Abstract
The aim of this project was to demonstrate that an oncolytic herpes simplex virus type 1 (HSV-1) can replicate in a tissue- and tumor-specific fashion through both transcriptional (prostate-specific promoter, ARR2PB) and translational (5′-untranslated regions (5′UTRs) of rFGF-2) regulation of an essential viral gene, ICP27. We generated two recombinant viruses, ARR2PB-ICP27 (A27) and ARR2PB-5′UTR-ICP27 (AU27) and tested their efficacy and toxicity both in vitro and in vivo. The ARR2PB promoter caused overexpression of ICP27 gene in the presence of activated androgen receptors (ARs) and increased viral replication in prostate cells. However, this transcriptional upregulation was effectively constrained by the 5′UTR-mediated translational regulation. Mice bearing human prostate LNCaP tumors, treated with a single intravenous injection of 5 × 107 plaque-forming units (pfu) of AU27 virus exhibited a >85% reduction in tumor size at day 28 after viral injection. Although active viral replication was readily evident in the tumors, no viral DNA was detectable in normal organs as measured by real-time PCR analyses. In conclusion, a transcriptional and translational dual-regulated (TTDR) viral essential gene expression can increase both viral lytic activity and tumor specificity, and this provides a basis for the development of a novel tumor-specific oncolytic virus for systemic treatment of locally advanced and metastatic prostate cancers.
Introduction
Prostate cancer remains the most commonly diagnosed non-skin-cancer and the second leading cause of cancer death in men.1 Although hormone withdrawal therapy is the only effective treatment for advanced/metastatic prostate cancer, the disease will eventually recur and become castration-resistant (hormone refractory). Although docetaxel-based combination therapies for treatment of hormone-refractory prostate cancer showed some improvements, the median survival is still only ~18 months (refs. 2,3). Hence, more effective, new treatment regimens are urgently needed to improve patient survival. Oncolytic viruses are promising therapeutic agents for treatment of various cancers as they have the potential to specifically replicate in and lyse tumor cells while sparing normal tissues.4 Current oncolytic herpes simplex virus type 1 (HSV-1) viral vectors in clinical trials contain deletions of genes that are involved in neurovirulence, which may result in a much more attenuated virus with reduced replication efficiency. Therefore, it may be better to maintain as much of the viral genome as possible by modifying only the regulator elements flanking essential viral genes to achieve tumor-specific replication. We hypothesize that HSV-1 replication can be further regulated at the translational level by employing the 5′-untranslated regions (5′UTRs) that form complex secondary structures.5 The 5′UTRs from genes that are commonly associated with malignant progression and metastasis are rich in GC residues and can form extensive secondary hairpin structures to repress the translation of their downstream mRNAs in normal cells.6,7,8 These secondary hairpin structures can be unwound in the presence of sufficient eukaryotic initiation factor (eIF)4E/eIF4F complexes, leading to translation initiation of the mRNA.9 The eIF4E protein, part of the eIF4F complex, plays a crucial role in the initiation of translation process by binding to the 5′cap (7-methylguanosine) of mRNA transcripts. Its overexpression has been reported in various cancer types, including prostate cancer, and can lead to increased translation of cancer-associated genes to enhance cell proliferation, suppress apoptosis, and induce malignant transformation.9,10,11 Recent data also suggest that elevated eIF4E activation is common in advanced prostate cancer and is associated with progression to a hormone-refractory state resulting in reduced patient survival.12
In this study, our aim was to demonstrate that through dual regulation of an essential viral gene at both the transcriptional and translational level by the prostate-specific promoter ARR2PB and the rFGF-2 5′UTR, respectively, a TTDR-oHSV (transcriptional and translational dual-regulated oncolytic HSV) would show high selectivity for tumor cells, resulting in tumor-specific oncolysis. Two recombinant viruses, ARR2PB-ICP27 (A27) and ARR2PB-5UTR-ICP27 (AU27) (Figure 1), were generated by inserting the ARR2PB promoter in front of the essential ICP27 gene with or without the 5′UTR from rFGF-2, and tested both in vitro and in vivo. Our results showed that dual regulation of HSV-1 viral replication significantly enhances tumor specificity while retaining high efficiency of tumor lysis.
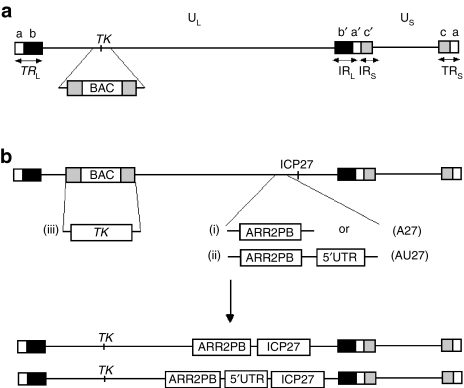
Figure 1.
Schematic diagram showing the construction of the recombinant viruses ARR2PB-ICP27 and ARR2PB-5′UTR-ICP27. (a) Bacterial artificial chromosome (BAC) was inserted into the thymidine kinase (TK) locus by homologous recombination. (b) ARR2PB-ICP27 (A27) and ARR2PB-5′UTR-ICP27 (AU27) recombinant viruses were generated by recombineering method, which replaced the endogenous ICP27 promoter with (i) ARR2PB promoter or (ii) ARR2PB promoter + 5′UTR from FGF-2. (iii) TK gene was then rescued by homologous recombination in 2-2 cells with hypoxanthine–aminopterin–thymidine selection.
Results
eIF4E is overexpressed in prostate cancer cells
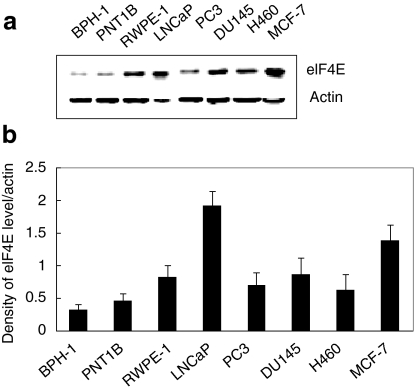
As we previously reported,11 the eIF4E expression level was higher in cancer cells than in noncancerous cells with the highest expression levels observed in LNCaP prostate and MCF-7 breast cancer cell lines (Figure 2). When compared to that in LNCaP cells, a six- and fourfold reduction in eIF4E levels was observed in BPH-1 and PNT1B cells, respectively (P < 0.002). Although both BPH-1 and PNT1B cells exhibited low eIF4E expression, nonmalignant PNT1B cells were chosen as a control cell line to study the transcriptional and translational regulation of ICP27 expression as PNT1B cells express the androgen receptor (AR), which is necessary for ARR2PB promoter activity.
Figure 2.
eIF4E expression level in cell lines. Total protein was extracted from normal human epithelial prostate cell lines (BPH-1, PNT1B, RWPE-1), human prostate cancer cell lines (LNCaP, PC-3, DU145), lung caner cell line (H460), and breast cancer cell line (MCF-7). (a) eIF4E and β-actin expression was determined by western blot analysis. (b) Density ratio of eIF4E levels normalized to β-actin (means ± SD, n = 3).
ICP27 expression is regulated at both transcriptional and translational level by the ARR2PB promoter and the rFGF-2 5′UTR, respectively.
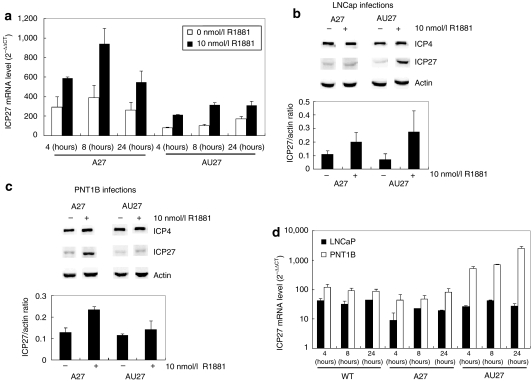
Transcriptional regulation. We have previously demonstrated prostate-specific expression of another essential viral gene, ICP4, in an amplicon/helper system using the highly androgen/AR-responsive ARR2PB promoter.13 To test the specificity of the ARR2PB promoter and its regulation of the ICP27 gene in the context of a whole virus, we first examined ICP27 mRNA levels by quantitative reverse transcriptase (RT)-PCR analysis in LNCaP cells infected with A27 and AU27 viruses in the presence of 0 or 10 nmol/l R1881. Consistent with our previous findings,13 ICP27 mRNA levels were more than twofold higher for both A27 and AU27 infections in the presence of 10 nmol/l R1881 when compared with no androgen treatment at 4, 8, and 24 hours postinfection (Figure 3a; P < 0.03). A trend was noticed, in both cancer and noncancer cells, that the complex 5′UTR may have a slight negative effect on transcriptional activity. These findings support transcriptional regulation of ICP27 gene expression within the HSV-1 viral genome by the ARR2PB promoter in an androgen/AR-dependent fashion.
