Abstract
Rationale
Transgenic mice with cardiac specific overexpression of mutated αB-crystallin (CryABR120G) display Desmin Related Myopathy (DRM) with dilated cardiomyopathy and heart failure. Our previous studies showed the presence of progressive mitochondrial abnormalities and activation of apoptotic cell death in CryABR120G transgenic hearts. However, the role of mitochondrial dysfunction and apoptosis in the overall course of the disease was unclear.
Objective
We tested the hypothesis that prevention of apoptosis would ameliorate CryABR120G pathology and decrease morbidity.
Methods and Results
We crossed CryABR120G mice to transgenic mice with cardiac specific overexpression of Bcl-2. Sustained Bcl-2 overexpression in CryABR120G hearts prolonged CryABR120G transgenic mice survival by 20%. This was associated with decreased mitochondrial abnormalities, restoration of cardiac function, prevention of cardiac hypertrophy, and attenuation of apoptosis. CryABR120G misfolded protein aggregation was significantly reduced in the double transgenic. However, inhibition of apoptotic signaling resulted in the upregulation of autophagy and alternative death pathways, the net result being increased necrosis.
Conclusion
While Bcl-2 overexpression prolonged life in this DRM model, in the absence of apoptosis, another death pathway was activated.
Keywords: heart disease, mitochondria, apoptosis, protein misfolding, autophagy, necrosis
Introduction
The R120G mutation in αB-crystallin (CryABR120G) causes Desmin Related Myopathy (DRM).1 A mouse model of DRM, created by cardiomyocyte-specific overexpression of CryABR120G, develops progressive dilated cardiomyopathy followed by a transition to heart failure (HF).2 We have previously shown that this model of DRM is characterized by early mitochondrial dysfunction and activation of intrinsic (mitochondrial-based) apoptotic signaling.3 While apoptotic cell death can directly cause HF,4 the sufficiency of apoptosis for DRM-mediated HF specifically, and in protein-conformation based heart disease in general has not been explored.
The intrinsic pathway of apoptosis is regulated by the Bcl-2 family,5 with Bcl-2 being the anti-apoptotic founding member.6 Complex interactions between Bcl-2 family members, such as Bak and Bax can result in permeabilization of the mitochondrial outer membrane.7 Together with MPTP opening, these two processes appear to be a general and, in most cases, terminal commitment step for the decision between cell life and death.8 It is still unclear as to how Bcl-2 protects cells from apoptotic death. Recent studies in experimental animal models demonstrated that overexpression of Bcl-2 prevents apoptosis and injury of cardiomyocytes in post-ischemia and reperfusion hearts.9,10 In addition, high levels of Bcl-2 were sufficient to prevent mitochondrial and contractile dysfunction in desmin null mice.11 Apart from its anti-apoptotic function, Bcl-2 also regulates release of Ca2+ from the endoplasmic reticulum and reduces mitochondrial Ca2+ uptake.12,13
We wished to define the role of apoptosis in DRM pathology and determine the effects of blocking the intrinsic apoptotic pathway in terms of impacting on DRM. By crossing the CryABR120G mice with transgenic (TG) mice harboring cardiac restricted overexpression of Bcl-2,9 we asked if Bcl-2 overexpression could decrease or abolish apoptosis in the CryABR120G cardiomyocytes and if inhibition of apoptosis was sufficient to prevent HF. We found that, in addition to preventing mitochondrial swelling, Bcl-2 overexpression resulted in the upregulation of autophagy, which in turn led to increased elimination of misfolded protein aggregates and a reduction in their overall accumulation. Cardiac-restricted overexpression of Bcl-2 in the CryABR120G mice attenuated cardiomyocyte apoptosis by blocking the intrinsic cell death pathway, but did not prevent their ultimate death as the death process shifted towards necrosis.
Materials and Methods
Transgenic Mice
CryABR120G mice and Bcl-2 overexpressing mice were used as described previously,2,9 except that the Bcl-2 TG mice were first was bred into the FVB/N background by backcrossing the original Bcl-2 TGs to FVB/N animals for at least 7 generations. Three experimental groups were studied: single CryABR120G, Bcl-2 overexpressing double TG CryABR120G/Bcl-2, and age matched non-transgenic (NTG) littermates. TG and double TG mice were identified by PCR analysis of genomic DNA isolated from tail clips. Animals were housed in an AAALAC-approved facility and experiments were approved by the Institution’s Animal Review Board.
Antibodies and Reagents
Anti-tubulin, and polyclonal anti-RIP1 were purchased from Santa Cruz; anti-caspase-3 was from Cell Signaling, monoclonal anti-RIP1 was purchased from BD Biosciences. Anti-Beclin 1 was obtained from Stressgen, and anti-LC3 from Novus. Recombinant human Tissue Necrosis Factor (TNF) was purchased from Sigma.
Evans Blue Staining
Fluorescent staining with Evans blue dye (EBD) was used to assess the impairment of sarcolemmal membrane integrity. Six-month-old mice were injected EBD (10 mg/ml in PBS) intraperitoneally with 0.1 ml per 10g body weight. Twenty-four hours later heart tissue was embedded in OCT Tissue Tek (Sakura Finetechnical), and snap-frozen in liquid nitrogen. Seven-μm sections were cut, washed with PBS, and counterstained by phalloidin (Invitrogen). Stained sections were visualized using the fluorescent microscope, and areas of red auto-fluorescence (EBD positive areas) were selected and imaged.
Cell Culture
3T3 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and antibiotics (25 U/ml penicillin, 25 μg/ml streptomycin) (Invitrogen) in 5% CO2 in air at 37 °C. Replication-deficient adenoviruses for CryAB R120G and Bcl-2 were generated using the AdEasy adenoviral system (Stratagene). Cells were typically infected with adenovirus at a multiplicity of infection of 10 for 2 hours at 37 °C. The cells were then cultured for a further 24 hours in virus-free media before treatment. Cell death was induced with 10 ng/ml of TNF for 10 minutes. For cell death analysis, the Cell Proliferation Kit from Roche was used according to the manufacturer’s protocol.
For autophagy assays, rat neonatal cardiomyocytes (RNC) were used. Cells were plated 24 hours before the infection. On the next day, cells were infected with GFP-LC3 adenovirus (provided by J. Sadoshima, University of Medicine and Dentistry of New Jersey), in combination either with AD-CryABR120G, AD-Bcl-2, or both. Forty-eight hours later, cells were washed, fixed, visualized by fluorescent microscope and analyzed by Image Stream.
Mitochondrial Swelling and Complex I Activity
Mitochondrial swelling experiments were performed as described previously.3 Complex I activity was measured as described.14 Fifty μg of isolated heart mitochondria were diluted in the reaction mixture consisted of 250 mmol/L sucrose, 1.0 mmol/L EDTA, 50 mmol/L Tris-HCl, pH 7.4, 10 umol/L decylubiquinone (Sigma), and 2 mmol/L KCN. The reaction was initiated by adding 50 μmol/L NADH, and monitored continuously at a wavelength of 272 minus 247 nm at 30 °C for 1 minute. Rotenone (5 μg) was added, and any rotenone-insensitive activity was measured for 1 minute.
ImageStream Data Acquisition and Analysis
This fluorescence image-based method for quantifying LC3 relies on differentiating the-diffuse and punctate forms of LC3 staining. Cells infected with adenovirus carrying GFP-LC3 were co-infected with AD-CryABR120G, AD-Bcl-2, or both. Forty-eight hours after infection, cells were trypsinized, fixed, excited with a 488 nm laser light and imaged on a time delay integration CCD camera. Images of fixed cells collected on the ImageStream (Amnis), were analyzed using ImageStream Data Exploration and Analysis Software (IDEAS). Because fluorescent probes have broad emission spectra, spectral compensation was digitally performed on a pixel-by-pixel basis prior to data analysis. After compensation, similarity analysis and spot counting was done on in-focus single cell images. Three samples from each group were analyzed using 10,000 cells per sample.
Statistical Analysis
All data are expressed as mean ± S.E. Comparisons between experimental were determined by 1-way or 2-way ANOVA where appropriate, followed by Student’s t-test. A P value of <0.05 was considered statistically significant.
Results
Overexpression of Bcl-2 Increases Lifespan and Ameliorates Cardiac Dysfunction in CryABR120G Mice
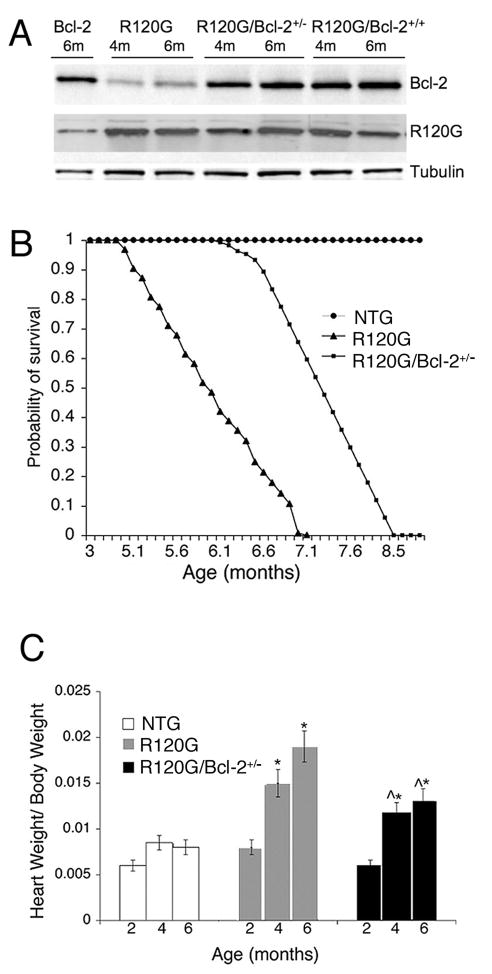
Mice that overexpress Bcl-2 specifically in the cardiomyocytes have been described.8 We crossed those mice repeatedly into the FVB/N background, the strain in which our other transgenic mice were made, in order to avoid strain specific phenotypes. Cardiomyocyte-restricted Bcl-2 overexpression in an FVB/N background was benign with no effects observed on heart structure or function (data not shown). The Bcl-2 transgene continued to be strongly expressed in the hearts of the FVB/N strain (Figure 1A) and when it was crossed into the CryABR120G background as well (Figure 1A), with quantitation of the signals indicating overexpression levels of approximately 5-fold in animals carrying one copy of the Bcl-2 transgene and 9-fold in animals with 2 copies (homozygous for the transgene). We used double transgenic animals that carried 1 copy of each transgene (Bcl-2 and CryABR120G) for all experiments in order to avoid any effects due to insertional mutagenic events. As shown previously, cardiac-restricted expression of CryABR120G leads to premature lethality by the age of 7–8 months (Figure 1B) due to HF.2 Life spans of the CryABR120G/Bcl-2 mice were significantly increased when compared to the CryABR120G mice. However, all of these mice also died prematurely with no survivors by 8.5–9 months of age (Figure 1B).
Figure 1.
A, Western blots showing expression levels in the single and double transgenic mice. When placed in the FVB/N background, the Bcl-2 transgene drove expression levels of between 5- (heterozygote) and 10-fold (homozygote, that is, two copies of the transgene) greater than present in NTG mice, with those levels maintained throughout the adult states (4 months and 6 month levels are shown). Placement of the Bcl-2 transgene in the CryABR120G background did not affect expression of either CryAB or Bcl-2. B, Kaplan-Meier curves for NTG, CryABR120G, and CryABR120G/Bcl-2 mice. C, Cardiac hypertrophy from 2 to 6 months in CryABR120G/Bcl-2 mice compared to CryABR120G. *P<0.05 versus NTG; ^P<0.05 CryABR120G.
We previously showed that overexpression of CryABR120G contributes to the LV dysfunction characterized by the progressive transition from concentric hypertrophy to dilated cardiomyopathy at the age of 6 months.2 In association with their prolonged life span, CryABR120G/Bcl-2 mice displayed an attenuated cardiac hypertrophic response (Figure 1C). To determine whether overexpression of Bcl-2 was sufficient to attenuate adverse cardiac remodeling in CryABR120G mice, we examined cardiac structure using standard morphometric analyses and 2D-directed M-mode echocardiography (Figure 1C and Table 1). Figure 1B shows that heart weight-to-body weight ratios of the single CryABR120G mice at 4 and 6 months were significantly greater than age-matched NTG and CryABR120G/Bcl-2 mice. In addition, marked disparities in cardiac function between the CryABR120G and CryABR120G/Bcl-2 mice were apparent as well (Table 1). At 6 months of age, when the CryABR120G animals were already in failure, the heart rate and shortening fraction of the CryABR120G/Bcl-2 mice were conserved with significantly better LV function (Table 1).
Table 1.
Echocardiography and Tissue Doppler measurements
| NTG | CryABR120G | CryABR120G/Bcl-2 | |
|---|---|---|---|
| IVSD | 0.079±0.005 | 0.115±0.004* | 0.142±0.04* |
| IVSs | 0.13±0.01 | 0.18±0.01* | 0.12±0.006^ |
| LVIDd | 0.365±0.007 | 0.387±0.014 | 0.354±0.007 |
| LVPWd | 0.105±0.002 | 0.119±0.004* | 0.117±0.002* |
| FS | 41.21±1.14 | 31.7±1.5* | 37.15±1.36^ |
| HR | 432±24.89 | 306.16±19.87* | 380.62±17.9^ |
| Septal Aa (mm/sec) | 15.66±3.2 | 4.5±0.3* | 8.02±1.8* |
| Septal Ea/Aa | 2.43±0.5 | 3.11±0.4* | 3.3±0.6 |
| Lateral Ea (mm/sec) | 26.2±2.2 | 12.71±2.0* | 20.32±2.6^ |
| Lateral Aa (mm/sec) | 12.84±1.7 | 7.44±1.8* | 8.45±1.8 |
Echocardiography and tissue Doppler measurements of NTG, CryABR120G, and CryABR120G/Bcl-2 mice. IVSD indicates interventricular septal thickness during diastole; IVSs, interventricular septal thickness during systole; LVIDd, LV internal dimension during diastole; LVPWd, posterior wall thickness during diastole; FS, % fractional shortening; HR, heart rate. Aa, late diastolic myocardial velocity, Ea, early diastolic myocardial velocity.
P<0.05 versus NTG;
P<0.05 versus CryABR120G.
Bcl-2 Expression Prevents Mitochondrial Swelling and Blunts the Apoptotic Response in CryABR120G mice
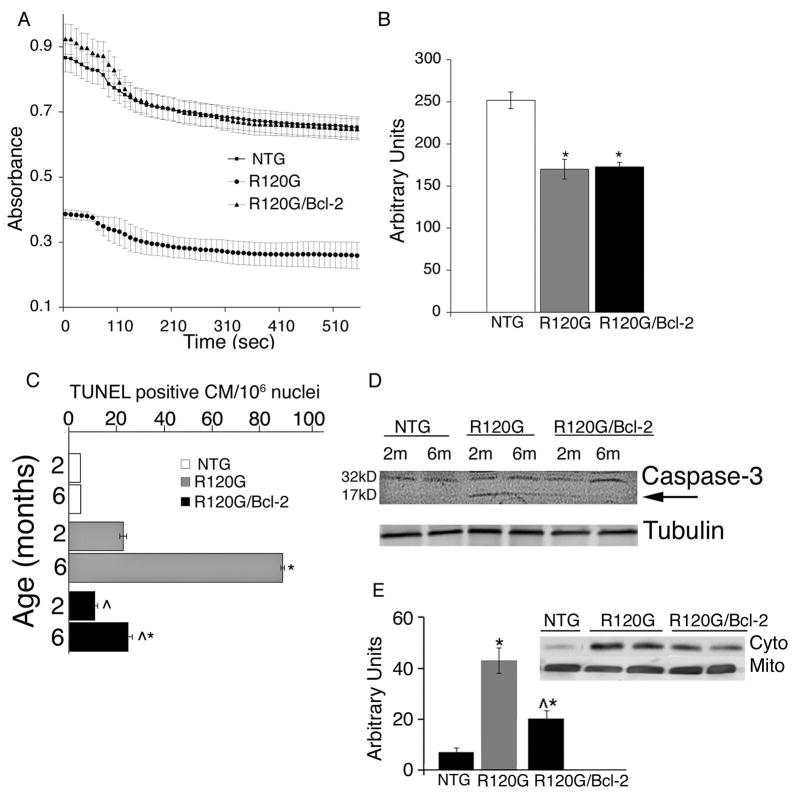
Constitutive overexpression of mutant CryABR120G leads to mitochondrial dysfunction, a precocious and lasting 50% reduction in Complex I activity, opening of the mitochondrial permeability transition pore (MPTP) and activation apoptotic cell death.3 MPTP opening results in mitochondrial swelling and irreversible loss of the matrix and intermembrane content.8 If this cannot be prevented, the outer mitochondrial membrane will eventually rupture, releasing potent apoptogens into the cytoplasm and triggering apoptotic cell death. Pro-apoptotic members of the Bcl-2 family such as Bax and Bak play important roles in mediating mitochondrial swelling. Since the anti-apoptotic family members, Bcl-2 and Bcl-XL are able to prevent the permeabilization of the outer mitochondrial membrane by directly inhibiting Bax and Bak,15 we first analyzed this potential beneficial effect in the CryABR120G/Bcl-2 hearts. Mitochondria isolated from 2 month old CryABR120G mice were extremely sensitive to Ca2+-induced swelling,3 but the mitochondria derived from the CryABR120G/Bcl-2 mice were essentially normal (Figure 2A). At the same time, mitochondrial complex I activity in the CryABR120G/Bcl-2 hearts showed the same deficits apparent in the CryABR120G-derived material, showing that Bcl-2 overexpression is not able to rescue the respiratory deficits in the CryABR120G mitochondria (Figure 2B).
Figure 2.
A, Ca2+-induced swelling of mitochondria from NTG, CryABR120G and CryABR120G/Bcl-2 hearts. Mitochondria from 2 month old CryABR120G hearts were noticeably swollen while mitochondria derived from NTG and CryABR120G/Bcl-2 hearts appeared normal. B, Complex I activity in 2 month old NTG, CryABR120G, and CryABR120G/Bcl-2 hearts. C, Quantification of TUNEL-positive cells in NTG, CryABR120G, and CryABR120G/Bcl-2 hearts at 2 and 6 months of age CM; cardiomyocytes. D, activation of caspase-3 (17 kD form, arrow) in TG, but not in CryABR120G/Bcl-2 hearts; E, The release of cytochrome c from mitochondria to the cytosol was determined by Western blotting in 5-month-old littermate NTG, CryABR120G, and CryABR120G/Bcl-2 hearts. Shown are representative Western blots next to the group data for cytoplasmic cytochrome c levels normalized to tubulin (not shown).
To begin to explore the mechanisms responsible for Bcl-2-mediated attenuation of cardiomyocyte apoptosis we evaluated the extent of DNA fragmentation longitudinally in the NTG, CryABR120G and CryABR120G/Bcl-2 hearts by quantitating TUNEL-positive nuclei located in identified cardiomyocytes. We observed significant decreases in the number of TUNEL positive nuclei in 2 and 6 month old CryABR120G/Bcl-2 hearts as compared to CryABR120G (Figure 2C). We confirmed that we were observing decreases in apoptosis by measuring reduced levels of activated caspase-3 in the CryABR120G/Bcl-2 but not in CryABR120G hearts (Figure 2D).
We then measured the release of cytochrome c from mitochondria into the cytosol in the hearts of 5-month-old CryABR120G and CryABR120G/Bcl-2 mice. Representative Western blots derived from the mitochondrial and cytosolic proteins showed that in CryABR120G/Bcl-2 mouse hearts, cytosolic cytochrome c levels were significantly lower than in the CryABR120G hearts although they remained higher than the level observed in the NTG littermates (Figure 2E).
Protein Aggregation in CryABR120G/Bcl-2 Mice is Reduced
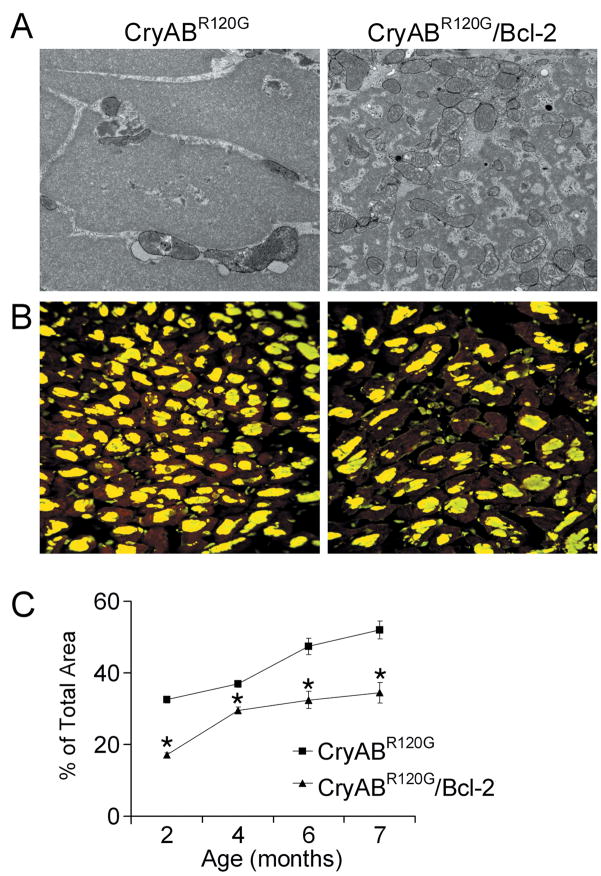
CryABR120G-mediated DRM is a protein misfolding disease characterized by accumulation of insoluble protein depositions, whose accumulations can have both toxic14 and protective functions.16 Although expression of Bcl-2 in the CryABR120G hearts did not completely prevent aggregate formation, electron microscopy revealed major disparities in protein deposition architecture. The aggresomes16,17 in the CryABR120G hearts appeared to be largely homogenous, consisting of uniformly solid, granulofilamentous bodies, while in the CryABR120G/Bcl-2 hearts they appeared as heterogeneous masses of small electron-dense material interspersed with mitochondria (Figure 3A). Immunofluorescent staining for CryAB was used to quantitate the cytoplasmic area occupied by the aggresomes (Figure 3B) and confirmed significant decreases in their accumulation at all ages measured (Figure 3C).
Figure 3.
Overexpression of Bcl-2 significantly reduced the accumulation of misfolded proteins in CryABR120G hearts. A, Electron microscopy images of protein aggregates from 5-month-old CryABR120G and CryABR120G/Bcl-2 hearts. B, Immunofluorescence staining for CryAB (green) with troponin I counterstaining (red) shows decreased aggregate levels. C, Time-dependent quantification of CryAB aggregates using MetaMorph software shows a decreased slope for aggregate accumulation in CryABR120G/Bcl-2 hearts. *P<0.05 versus CryABR120G.
Protein aggregates may form by abnormal folding or by disturbance of intracellular protein degradation pathways. While autophagy is known to play a critical role in the major degradation pathways for cellular protein and organelles, it is becoming increasingly apparent that autophagy is an important cellular process that can be activated in response to the accumulation of misfolded proteins.18 Autophagy is upregulated in rat neonatal cardiomyocytes infected with CryABR120G adenovirus and blunting autophagy in CryABR120G hearts accelerated and increased development of the cardiac pathology.19
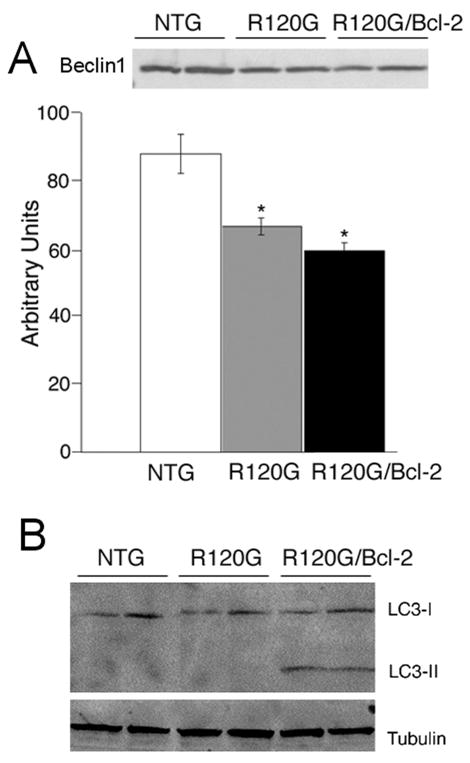
Given those data and the observation that CRYABR120G/Bcl-2 hearts displayed significant decreases in protein aggregate levels, we wished to evaluate and compare the autophagic response in CryABR120G and CryABR120G/Bcl-2 hearts. Beclin 1 is considered to be a key player in autophagy. Beclin 1 promotes autophagic activity20 and is involved in the recruitment of membranes to form the autophagosome. However, in contrast to our expectation that autophagy would be increased in both the CryABR120G and CryABR120G/Bcl-2 hearts, we observed modest but significant reductions in Beclin 1 levels in both the CryABR120G and CryABR120G/Bcl-2 hearts relative to NTG (Figure 4A).
Figure 4.
Autophagy in CryABR120G and CryABR120G/Bcl-2 mice. A, Representative Western Blot images and quantification for Beclin 1 in 6-month-old CryABR120G and CryABR120G/Bcl-2 hearts. B, Appearance of LC3II as a marker of autophagy. Western Blots were probed with anti-LC3 antibody. Tubulin was used as a loading control, n=4.
Given the complex nature of autophagy, we analyzed additional markers of the process. Formation and maturation of autophagosomes is accompanied by intracellular translocation and processing of microtubule-associated protein 1 light chain 3 (LC3),21 an important component of the autophagosome membrane and one of the most reliable markers of autophagy. Western Blot analysis showed accumulation of cleaved LC3-II exclusively in the hearts of CryABR120G/Bcl-2 but not CryABR120G mice, suggesting a significant augmentation of an autophagic response in the double transgenic mice (Figure 4B).
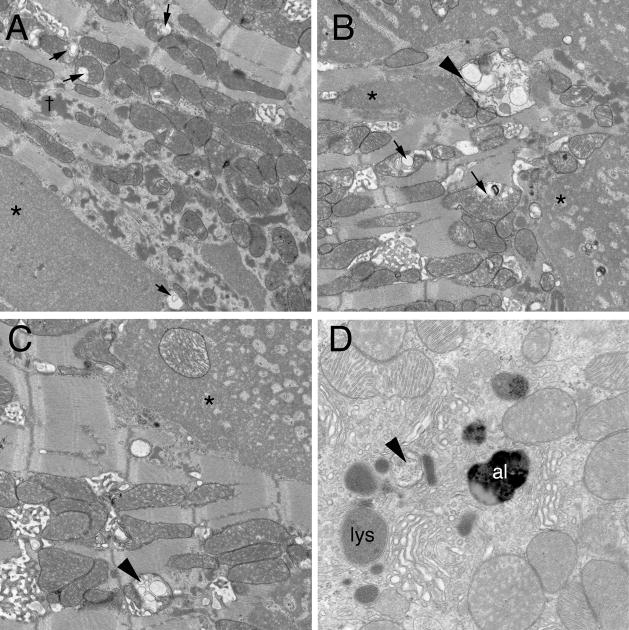
In order to gain a qualitative assessment of autophagy, we carried out transmission electron microscopy on hearts derived from the single and double transgenic hearts at 5 months. As expected, the CryABR120G hearts showed the usual pathology at this stage with significant accumulations of large, granulofilamentous aggregates, disrupted mitochondria with massive cristae lysis and sarcomere disruption. However, strikingly, there was a paucity of any structures that could be identified as either intermediates or late stages in the autophagic pathway (Figure 5A). In contrast, the fields from the double transgenic hearts contain numerous intermediates/participants in the autophagic pathway, including amphisomes, the autophagic vacuoles formed upon fusion between autophagosomes and endosomes, lysosomes and autolysosomes (Figure 5B–D).
Figure 5.
EM analyses of CryABR120G and CryABR120G/Bcl-2 transgenic hearts. Heart were harvested at 5 months and subjected to TEM analyses. Fields were randomly selected from multiple samples from each experimental cohort and viewed at 20,000× or 40,000× (panel D) magnification. A, Typical morphology observed in a 5 month, CryABR120G ventricle midwall. Type 1 (*) and Type 2 (†) aggregates and the frequent abnormal mitochondria showing cristae lysis and abnormal internal membrane whorls (arrows) are readily apparent.12 B-D, Three fields from sections derived from the CryABR120G/Bcl-2 transgenic hearts. Amphisomes (arrowheads), autolysosomes (al) and lysosomes (ly) were invariably present.
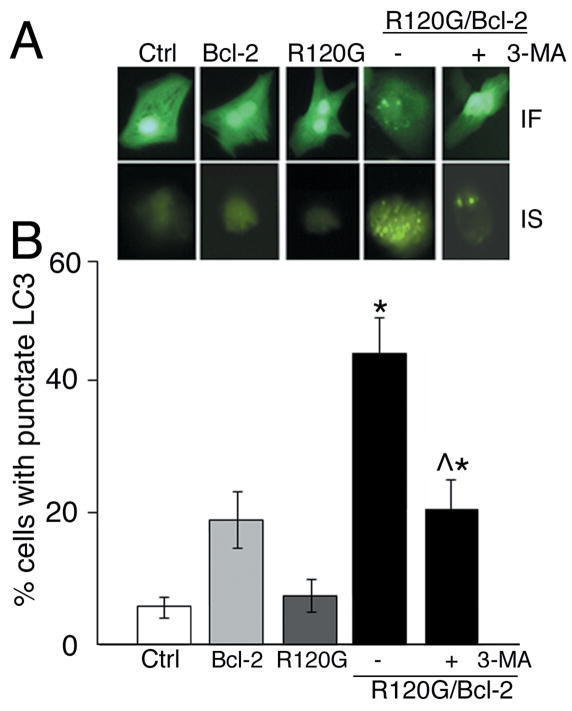
To quantitate the increase in autophagic activity in response to Bcl-2 overexpression, neonatal rat cardiomyocytes were infected with GFP-LC3 - autophagy–reporter adenovirus, followed by infection with AD-CryABR120G, AD-Bcl-2 or a combination of both. Forty-eight hours later cells were fixed, visualized by fluorescent microscope and analyzed using the Image Stream system (Figure 6). LC3 undergoes a significant redistribution during autophagy, changing from a diffused cytosolic signal to punctate dots that mark the mature autophagosome.22 After 48 hours, the abundance of punctuated LC3 was almost 5-fold higher in the cardiomyocytes infected with both AD-Bcl-2 and AD-CryABR120G in comparison to AD-CryABR120G only (Figure 6B). Blockade of autophagosome formation using 3-MA significantly reduced the accumulation of punctuated LC3 in R120G/Bcl-2 infected cardiomyocytes.
Figure 6.
Immunofluorescence (IF) for GFP-LC3 and ImageStream (IS) analyses of autophagic activity. A, Representative images of individual infected cells obtained using fluorescent microscope and the ImageStream cytometer. Rat neonatal cardiomyocytes expressing GFP-tagged LC3 only (Control; Ctrl) or in combination with Bcl-2, CryABR120G (R120G) as well as R120G and Bcl-2 (R120G/Bcl-2) together in the absence and presence of 10 μM 3-MA. B, quantification of % of cells with punctate LC3 using IDEAS software. For each cell, the punctate LC3 was differentiated from the diffused signal and the number of spots calculated. *P<0.005 versus NTG, *^P<0.005 versus CryABR120G.
Blunting the Apoptotic Response Leads to Necrosis in CryABR120G/Bcl-2 Mice
Our data showed that overproduction of the anti-apoptotic protein Bcl-2 increased the life span of the CryABR120G animals, indicating that the intrinsic apoptotic pathway does play a pathogenic role in DRM. However, the animals still die prematurely, so abrogation of apoptosis is clearly not sufficient to rescue the phenotype. Historically, apoptosis has been defined as a type of cell death that is morphologically distinct from other types of cell death such as necrosis.23 Despite the long held view that necrosis is an accidental and uncontrolled form of cell death, recent studies have revealed that necrosis is probably tightly controlled, and intriguing interactions between the apoptotic and necrotic machinery have been uncovered.24 Eliminating genes encoding proteins controlling apoptosis resulted in the conversion of an apoptotic phenotype to a necrotic one, both in vitro and in vivo.25 We hypothesized that by overexpressing Bcl-2 and decreasing apoptotic flux in the absence of not affecting the primary genetic lesion (expression of CryABR120G); we might have shifted the cell death balance to necrosis.
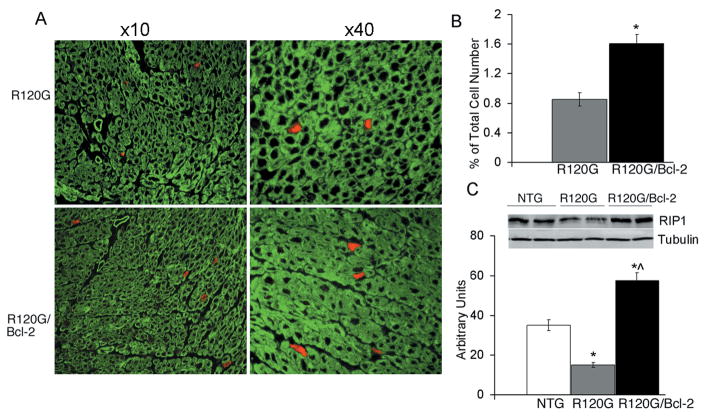
Evans Blue dye (EBD) analyses were performed in order to detect necrotic cell death-related membrane abnormalities in the myocardium of NTG, CryABR120G and CryABR120G/Bcl-2 hearts (Figure 7A). NTG cardiomyocytes were impervious to EBD (not shown). In the hearts from 6 month CryABR120G mice, which are undergoing extensive apoptotic cell death,3 sporadic EBD-positive myocytes were observed (Figure 7A). However, the number of EDB-positive cells in CryABR120G/Bcl-2 hearts were significantly increased (Figure 7A, lower panel and B).
Figure 7.
A, Evans blue permeability of cardiomyocytes derived from CryABR120G and CryABR120G/Bcl-2 hearts. Sections were counterstained with phalloidin (green) and visualized by fluorescent microscopy at magnifications of ×10 and ×40. B, Quantification of EBD-positive cells. *P<0.005 versus CryABR120G. C, Protein derived from CryABR120G and CryABR120G/Bcl-2 hearts were electrophoresed in SDS-PAGE, Western blotted and probed with anti-RIP1 and anti-tubulin antibodies. D, Quantification of RIP1 levels using ImageJ software, *P<0.005 versus NTG, ^P<0.005 versus CryABR120G.
Pharmacological and genetic evidence suggests that necrosis can occur in a tightly regulated manner.26 Cell death inducers such as TNF promote either apoptosis or necrosis depending on the specific experimental setting.27 When apoptotic cell death is inhibited by anti-apoptotic factors, a process of programmed necrotic death can occur.28 Although the proteins regulating necrosis are largely undefined, recent data indicate that some regulatory mechanisms may be shared or intersect with apoptosis,29 and RIP1 kinase may play an important role in deciding between necrosis and survival when the apoptotic signal is blocked.29 Consistent with this hypothesis, we found significantly higher RIP1 levels in 6 month old CryABR120G/Bcl-2 hearts compared to NTG and CryABR120G samples (Figure 7C and D). Interestingly, CryABR120G hearts showed markedly reduced RIP1 levels.
We tested the hypothesis that co-expression of Bcl-2 and CryABR120G is sufficient to cause increased levels of RIP1. 3T3 cells were infected with adeno-CryABR120G (R group), adeno-Bcl-2 (B group), or with both (RB group) and RIP1 accumulation determined 24 hours later by immunohistochemistry (Online Figure IA). Confirming the hypothesis, RB cells showed more than a 7-fold accumulation of RIP1 compared to R, B and control cells (Online Figure IB).
We next tested whether accumulation of RIP1 was sufficient to increase the sensitivity of infected 3T3 cells to TNF-mediated necrosis (Online Figure IC). Cells were treated with low levels of TNF (Materials and Methods) and the level of cell death was evaluated. While non-transfected, R120G and Bcl-2-infected cells showed only minor responses to TNF, cell death in RB cells increased by 100%, indicating a significantly elevated sensitivity of RB cells to TNF-mediated necrosis. However, when the effect of TNF was analyzed in the presence of necrostatin, a specific inhibitor of RIP1, the sensitizing effect was completely abrogated.
Discussion
Several etiologic factors have been linked to the onset and development of dilated cardiomyopathy and HF in the CryABR120G mice. These include mitochondrial abnormalities, myofilament disarray, reduction in contractile function due to accumulation of insoluble aggregates, and apoptotic cell loss.3,14,17,30 Each of these parameters contributes to CryABR120G cardiac pathogenesis. In this study, we wished to explore the role of apoptosis in the progression of HF in CryAB-mediated DRM and took a genetic approach to inhibit cardiomyocyte apoptosis by driving overexpression of anti-apoptotic Bcl-2 in the hearts of CryABR120G mice.
There are considerable data suggesting that apoptotic cell death can play an important or even determining role in progressive cardiac remodeling,31,32 with overexpression of either intrinsic or extrinsic apoptotic pathway components causing dilated cardiomyopathy.28 Previous studies suggest a beneficial effect for increased Bcl-2 expression in different genetic models of cardiomyopathy, including the desmin nulls, a model of ischemic/reperfusion injury, as well as in CryABR120G mice with cardiac restricted overexpression of secretable TNF.10,11,31 We show here that overproduction of Bcl-2 in the heart not only reduces the level of CryABR120G-induced cardiac apoptosis but also impacts, albeit temporarily, on adverse cardiac remodeling, progressive hypertrophy and decreased fractional shortening. While abrogation of apoptosis slows loss of cardiac function, it does not halt a pathogenic progression that results in eventual dilation and HF.
In addition to clarifying the role of apoptosis in this model, our data provide several new insights into the mechanism of CryABR120G-mediated pathology. As is the case for most protein conformation-based disease, CryABR120G-DRM is characterized by the accumulation of abnormal protein aggregates.2 Autophagy can be involved in the intracellular degradation of aggregation–prone proteins, such as alpha-synuclein and huntingtin,33,34 and Beclin 1 is one of the key players in autophagosome formation.35 However, despite the increase in the number of lysosomes and autophagosomes in both CryABR120G and CryABR120G/Bcl-2 mice, the level of Beclin 1 was significantly reduced in these models. This is in agreement with studies from other groups showing Beclin 1 reduction in other protein misfolding diseases such as Alzheimer or Huntington.36 How Beclin 1 is regulated in cardiomyocytes and why it is decreased in the latter stages of the DRM model remains unclear. Overexpression of mutant CryABR120G itself did not directly result in reduced Beclin 1 levels. However, the BH3 domain of Beclin 1 can bind directly to Bcl-2,37 with the interaction actually inhibiting autophagy but leaving the anti-apoptotic activity of Bcl-2 intact.38 In beginning to ask how overexpression of Bcl-2 affects the autophagic status of CryABR120G mice, we find it intriguing that critical players in the apoptotic and autophagic pathways can interact. Unlike other studies showing the anti-autophagic activity of Bcl-2,39 the autophagic response remained largely intact as evidenced by increased LC3-II levels and the significant reduction in protein aggregate accumulation. Activation of autophagy under conditions of inhibited apoptotic cell death has been previously documented in Bak/Bax knockout mice.40
The precise role of autophagy in the progression of HF remains unclear. In some HF models, amplification of the autophagic response led to pathologic hypertrophy, decreased cardiac performance, and promotion of autophagic cell death.41 In other studies, autophagy protected the heart by scavenging and eliminating misfolded proteins.42 It appears that autophagy can lead to different outcomes depending upon disease context and severity. Future studies using genetic approaches are underway to define the exact role of autophagy in our model at the different disease stages.
Necrosis has been often viewed as an accidental and unregulated event but data are now rapidly accumulating that suggest necrosis can also be thought of as programmed cell death.43 The cell death program, whether by apoptosis or by necrosis, is mediated through an integrated cascade, which can be assessed at multiple junction sites and propagated through numerous branch points.29 Recent studies point to RIP1 as a possible “molecular switch” that can determine whether the cell succumbs through apoptosis or necrosis.44 In our model, overproduction of Bcl-2 led to increased RIP1 with a concomitant increase in necrosis and these admittedly preliminary but intriguing data point the way for further exploration of whether these markers are general or more specific to different but related cell death processes.
That overexpression of Bcl-2 leads to a reversal of calcium sensitivity of the mitochondria and may lead to a switch from apoptosis to autophagy and necrosis argues strongly for the existence of complex regulatory interactions between these scavenger/cell death mechanisms and could explain the limited success of anti-apoptotic therapies. An additional complication is the different roles these processes could play at different times in disease progression and presentation. Although considerable work will be required to explain the observations outlined in this manuscript, an appreciation of the complexities and understanding the underlying molecular pathways will help define effective combinational therapies.
Novelty and Significance.
What is Known?
Mutations in αB-crystallin can cause a dilated cardiomyopathy and heart failure.
Although apoptotic cell death contributes to the development of cardiac pathology, its necessity and sufficiency in the cardiac pathogenesis is not known.
What New Information Does This Article Contribute?
In vivo evidence that death pathways other than apoptosis are capable of causing cardiomyopathy and heart failure in this animal model
First demonstration in this animal model that abrogating the intrinsic pathway of apoptosis in the heart is possible and that, while life is prolonged, the animal still dies prematurely
Indirectly supports a model for the interlinking of different death pathways in the pathogenic process that leads to cardiac death.
The missense R120G mutation in the small heat shock protein alpha-B-crystallin (CryABR120G) causes a desmin-related myopathy (DRM). This skeletal and cardiac disease is characterized by the formation of desmin- and CryAB-containing aggregates within muscle fibers, including those within the heart. Mice with cardiac-specific overexpression of CryABR120G develop severe cardiomyopathy at 3 months of age and die at 6–7 months from heart failure. Previous studies showed that overexpression of CryABR120G results in formation of perinuclear aggresomes, and accumulation of pre-amyloid oligomer (PAO). PAO is considered to be the cytotoxic entity in many of protein misfolding-based diseases and we noted extensive apoptosis in these hearts. Because of this, we asked whether we could impact materially on disease progression by interfering with apoptosis and so, we crossed the CryABR120G mice with a mouse that specifically expressed the anti-apoptotic molecule, Bcl-2, in cardiomyocytes. To our surprise, while apoptosis was largely ameliorated, the mice still died although they lived 20% longer than mice that did not express increased levels of Bcl-2. In order to understand the cause of death, we explored whether other death pathways were activated and found that, indeed, while apoptosis was shut down, necrosis was activated. These findings have implications for using the death pathways as therapeutic targets and indicate that multiple pathway interactions will need to be considered and tested for any potential therapeutics aimed at a single pathway.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants P01HL69799, P50HL074728, P50HL077101, P01HL059408, R01HL087862 (J.R.)
Abbreviations
- HF
heart failure
- CryAB
αB-crystallin
- EBD
Evans Blue Dye
- RNC
rat neonatal cardiomyocytes
- MPTP
Mitochondrial Permeability Transition Pore
- RIP1
Receptor Interacting Protein 1
- TNF
Tumor Necrosis Factor
- TG
transgenic
- NTG
non-transgenic
Footnotes
Disclosures
None
References
- 1.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 3.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation. 2005;112:3451–3461. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirshenbaum LA, de Moissac D. The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation. 1997;96:1580–1585. doi: 10.1161/01.cir.96.5.1580. [DOI] [PubMed] [Google Scholar]
- 6.Garland JM, Halestrap A. Energy metabolism during apoptosis. Bcl-2 promotes survival in hematopoietic cells induced to apoptose by growth factor withdrawal by stabilizing a form of metabolic arrest. J Biol Chem. 1997;272:4680–4688. doi: 10.1074/jbc.272.8.4680. [DOI] [PubMed] [Google Scholar]
- 7.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 10.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 11.Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A. 2004;101:769–774. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloyan A, Osinska H, Lammerding J, Lee RT, Cingolani OH, Kass DA, Lorenz JN, Robbins J. Biochemical and mechanical dysfunction in a mouse model of desmin-related myopathy. Circ Res. 2009;104:1021–1028. doi: 10.1161/CIRCRESAHA.108.193516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 16.Sanbe A, Osinska H, Villa C, Gulick J, Klevitsky R, Glabe CG, Kayed R, Robbins J. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2005;102:13592–13597. doi: 10.1073/pnas.0503324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–10136. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 21.Zakeri Z, Melendez A, Lockshin RA. Detection of autophagy in cell death. Methods Enzymol. 2008;442:289–306. doi: 10.1016/S0076-6879(08)01415-8. [DOI] [PubMed] [Google Scholar]
- 22.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanlangenakker N, Berghe TV, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 25.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 26.Golstein P, Kroemer G. A multiplicity of cell death pathways. Symposium on apoptotic and non-apoptotic cell death pathways. EMBO Rep. 2007;8:829–833. doi: 10.1038/sj.embor.7401042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 29.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloyan A, Gulick J, Glabe CG, Kayed R, Robbins J. Exercise reverses preamyloid oligomer and prolongs survival in alphaB-crystallin-based desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:5995–6000. doi: 10.1073/pnas.0609202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117:2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravikumar B, Sarkar S, Rubinsztein DC. Clearance of mutant aggregate-prone proteins by autophagy. Methods Mol Biol. 2008;445:195–211. doi: 10.1007/978-1-59745-157-4_13. [DOI] [PubMed] [Google Scholar]
- 34.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 35.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 36.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 38.Ciechomska IA, Goemans GC, Skepper JN, Tolkovsky AM. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28:2128–2141. doi: 10.1038/onc.2009.60. [DOI] [PubMed] [Google Scholar]
- 39.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 43.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Galluzzi L, Kepp O, Kroemer G. RIP Kinases Initiate Programmed Necrosis. J Mol Cell Biol. 2009 doi: 10.1093/jmcb/mjp007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.