Abstract
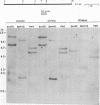
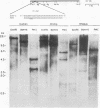
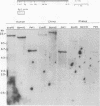
Endogenous retrovirus-related sequences exist within the normal genomic DNA of all eukaryotes, and these endogenous sequences have been shown to be important to the nature and biology of related exogenous retroviruses and may also play a role in cellular functions. To date, no endogenous sequences related to human immunodeficiency virus type 1 (HIV-1) have been reported. Herein we describe the first report of the presence of nucleotide sequences related to HIV-1 in human, chimpanzee, and rhesus monkey DNAs from normal uninfected individuals. We also present the isolation and characterization of two of these endogenous HIV-1-related sequences, EHS-1 and EHS-2. With use of low-stringency Southern blot hybridization, complex banding patterns were detected in human DNA with 5' and 3' HIV-1-derived probes. When an HIV-1 env region probe was used, we detected a less complex, conserved banding pattern in human DNA as well as a related but distinct banding pattern in chimpanzee and rhesus monkey DNAs. EHS-1 and -2 were cloned from normal human genomic DNA libraries by using the env region probe. Clone EHS-1 shows sequence similarity with the domain of the envelope cellular protease cleavage site of HIV-1, while EHS-2 has sequence similarity to the overlapping reading frame for Rev and gp41. Stringent hybridization of EHS-1 back to primate genomic DNA indicates two distinct EHS-1 loci in normal human DNA, an identical band pattern in chimpanzee DNA, and a single locus in rhesus monkey DNA. Likewise, EHS-2 is present as a single highly conserved locus in all three species. An oligonucleotide derived from EHS-2 across a region of near identity to HIV-1 detects a complex banding pattern in all primates tested similar to that seen with the 3' HIV-1 probe. These data suggest that most of the HIV-1-related sequences identified in primate DNA share a common core of nucleic acid sequence found in both EHS-2 and rev and that some of these HIV-1-related sequences have additional larger regions of sequence similarity to HIV-1.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham C. R., Daenke S., Phillips R. E., Cruickshank J. K., Bell J. I. Enzymatic amplification of exogenous and endogenous retroviral sequences from DNA of patients with tropical spastic paraparesis. EMBO J. 1988 Dec 20;7(13):4179–4184. doi: 10.1002/j.1460-2075.1988.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V., Pawlita M. Mutational analysis of the human immunodeficiency virus type 1 env gene product proteolytic cleavage site. J Virol. 1990 May;64(5):2337–2344. doi: 10.1128/jvi.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Tronick S., Schlom J. Detection and cloning of human DNA sequences related to the mouse mammary tumor virus genome. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5503–5507. doi: 10.1073/pnas.79.18.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien U. H., Lai M., Shih T. Y., Verma I. M., Scolnick E. M., Roy-Burman P., Davidson N. Heteroduplex analysis of the sequence relationships between the genomes of Kirsten and Harvey sarcoma viruses, their respective parental murine leukemia viruses, and the rat endogenous 30S RNA. J Virol. 1979 Sep;31(3):752–760. doi: 10.1128/jvi.31.3.752-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Drivas G. T., Shih A., Coutavas E., Rush M. G., D'Eustachio P. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol Cell Biol. 1990 Apr;10(4):1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie C. T., Resnick R., Boyce-Jacino M., Alegre J. N., Faras A. J. Molecular cloning and characterization of gag-, pol-, and env-related gene sequences in the ev- chicken. J Virol. 1986 Sep;59(3):669–675. doi: 10.1128/jvi.59.3.669-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie C., Faras A. J. Presence of retrovirus reverse transcriptase-related gene sequences in avian cells lacking endogenous avian leukosis viruses. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5097–5101. doi: 10.1073/pnas.82.15.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Mutational analysis of the cleavage sequence of the human immunodeficiency virus type 1 envelope glycoprotein precursor gp160. J Virol. 1989 Nov;63(11):4670–4675. doi: 10.1128/jvi.63.11.4670-4675.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Harada F., Tsukada N., Kato N. Isolation of three kinds of human endogenous retrovirus-like sequences using tRNA(Pro) as a probe. Nucleic Acids Res. 1987 Nov 25;15(22):9153–9162. doi: 10.1093/nar/15.22.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Kröger B., Horak I. Isolation of novel human retrovirus-related sequences by hybridization to synthetic oligonucleotides complementary to the tRNA(Pro) primer-binding site. J Virol. 1987 Jul;61(7):2071–2075. doi: 10.1128/jvi.61.7.2071-2075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S., Siomi H., Satoh T., Endo S., Maki M., Hatanaka M. Functional similarity of HIV-I rev and HTLV-I rex proteins: identification of a new nucleolar-targeting signal in rev protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):963–970. doi: 10.1016/0006-291x(89)90767-5. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Lewis N., Williams J., Rekosh D., Hammarskjöld M. L. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J Virol. 1990 Apr;64(4):1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Hauber J., Cullen B. R. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Fenrick R., Cullen B. R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Tiley L. S., McCarn D. F., Rusche J. R., Hauber J., Cullen B. R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990 Feb 23;60(4):675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C., O'Brien S., Nash W. G., Cohen M. ERV3, a full-length human endogenous provirus: chromosomal localization and evolutionary relationships. Virology. 1984 Oct 30;138(2):225–235. doi: 10.1016/0042-6822(84)90347-7. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Perl A., Rosenblatt J. D., Chen I. S., DiVincenzo J. P., Bever R., Poiesz B. J., Abraham G. N. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989 Sep 12;17(17):6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Shibata R., Miura T., Hayami M., Ogawa K., Kiyomasu T., Ishimoto A., Adachi A. Complementation of the rev gene mutation among human and simian lentiviruses. J Virol. 1990 May;64(5):2202–2207. doi: 10.1128/jvi.64.5.2202-2207.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargan D. R., Bennet I. D. A transcriptional map of visna virus: definition of the second intron structure suggests a rev-like gene product. J Gen Virol. 1989 Aug;70(Pt 8):1995–2006. doi: 10.1099/0022-1317-70-8-1995. [DOI] [PubMed] [Google Scholar]
- Shih A., Misra R., Rush M. G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989 Jan;63(1):64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26(1-2):99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Derse D., Rice N. R. Cloning and characterization of cDNAs encoding equine infectious anemia virus tat and putative Rev proteins. J Virol. 1990 Aug;64(8):3716–3725. doi: 10.1128/jvi.64.8.3716-3725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh L. K., Chinnadurai G. Mutants in a conserved region near the carboxy-terminus of HIV-1 Rev identify functionally important residues and exhibit a dominant negative phenotype. Virology. 1990 Sep;178(1):327–330. doi: 10.1016/0042-6822(90)90414-m. [DOI] [PubMed] [Google Scholar]
- Venkatesh L. K., Mohammed S., Chinnadurai G. Functional domains of the HIV-1 rev gene required for trans-regulation and subcellular localization. Virology. 1990 May;176(1):39–47. doi: 10.1016/0042-6822(90)90228-j. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Sakai K., Stevenson M., Dewhurst S. Retroviral etiology of the acquired immune deficiency syndrome (AIDS). AIDS Res. 1986 Dec;2 (Suppl 1):S35–S48. [PubMed] [Google Scholar]