Figure 3.
Transcriptional and translational regulation of ICP27. (a) LNCaP cells were infected with A27 and AU27 viruses at a multiplicity of infection of three in the presence of 0 or 10 nmol/l R1881. Total RNA was extracted at 4, 8, and 24 hours postinfection and ICP27 mRNA level was determined by RT-PCR and normalized with β-actin. (b) LNCaP and (c) PNT1B cells were infected with A27 and AU27 viruses as described above. Total protein was extracted at 8 hours postinfection, and ICP4, ICP27, and β-actin expression were determined by western blot analysis. Density ratio of eIF4E levels normalized to β-actin (means ± SD). (d) ICP27 mRNA level at 4, 8, and 24 hours postinfection was determined by quantitative RT-PCR using β-actin as an endogenous control.
Translational regulation. We next determined ICP27 protein expression level in both normal PNT1B and LNCaP cancer cells infected with A27 and AU27 viruses by western blot analysis. For A27 virus infections, addition of 10 nmol/l R1881 resulted in a >80% increase in the ICP27 protein in both cell lines at 8 hours postinfection (Figure 3b,c; P < 0.04). Similar results were also observed for AU27 virus infections in LNCaP cells, with an approximately fourfold increase in ICP27 protein level in the presence of 10 nmol/l R1881 at 8 hours postinfection (Figure 3b). However, A27 and AU27 behave quite differently in PNT1B cells. At the same multiplicity of infection (MOI), the ICP27 protein level was >60% higher after A27 infection than after AU27 infection at 8 hours postinfection (Figure 3c). The expression of another HSV-1 immediate-early gene, ICP4 with its endogenous promoter, remained unchanged in both cells lines. Clearly, the expression of ICP4 was neither dependent on eIF4E level nor AR.
To verify that reduced ICP27 expression from AU27 viral infected PNT1B cells was not due to a reduction in transcriptional activity, quantitative RT-PCR analysis of ICP27 mRNA levels was performed in PNT1B and LNCaP cells infected with wild-type, A27, or AU27 viruses. Surprisingly, levels of ICP27 mRNA were consistently higher in PNT1B cells than in LNCaP cells (Figure 3d). Thus, the reduction in ICP27 protein levels in PNT1B cells is consistent with downregulation at the translational level. Together, these results demonstrate that viral ICP27 gene expression from the AU27 virus can be transcriptionally and translationally regulated by the ARR2PB promoter and the 5′UTR element, respectively. Furthermore, although the ARR2PB promoter was able to direct the expression of the ICP27 gene in an androgen/AR-responsive fashion in the presence of a complex 5′UTR, this transcriptional upregulation by androgen could not be translated into increased protein production in cells with low levels of eIF4E.
Replication of the AU27 virus is androgen/ AR-regulated and tumor-specific
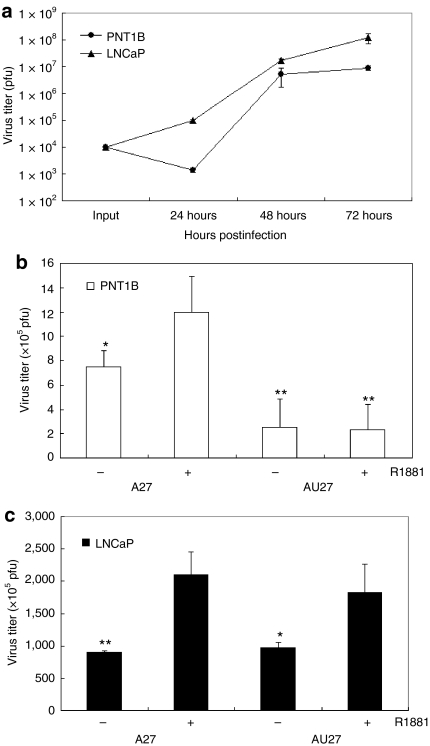
Wild-type (KOS) virus was able to infect and replicate in both LNCaP and PNT1B cells with similar efficiency when infected at an MOI of 0.01 (Figure 4a), and androgen treatment had no effect on the virus titer of the wild-type virus (data not shown). Despite a slight delay in viral growth in PNT1B cells for the first 24 hours, there was no significant difference in viral titers between PNT1B and LNCaP cells by 48 hours postinfection. Hence, both cells have similar infectivity and viral replicability for wild-type HSV-1 virus. At 72 hours the virus titer was significantly higher in LNCaP cells, which may be due to a higher cell proliferation rate of LNCaP cells than PNT1B cells. It should be noted that when cells are infected at MOI = 0.1, the two cell lines showed no difference in viral yield at all time points (data not shown). The A27 viral titer was approximately twofold higher in the presence of 10 nmol/l R1881 when compared to the absence of R1881 in both PNT1B and LNCaP cells. Interestingly, for the AU27 virus, adding 10 nmol/l R1881 caused an approximately twofold increase in virus titer in LNCaP cells, but not in PNT1B cells (Figure 4b,c; P < 0.05). Thus, the replication of both viruses was androgen/AR-responsive in cancer cells, but this androgen-driven transcriptional upregulation in PNT1B cells was overridden by translational suppression by the complex 5′UTR in the AU27 virus in cells with low eIF4E levels, resulting in a 700-fold lower viral titer in PNT1B cells than in LNCaP cells. This demonstrates that the AU27 virus can differentially replicate in LNCaP cells due to differences in endogenous eIF4E level while sparing normal cells with low eIF4E levels.
Figure 4.
Tumor-specific replication of AU27 virus in vitro. (a) PNT1B and LNCaP cells were infected with wild-type virus at a multiplicity of infection (MOI) of 0.01 (104 plaque-forming units) and virus titer was determined at 24, 48, and 72 hours postinfection. (b) PNT1B and (c) LNCaP cells were infected with A27 or AU27 at an MOI of 0.01 with ±10 nmol/l R1881 treatment and virus titer was determined at 48 hours postinfection. Virus titer was plotted as mean ± SD from four determinants (*P < 0.05; **P < 0.005). The P values are compared to the virus titer of A27 virus in the presence of 10 nmol/l R1881 in b, and the P values are determined by comparing the virus titers of A27 or AU27 with R1881 treatment to virus titers without R1881 treatment in c.
Intratumoral injection of AU27 virus reduces tumor size and prolongs survival
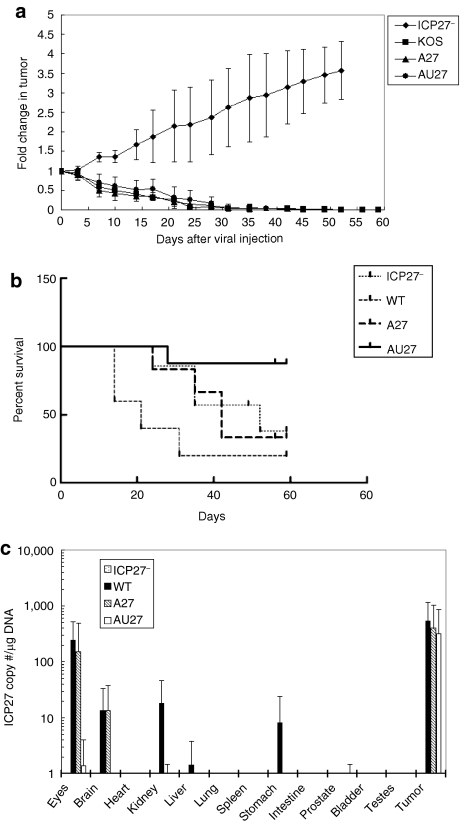
Nude mice with established LNCaP xenograft tumors (>100 mm3) were treated with one intratumoral injection of 2 × 106 plaque-forming units (pfu) of ICP27–, wild-type, A27, or AU27 viruses. In comparison to day 0 after viral injection, a >2.5-fold increase in tumor size was observed in mice treated with ICP27– virus at day 31 after viral injection, although a >90% reduction of tumor volumes was evident in mice treated with wild-type, A27, or AU27 virus (Figure 5a). In many cases, the tumors became barely palpable and complete eradication of the tumors was observed in three out of six mice treated with A27 virus and in four out of eight mice treated with AU27 virus. The treated mice were monitored for 2 months after initial viral injection. Approximately 60% of the mice treated with ICP27– were killed by day 50 post-treatment due to aggressive tumor growth. By comparison, 80% of the mice treated with wild-type virus exhibited signs of toxicity in the gastrointestinal (GI) tract and were killed by day 31 after viral injection, whereas 83% of the mice treated with A27 virus survived 31 days of treatment period (Figure 5b). However, only 33% survived for 2 months due to similar virulence in the GI tract. On the other hand, 88% of the mice treated with AU27 virus exhibited no signs of morbidity and survived for 2 months, at which time the experiments were terminated. Only one out of eight AU27-treated mice showed toxicity in the intestine at day 28 after viral injection and was killed early. Thus, the AU27 virus was effective in tumor eradication with remarkably reduced toxicity when compared to wild-type and A27 viruses, resulting in a significantly improved survival (P < 0.01).
Figure 5.
AU27 virus inhibited tumor growth without extensive toxicity to normal tissues in vivo. (a) Nude mice with LNCaP tumors were treated with one intratumoral injection of 2 × 106 plaque-forming units of ICP27− (n = 7), WT (n = 5), A27 (n = 6), or AU27 (n = 8) at day 0 when tumor size reached ~100 ± 20 mm3. Tumor size was determined by caliper measurements. (b) Survival rate of the treated mice mentioned above was monitored for ~2 months and any mouse that showed signs of morbidity due to viral toxicity was killed immediately. Mice in ICP27− treatment group were killed due to tumor burden when tumor size exceeded a fourfold increase. (c) Total DNA was extracted from various organs of the treated mice at the end of the experiment. ICP27 copy number (per µg of DNA) was determined by real-time PCR assays to indicate the presence of virus in the tissues. The n values indicated the number of mice treated and each tumor was treated individually. WT, wild type.
To examine the extent of viral biodistribution, ICP27 gene copy number (per µg of DNA) in various organs was determined by quantitative RT-PCR analysis. The majority of the virus was detected in the tumors of all treatment groups (Figure 5c). In mice treated with wild-type virus, a significant amount of virus was detected in the eyes and small traces were also found in the brain, kidneys, and stomach. Similar results were observed in mice treated with A27 virus with detectable virus in the eyes (in one of five mice) and brain (in two of five mice). By comparison, no virus was detected in the normal tissues of mice treated with AU27 virus, except that a trace of virus was detected in the stomach and intestine of one mouse that showed virus-related toxicity at day 28 after viral injection. A small trace of viral genome was also detected in the eyes of the AU27-treated mice, but did not cause any obvious morbidity or mortality.
Systemic administration of AU27 virus results in tumor-specific targeting and killing
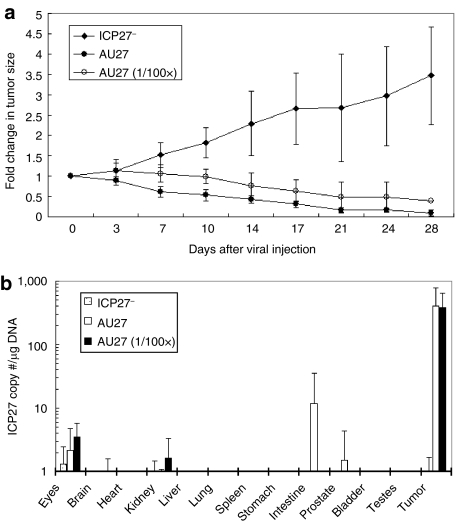
Nude mice with LNCaP tumors were treated with one intravenous injection of low (5 × 105 pfu) or high (5 × 107 pfu) doses of AU27 virus. In the control group, 5 × 107 pfu replication-defective ICP27– virus was injected intravenously and a more than three fold increase in tumor size was observed at day 28 after viral injection (Figure 6a). In contrast, a >60% and >85% reduction in tumor size was observed in mice treated with a single intravenous injection of 5 × 105 and 5 × 107 pfu of AU27 virus, respectively. The P value between the two AU27 doses was 0.0511 as determined by one-way analysis of variance analysis, so it was borderline statistically significant. Thus, there is a potential to achieve therapeutic effect. This demonstrated that systemic targeting of the virus may be dose-dependent. No sign of morbidity or mortality was observed in any of the three treatment groups, which is most likely due to quick clearance of the virus through systemic delivery. Quantitative real-time PCR analysis of various organs harvested from the treated mice showed that the virus was found significantly higher levels in the tumors (Figure 6b). A very small amount of virus was detected in the intestine of one mouse treated with 5 × 107 pfu of AU27 virus, but there was no noticeable toxicity.
Figure 6.
AU27 virus targeted and killed tumor cells after systemic administration. (a) Nude mice with LNCaP tumors were treated with a single intravenous injection of 5 × 107 plaque-forming units (pfu) of ICP27− (n = 4), AU27 (n = 5), or 5 × 105 pfu (100× less) of AU27 (n = 4) at day 0 when tumor size reached ~140 ± 65 mm3. (b) At day 28 after viral injection, total DNA was extracted from various organs of the treated mice. ICP27 copy number (per µg of DNA) was determined by real-time PCR assays.
Discussion
Prostate cancer patients with locally invasive and metastatic disease have relatively few treatment options, and even these are more palliative than curative in intent.14 Systemic delivery of chemotherapeutic drugs may be limited by toxicity to normal tissues, whereas targeted oncolytic virotherapy can greatly enhance the therapeutic index when the virus is engineered to specifically replicate in and kill tumor cells while sparing normal tissues. In this study, we demonstrated the targeting specificity of a TTDR-oHSV through both transcriptional and translational regulation of the essential ICP27 viral gene. We found that the eIF4E protein is overexpressed in LNCaP cells by at least fourfold when compared to a nonmalignant, normal epithelial prostate cell line, PNT1B (Figure 2). In addition, androgen stimulation resulted in a consistent approximately twofold increase in ICP27 mRNA transcripts and virus titers when LNCaP cells were infected with A27 or AU27 virus (Figures 2 and 3). However, some leaky ICP27 expression from the ARR2PB promoter in the absence of androgen was observed and was most likely due to the activity of the promiscuous viral transactivator ICP0 protein, which may interact nonspecifically with a variety of viral genes.15,16 ICP27 mRNA was transcribed efficiently in both PNT1B and LNCaP cell lines, but protein expression was hindered by the complex rFGF-2 5′UTR only in PNT1B cells (Figure 3). Moreover, AU27 virus replicated well in LNCaP cells, but not in PNT1B cells irrespective of androgen status (Figure 4), demonstrating differential replicability of the virus in normal versus tumor cells. We also showed that after in vivo administration, the AU27 virus was very effective in killing LNCaP xenograft tumor cells and extending survival without causing extensive toxicity to normal tissues (Figures 5 and 6). Mice were killed when they exhibited signs of GI toxicity. Symptoms of GI tract problems included mild diarrhea and a bulged abdomen. The most prominent symptom was a congested intestine, indicating a disruption of intestinal muscle contraction abilities. Weight loss was not observed and morbidity was a result of the inability of the mice to digest food. We stained a dozen or more normal tissues for eIF4E and found that there is considerable focal eIF4E in the gut, liver, and kidney (data not shown). This may explain the toxicity seen in the GI tract. Perhaps a much smaller dose of AU27 virus could have been used in the intratumoral treatment experiments; however, it might take longer for the tumor to regress as seen in the intravenous treatment groups. Wild-type virus was very toxic to the mice, causing a major impact on the GI system, although AU27 was considerably safer and amplified mainly inside the tumor.
In Figure 4c, the virus titer for A27 infection seemed to be higher in LNCaP cells than for KOS infection in the absence of R1881, whereas the titers are similar in PNT1B cells. A possible explanation is that LNCaP cells may have a higher basal level activity of AR even without androgen. We have shown in our previous work that knocking down AR using small interfering RNA can reduce tumor burden in the absence of androgen in LNCaP cells.17 The ARR2PB promoter may be responsive to the basal activity of AR in LNCaP cells and thereby cause higher viral production.
Interestingly, the transcriptional upregulation of ICP27 expression by androgen stimulation of the ARR2PB promoter was overpowered by translational suppression by the complex 5′UTR in noncancer cells. This dominance of translational regulation over transcription provides a significant implication in oncolytic virus design; in that viral gene expression and replication can be selectively enhanced through transcriptional upregulation in tumor cells, as evidenced by the increase in viral titer in the presence of activated AR, while at the same time being translationally repressed in normal tissues. Previous clinical studies have reported that progression of prostate cancer to a castration-resistant state is frequently accompanied by overexpression or overactivation of the AR, which allows tumor-cell proliferation in the absence of androgen.18,19,20,21 In such cases, the oncolytic effect of AU27 virus can be augmented in tumor cells that retain elevated AR activity without compromising its safety in noncancer tissues due to translational override. This finding provides a new avenue to solve a long-existing conflict in oncolytic viral vector design—a balance between the aggressiveness of an oncolytic virus for cancer cell killing and the safety concern of uncontrolled viral lysis.
Although a more than fourfold elevation of eIF4E levels is observed in LNCaP cells when compared to nonmalignant BPH-1 and PNT1B cells, there may be cases where the level of eIF4E is not as dramatically elevated in tumor cells relative to normal tissues (approximately twofold increase22). In such cases, other tumor-specific elements can also be used in combination with this targeting strategy to enhance specificity. For instance, recent strategies utilizing endogenous microRNAs to regulate oncolytic viral replication using various viral vectors also showed great promise.23,24 In our present approach, we have demonstrated that employment of a complex 5′UTR element could confer stringent tumor-specificity and enhance tissue-specificity of an oncolytic HSV-1 virus. This targeting strategy has also been demonstrated using adenoviruses25 and may be particularly useful in treating the most aggressive cancers, as recent data indicate that elevated activation of eIF4E is associated with poor patient survival.12 Furthermore, the advantage of using oncolytic HSV-1 is the lack of liver toxicity, making it superior to adenoviral vectors since liver damage is the major clinical limitation of adenoviral therapy.
Materials and Methods
Construction of recombinant virus. The ARR2PB-ICP27 (A27) and ARR2PB-5′UTR-ICP27 (AU27) recombinant viruses were generated by the recombineering method,26,27 which replaced the endogenous ICP27 promoter with the ARR2PB ± promoter the 5′UTR (619 bp) of rat FGF-2 gene28 in BAC.p25 (Figure 1). A 538-bp region between UL53 and ICP27(UL54) was deleted to insert the promoter ± 5′UTR. The bacterial artificial chromosome (BAC) was inserted into the thymidine kinase (TK) locus by homologous recombination to generate BAC.p25 containing the whole HSV-1 genome.29 This recombineering technique generated two BACs: BAC.AR27 and BAC.ARU27. The TK gene was then rescued by homologous recombination in 2-2 cells with hypoxanthine–aminopterin–thymidine selection.30 The packaged A27 and AU27 viruses were then plaque-purified three times in 2-2 cells to ensure successful TK rescue. The presence of the TK gene was then confirmed by PCR and western blotting for TK expression. The recombinant viruses were then propagated in 2-2 cells. The wild-type virus (KOS strain) was propagated in Vero cells and the replication-defective virus ICP27– was grown in 2-2 cells. Virus titer was determined by plaque-forming assay as previously described.13
Cell culture. RWPE-1 (ref. 31), LNCaP (ref. 32), DU145 (ref. 33), PC-3 (ref. 34), H460 (ref. 35), MCF-7 (ref. 36), and Vero cells were obtained from American Type Culture Collection (ATCC, Manasas, VA). BPH-1 (ref. 37) and PNT1B (ref. 38) cells were gifts from Dr Hayward (Vanderbilt University) and Dr Maitland (York, UK), respectively. The 2-2 cell line was kindly provided by Dr Schaffer (University of Pennsylvania School of Medicine). All cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics containing penicillin and streptomycin except for RWPE-1 cell line, which was maintained in keratinocyte serum-free medium with 0.05 mg/ml bovine pituitary extract and 5 ng/ml human recombinant epidermal growth factor (Invitrogen, Carlsbad, CA). PNT1B and LNCaP cells are human normal and tumor prostate cancer cell lines, respectively. They are positive for AR and are both androgen-responsive. The 2-2 cell line (transfected with ICP27 gene) is a derivative cell line generated from Vero cells. For the androgen-responsive experiments, PNT1B and LNCaP cells were maintained in Dulbecco's modified Eagle's medium with 10% charcoal-stripped serum (HyClone; VWR, West Chester, PA) for 2 days before infection and then 10 nmol/l R1881 (Perkin Elmer, Boston, MA) was added to the medium 1 hour after viral infection.
Western blotting. PNT1B and LNCaP cells were infected with wild-type, A27 and AU27 viruses at an MOI of 3, and total proteins were collected at 8 and 12 hours postinfection. PNT1B and LNCaP cells were lysed with 2× sample buffer and protein samples were subjected to western blot analysis as previously described.39 Primary antibodies were prepared in 5% bovine serum albumin in Tris-buffered saline Tween-20 at the following dilutions: anti-ICP4 (EastCoast Bio, North Berwick, ME) at 1:800 (ref. 40), anti-ICP27 (Virusys, Sykesville, MD) at 1:800 (refs. 40,41), anti-TK (a gift from Dr Summers, Yale University) at 1:500, anti-eIF4E (BD Transduction Laboratories, Mississauga, ON) at 1:1,000, and anti-β-actin (Cell Signaling, Danvers, MA) at 1:1,000. Anti-mouse and anti-rabbit secondary antibodies were prepared in Tris-buffered saline Tween-20 at a 1:2,000 dilution (Cell Signaling). Band intensities were quantified using ImageJ software (NIH, Bethesda, MD).
DNA, RNA extraction, and RT-PCR. Total RNA was extracted from cell lines and mouse organs using Trizol (Invitrogen) following the manufacturer's protocol. To determine ICP27 and β-actin mRNA levels in co-transfection studies, 200 ng RNA was used in one-step real-time RT-PCR as previously described.13 All RT-PCR reactions were performed in 25 µL SYBR-green mixture containing the MultiScribe Reverse Transcriptase, using the ABI prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA). The ICP27 mRNA levels were first normalized to the β-actin mRNA level (ΔCT = CTICP4 − CTActin) and then compared to the negative control group where LNCaP cell was infected with ICP27– virus only (ΔΔCT = ΔCT − ΔCTICP27–). The results were expressed as 2–ΔΔCT. DNA was extracted from the organs of the mice treated with viruses using the phenol–chloroform and ICP27 copy number was determined by quantitative real-time PCR using the ICP27 primers (5′-GTCTGGCGGACATTAAGGACA-3′ and 5′-TGGCCAGAATGACAAACACG-3′).
Virus growth assays. PNT1B and LNCaP cells were infected with viruses at an MOI of 0.01 with or without 10 nmol/l R1881 treatment. Total virus was collected at 24, 48, and 72 hours postinfection, and virus titer was determined by the plaque-forming assay in 2-2 cells.
LNCaP xenograft mouse model. Athymic nude mice were purchased from Harlan Sprague Dawley (Indianapolis, IN) and were inoculated with LNCaP cells subcutaneously by injecting 5 × 106 cells in 100 µl of medium with matrigel at two different flank sites. Tumor volumes were determined by caliper measurements and calculated using the formula volume = width × length × thickness × π/6 (ref. 13). