TX | Pre-WL | Post-WL | ||||
| Anxiety-related psychopathology | |||||||
| PDSS (0–4) | 2.14 (0.65) | 0.69 (0.45) | 1.98 (0.87) | 1.95 (0.67) | 2,34 | 63.10*** | 2.21 |
| SDS (0–10) | 2.47 (2.45) | 0.73 (0.92) | 3.20 (2.25) | 2.77 (2.17) | 2,33 | 21.05*** | 1.22 |
| ASI (0–4) | 1.86 (0.83) | 0.92 (0.64) | 1.86 (0.86) | 1.58 (0.72) | 2,33 | 23.45*** | 0.97 |
| MI-AAL(1–5) | 1.86 (0.60) | 1.45 (0.46) | 2.20 (0.65) | 2.06 (0.71) | 2,33 | 7.81** | 1.02 |
| BDI (0–63) | 11.15 (8.41) | 4.15 (3.51) | 13.47 (7.51) | 11.44 (7.79) | 2,33 | 14.45** | 1.21 |
| Basal respiration during quiet sitting | |||||||
| QS/pCO2 | 32.16 (4.79) | 34.59 (4.98) | 32.22 (3.98) | 31.52 (5.41) | 2,34 | 3.72+ | 0.59 |
| QS/RR | 11.57 (5.03) | 9.27 (4.10) | 12.26 (3.15) | 13.98 (3.57) | 2,34 | 19.94*** | 1.23 |
| Respiratory activation during recovery from hyperventilation | |||||||
| VH/pCO2 | 30.29 (5.05) | 36.18 (4.22) | 31.63 (4.33) | 30.75 (9.05) | 2,31 | 5.11* | 0.77 |
| VH/RR | 11.82 (4.37) | 9.21 (4.23) | 11.68 (3.43) | 12.68 (4.27) | 2,32 | 9.88** | 0.82 |
BRT, Capnometry-assisted breathing training; WL, waitlist control; PDSS, Panic Disorder Severity Scale; SDS, Sheehan Disability Scale; ASI, Anxiety Sensitivity Index; MI-AAL, Mobility Inventory (alone); BDI, Beck Depression Inventory; QS/pCO2, pCO2 during quiet sitting; QS/RR, respiration rate during quiet sitting, VH/pCO2, recovery pCO2 after hyperventilation; VH/RR, recovery respiration rate after hyperventilation.
Cohen’s d was calculated to determine the effect size for differences between BRT and WL groups at posttreatment
p<.001;
p<.01,
p<.05,
p<.10
3.3. Changes Across Treatment Weeks 2
Linear trends on physiological variables confirmed a steady improvement across the four weeks of training (Table 2). WL patients who continued to meet the trial inclusion criteria after the waiting period, and were then enrolled in treatment, were included in these analysis. The WL patients did not differ from the active group on any measure at any time point across treatment or follow-up. Repeated measures MANOVAs showed significant effects of time for respiratory measures, F(18,17)=28.97, p<.001. In follow-up univariate repeated measures ANOVAs, significant linear trends were observed for all variables (Table 2). ANOVA time effects showed a similar pattern of significance across weeks. RR and pCO2 showed significant improvements for all three phases of the exercises: the overall effect of weeks was F(3,102)=50.68 and 5.53, p<.020 and p<.001, ε=.70 and .61, respectively. Although linear trends showed that RR decreased and pCO2 increased significantly for each phase, these trends across weeks were steeper for paced breathing and the transfer phase than for baseline, as indicated by significant interactions of Phase by Week effects for RR and pCO2, F(6,204)=5.76 and 8.79, p<.005 and p<.001, ε=.36 and .41, respectively. Post-hoc tests showed that pCO2 dropped significantly below baseline level during paced breathing and transfer in week 1 (34.7 and 34.5 mmHg). It reached the highest levels (around 38 mmHg) for all three phases during week 4. Patients were successful in following the pacing tones throughout week 3 (13, 12, and 9 breaths/min), but were unable to maintain the required 6 breaths/min in week 4. The fact that RR and pCO2 changed in parallel during transfer and paced breathing shows that patients were able to maintain control over their breathing pattern without guiding tones.
Table 2.
Means, standard deviations, and significance of linear trends for PCO2 and RR data across treatment weeks
| Measures | Week 1 | Week 2 | Week 3 | Week 4 | df | F ratio for linear trend | η2 |
|---|---|---|---|---|---|---|---|
| End-tidal pCO2 | |||||||
| Baseline | 36.67 (3.04) | 36.76 (2.53) | 37.23 (2.64) | 37.88 (2.82) | 1,34 | 7,99*a | .19 |
| Pacing tones plus feedback | 34.69 (5.14) | 36.70 (2.69) | 37.56 (3.07) | 38.75 (3.44) | 1,34 | 35,95**a | .51 |
| Feedback only | 34.53 (5.03) | 35.98 (2.71) | 36.72 (3.07) | 37.67 (3.66) | 1,34 | 19,63**a | .37 |
| Respiration Rate | |||||||
| Baseline | 14.75 (3.55) | 14.08 (2.63) | 12.74 (3.10) | 12.23 (3.76) | 1,34 | 12,95**b | .28 |
| Pacing tones plus feedback | 12.95(0.93) | 11.31 (1.57) | 9.05 (1.50) | 7.76 (2.61) | 1,34 | 272,47**b | .89 |
| Feedback only | 13.30 (1.94) | 11.77 (1.94) | 9.85 (2.22) | 8.52 (2.95) | 1,34 | 105,77**b | .76 |
p<.001;
p<.01,
p<.05,
p<.10 (Greenhouse-Geisser εcorrected)
Bonferroni-corrected (overall p<.05 or .01/3) significance levels
p<.01,
p<.05
3.4 Overall Outcome Across Posttreatment and Follow-up Assessments
Analysis of change from pretreatment through 12-month follow-up resulted in highly significant rates of improvement on all clinical measures, and significant or marginally significant rates on respiratory measures (Table 3). Figure 2 and 3 illustrate changes of patients who continued up to 12 months follow-up.
Table 3.
Slope as outcome measure for clinical and respiratory measures: Pretreatment through follow-up
| Measures | PDSS | SDS | ASI | MQ | BDI | QS/pCO2 | QS/RR | VH/pCO2 | VH/RR |
|---|---|---|---|---|---|---|---|---|---|
| Slope | −2.86 | −3.20 | −2.15 | −0.52 | −6.24 | 8.02 | −6.76 | 15.49 | −10.91 |
| z-score | −4.75 | −3.63 | −3.55 | −4.01 | −2.95 | −1.87 | −1.88 | −2.60 | −2.77 |
| P | .000 | .000 | .000 | .000 | .003 | .062 | .060 | .009 | .006 |
PDSS, Panic Disorder Severity Scale; SDS, Sheehan Disability Scale; ASI, Anxiety Sensitivity Index; MI-AAL, Mobility Inventory (alone); BDI, Beck Depression Inventory; QS/pCO2, pCO2 during quiet sitting; QS/RR, respiration rate during quiet sitting, VH/pCO2, recovery pCO2 after hyperventilation; VH/RR, recovery respiration rate after hyperventilation.
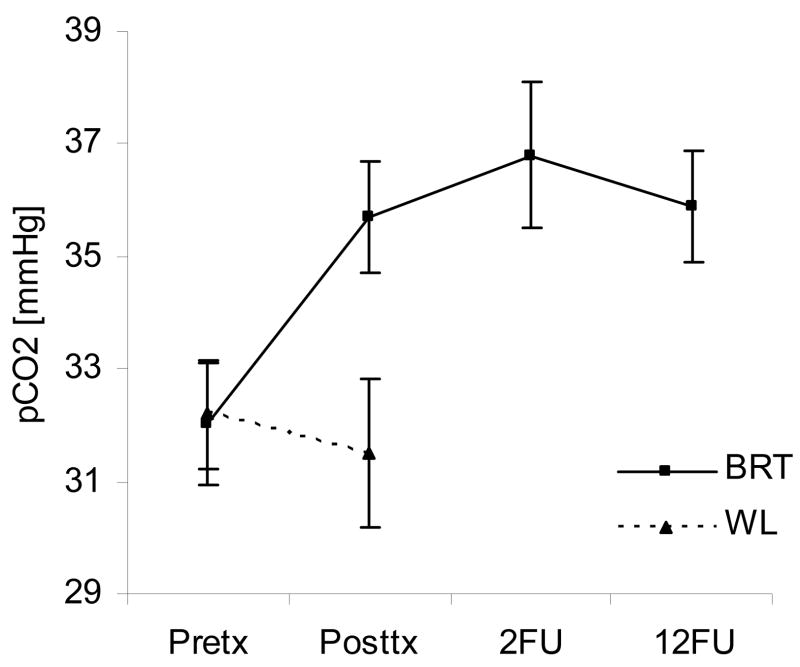
Figure 2.
This figure shows a comparison between mean (+ SEM) pCO2 values at different time points in patients who were treated with BRT. Wait-list values for those assigned to the control group are shown for comparison
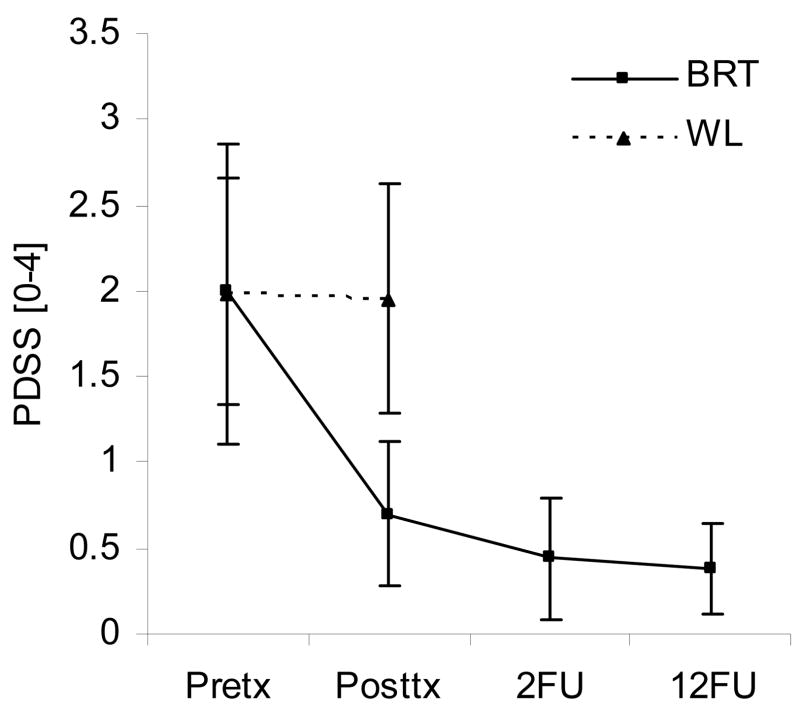
Figure 3.
This figure shows a comparison between mean (+ SEM) PDSS scores at different time points in patients who were treated with BRT. Wait-list values for those assigned to the control group are shown for comparison
Patients classified as “respiratory subtype” did not benefit more from therapy on any of the above measures than patients who reported few or no respiratory symptoms before treatment (Mann-Whitney U=114–147, ps>.25). Furthermore, initial pCO2 and RR during QS were unrelated to this classification, t(35)=0.43 and 1.36, p>.10, respectively (respiratory subtype pCO2=31.4+/− 3.6 mmHg, RR=12.1+/−4.4 breaths/min, non-respiratory pCO2=33.4+/−5.2 mmHg, RR=11.5+/−4.1). However, pCO2 at the end of VH recovery initially tended to be lower in the respiratory (M=29.7+/−4.3) than in the non-respiratory (M=32.8+/−4.9) subgroup, t(34)=2.02, p<.06). Furthermore, no significant differences in treatment outcome for either respiratory or psychological measures were found when dividing patients into a non-hypocapnic vs. hypocapnic group using a cut-off score of 35 mmHg. Treatment outcome was not affected by medication status (on stable psychotropic medication or not).
We correlated slopes from individual linear regressions over time for selected panic and anxiety measures. Significant or marginally significant correlations were found on the one hand between PDSS change and change in VH/pCO2, VH/RR, and QS/RR, r=−.33, .41, and .31, p= .052, .014, and .066 respectively, and on the other hand between SDS change and change in VH/RR and QS/RR, r=.44 and .50, p=.008 and .002, respectively. Thus, patients who continued to show abnormal levels of pCO2 or RR during recovery from VH were less likely to have sustained improvement in PD severity or avoidance.
3.5. Clinical Significance of Outcome
At posttreatment 40% had experienced no further panic attack during the four week period. At 2-month follow-up 62% had experienced no further panic attack since the end of treatment and 68% were panic-free at 12-month follow-up. Eighty-eight percent at 2-month follow-up and 96% at 12-month follow-up were either “much improved” or “very much improved” (CGI >=3). A forty percent reduction in initial PDSS scores (see Barlow et al., 2000) was achieved by 68% of the participants at posttreatment, 79% at 2-month follow-up, and 93% at 12-month follow-up.
4. Discussion
4.1. Efficacy of a Capnometry-Assisted Breathing Training for Panic Disorder
The results of this study suggest that a new, brief, capnometry-assisted breathing therapy (BRT), which specifically teaches patients to raise pCO2 levels by regular slow and shallow breathing, can be therapeutic in PD. Significant improvements were seen in treated but not untreated patients, with respect to PD severity, agoraphobic avoidance, anxiety sensitivity, disability, and respiratory measures. Psychological measures continued to be improved or improved further at 2-month and 12-month reassessments. Mean pCO2 increased from hypocapnic to normocapnic levels over the course of treatment and remained normocapnic at follow-up. Sixty-eight percent of participants no longer reported panic attacks at 12-month follow-up. Effect sizes (treatment group compared with wait-list group) were large for psychological measures (Cohen’s d=0.97–2.21) and moderate to large for respiratory measures (d=0.59–1.23). Compliance was high for both session attendance and completion of homework exercises; dropouts were few. Compliance was likely enhanced by electronic monitoring of homework exercises and extensive feedback of physiological results, both during homework and during the therapist-guided sessions.
4.2 Potential Mechanisms of Change
A number of mechanisms may have contributed to patient improvement. Our working hypothesis was that dysregulated respiratory control is a central aspect of PD and clinical improvement was a consequence of the direct manipulation on this dysregulation provided by the treatment. The clinical improvement produced by a brief treatment that focused exclusively on breathing and contained none of the explicit cognitive restructuring of cognitive therapy (Clark et al., 1999) or exposure-based techniques of standard CBT for panic (Barlow et al., 2000) is consistent with this hypothesis. However, this study was designed to test primarily overall effects of the BRT intervention; thus, any interpretation of our findings in terms of mechanisms therefore is speculative. Repeated elevation of pCO2 during homework sessions may have desensitized a hypersensitive suffocation alarm system (Klein, 1993), reducing panic vulnerability. Such desensitization could increase tolerance for incidental increases of arterial pCO2 during daily life and result in fewer compensatory hyperventilatory episodes. Alternatively, in so far as hyperventilation itself can cause panic attacks (Ley, 1985), practiced skill at raising pCO2 could directly reduce risk. The fact that an inability to normalize breathing quickly after paced hyperventilation was associated with less clinical recovery suggests that respiratory and clinical outcomes were linked.
However, treatment success was not related to respiratory symptom subtype or initial level of baseline or recovery pCO2. Thus, non-respiratory mechanisms may also have played a role in patient improvement. For instance, the treatment rationale provided to patients included cognitive components that may have counteracted catastrophic thinking and given patients a greater sense of control. The paced breathing exercises, which often triggered uncomfortable sensations similar to those experienced during panic attacks (Meuret et al., 2003), may have produced interoceptive exposure and desensitization to bodily cues that was not respiration-specific (Craske et al., 1997). The slight decreases of pCO2 during home-exercises could be indicative of such an unpleasant exposure effect. However, they were contrasted with gradual increases in baseline levels before exercises across weeks. Finally, what was taught in the exercises may have served as an anxiety-reducing distraction. Indeed, it has been argued that breathing training may distract from experiencing anxiety in exposure therapy and thereby reduce its efficacy (Craske et al., 1997; Schmidt et al., 2000; Garssen et al., 1992). However, even if distraction had been part of the mechanism of action in our training, its effects do not seem to have interfered substantially with the overall beneficial outcome of the training, which was comparable to that of standard CBT protocols. Future studies should be directed at testing the viability of these alternative explanations for patients’ improvement.
4.3. Limitations
This study is limited by the lack of an active control intervention or inactive, “placebo” therapy. We thus cannot rule out the possibility that clinical improvement was secondary to non-specific therapy factors that are unrelated to the specific respiratory training employed or the sustained changes in respiration observed. Furthermore, the long-term follow-up data were entirely uncontrolled. However, we do know that immediately following treatment, BRT does produce significantly more improvement than no treatment at all, and this improvement is sustained over a prolonged follow-up. The effect sizes seen for the wait list comparisons and the 68% panic-free rate at 12-month follow-up do suggest that this is a potentially potent therapy that warrants more definitive testing.
4.4. Practicality and Economy of Implementation
The potential scientific value of this new treatment is intriguing, but its practical value as a treatment innovation also deserves attention. Substantial and lasting clinical improvement was seen with a relatively brief intervention. Patient acceptance and compliance were high. Though initial costs may seem high due to the cost of purchasing capnometers, their price has been coming down (now less than $1200). The technology is reliable so each instrument should last for years and treat many patients, reducing the total costs over time below those for a standard 13-session CBT. Direct comparison trials will be needed, measuring both long-term clinical outcomes and treatment costs to determine the true value of this potential innovation in the non-pharmacological treatment of panic.
4.5 Conclusion
Our results provide initial evidence that raising end-tidal pCO2 by means of capnometry-assisted feedback is therapeutically beneficial for panic patients. Capnometry feedback is a direct and convincing way to correct problematic breathing patterns associated with hyperventilation, ensuring that the primary goal of breathing therapy is being attained. As with most initial studies, our findings await replication and extension with more comprehensive designs. If the initial therapeutic promise seen here is supported in better controlled trials, further work will be needed to determine the true mechanisms of efficacy. Mechanistic studies will be needed to sort out the relative contributions of a range of cognitive, behavioral and respiratory factors that may have fostered improvement in this cohort of patients. We hope that the therapeutic success in this preliminary report will stimulate further study. We also hope it will provide a model for the potential value of attempting to translate basic biological findings in psychiatric disorders into innovative treatment approaches–psychotherapeutic as well as pharmacological.
Acknowledgments
Authors note: This research was supported by the National Institutes of Mental Health and the Department of Veterans Affairs. We gratefully acknowledge Dr. James Abelson and Dr. David H. Barlow for their feedback on an earlier draft of this article and Dr. Helena C. Kraemer for her statistical consultation on this study.
Footnotes
Calculating 2-way repeated measures ANOVAs with group (BRT, WL) as between-individuals variable and time (pre-therapy, post-therapy) as within-subject variables, Group by Time interactions revealed a similar pattern of significance levels.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry. 2001;49:588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. The Journal of the American Medical Association. 2000;17:2573–2574. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;41:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biedler AE, Wilhelm W, Kreuer S, Soltesz S, Bach F, Mertzlufft FO, Molter GP. Accuracy of portable quantitative capnometers and capnographs under prehospital conditions. The American Journal of Emergency Medicine. 2003;21:520–524. doi: 10.1016/j.ajem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Briggs AC, Stretch DD, Brandon S. Subtyping of panic disorder by symptom profile. British Journal of Psychiatry. 1993;163:201–209. doi: 10.1192/bjp.163.2.201. [DOI] [PubMed] [Google Scholar]
- Caldirola D, Bellodi L, Caumo A, Migliarese G, Perna G. Approximate entropy of respiratory patterns in panic disorder. American Journal of Psychiatry. 2004;161:79–87. doi: 10.1176/appi.ajp.161.1.79. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Bright P, Gallaher R. Assessment of fear of fear in agoraphobics: The Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Hackmann A, Wells A, Ludgate J, Gelder M. Brief cognitive therapy for panic disorder: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 1999;67:583–589. doi: 10.1037//0022-006x.67.4.583. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Craske MG, Rowe M, Lewin M, Noriega-Dimitri R. Interoceptive exposure versus breathing retraining within cognitive-behavioural therapy for panic disorder with agoraphobia. British Journal of Clinical Psychology. 1997;36:85–99. doi: 10.1111/j.2044-8260.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. American Journal of Psychiatry. 1995;152:666–672. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, P-Version. New York: Biometrics Research Department; 1994. [Google Scholar]
- Garssen B, de Ruiter C, Van Dyck R. Breathing retraining: A rational placebo? Clinical Psychology Review. 1992;12:141–153. [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF. Ventilatory physiology of patients with panic disorder. Archives of General Psychiatry. 1988;45:31–39. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biological Psychiatry. 2004;56:862–867. doi: 10.1016/j.biopsych.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Guy W. Assessment Manual for Psychoparmacology,rev. Rockville, Md: US Dept of Health, Education, and Welfare; 1976. pp. 217–222. [Google Scholar]
- Hegel MT, Ferguson RJ. Psychophysiological assessment of respiratory function in panic disorder: evidence for a hyperventilation subtype. Psychosomatic Medicine. 1997;59:224–230. doi: 10.1097/00006842-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Holt PD, Andrews G. Hyperventilation and anxiety in panic disorder, social phobia, GAD, and normal controls. Behaviour Research and Therapy. 1998;27:453–460. doi: 10.1016/0005-7967(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Keller MB, Yonkers KA, Warshaw MG, Pratt LA, Gollan JK, Massion AO, White K, Swartz AR, Reich J, Lavori PW. Remission and relapse in subjects with panic disorder and panic with agoraphobia: a prospective short-interval naturalistic follow-up. Journal of Nervous and Mental Disease. 1994;182:290–296. doi: 10.1097/00005053-199405000-00007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM, Ouellette R, Johnson J, Greenwald Panic attacks in the community. Social morbidity and health care utilization. The Journal of the American Medical Association. 1991;13:742–746. [PubMed] [Google Scholar]
- Kraemer HC, Thiemann S. A strategy to use soft data effectively in randomized controlled clinical trials. Journal of Consulting and Clinical Psychology. 1989;57:148–154. doi: 10.1037//0022-006x.57.1.148. [DOI] [PubMed] [Google Scholar]
- Leon AC, Portera L, Weissman MM. The social costs of anxiety disorders. British Journal of Psychiatry Suppl. 1995:19–22. [PubMed] [Google Scholar]
- Ley R. Blood, breath, and fears: A hyperventilation theory of panic attacks and agoraphobia. Clinical Psychology Review. 1985;5:271–285. [Google Scholar]
- Ley R. The efficacy of breathing retraining and the centrality of hyperventilation in panic disorder: a reinterpretation of experimental findings. Behaviour Research and Therapy. 1991;29:301–304. doi: 10.1016/0005-7967(91)90121-i. [DOI] [PubMed] [Google Scholar]
- Marks IM, Dar R. Fear reduction by psychotherapies. Recent findings, future directions. British Journal of Psychiatry. 2000;176:507–511. doi: 10.1192/bjp.176.6.507. [DOI] [PubMed] [Google Scholar]
- Mavissakalian M, Michelson L. Two-year follow-up of exposure and imipramine treatment of agoraphobia. American Journal of Psychiatry. 1986;143:1106–1112. doi: 10.1176/ajp.143.9.1106. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT. Voluntary hyperventilation in the treatment of panic disorder. Clinical Psychology Review. 2005;25:285–306. doi: 10.1016/j.cpr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training in panic disorder treatment: Useful intervention or impediment to therapy? Behavior Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory biofeedback-assisted therapy in panic disorder. Behavior Modification. 2001;25:584–605. doi: 10.1177/0145445501254006. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. Journal of Clinical Psychology. 2004;60:197–207. doi: 10.1002/jclp.10245. [DOI] [PubMed] [Google Scholar]
- Oakes DF. Clinical practitioner’ pocket guide to respiratory care. 4. Old Town, MN: Health Educator Publications, Inc; 1996. [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, de Jesus MJ, Ross D, Goetz R, Gorman JM. Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. American Journal of Psychiatry. 1997;154:1557–65. doi: 10.1176/ajp.154.11.1557. [DOI] [PubMed] [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, Gorman JM. Rebreathing tests in panic disorder. Biological Psychiatry. 1995;15:240–245. doi: 10.1016/0006-3223(94)00296-F. [DOI] [PubMed] [Google Scholar]
- Perna G, Bertani A, Caldirola D, Gabriele A, Cocchi S, Bellodi L. Antipanic drug modulation of 35% CO2 hyperreactivity and short-term treatment outcome. Journal of Clinical Psychopharmacology. 2002;22:300–308. doi: 10.1097/00004714-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Jones DR, Clark DM. Respiratory control in the treatment of panic attacks: replication and extension with concurrent measurement of behaviour and pCO2. British Journal of Psychiatry. 1986;148:526–532. doi: 10.1192/bjp.148.5.526. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J. Dismantling cognitive-behavioral treatment for panic disorder: Questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, et al. Multicenter collaborative Panic Disorder Severity Scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. International Clinical Psychopharmacology. 1996;11:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Alpers GW, Meuret AE, Roth WT. Respiratory pathophysiology of clinical anxiety outside the laboratory: Assessment of end-tidal pCO2, respiratory pattern variability, and transfer function RSA. In: Fahrenberg J, editor. Progress in ambulatory assessment. Göttingen: Hogrefe & Huber; 2001. pp. 313–343. [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Physiological instability in panic disorder and generalized anxiety disorder. Biological Psychiatry. 2001;49:596–605. doi: 10.1016/s0006-3223(00)01000-3. [DOI] [PubMed] [Google Scholar]
- Woods SW, Charney DS, Loke J, Goodman WK, Redmond DE, Heninger GR. Carbon dioxide sensitivity in panic anxiety. Ventilatory and anxiogenic response to carbon dioxide in healthy subjects and patients with panic anxiety before and after alprazolam treatment. Archives of General Psychiatry. 1986;43:900–909. doi: 10.1001/archpsyc.1986.01800090090013. [DOI] [PubMed] [Google Scholar]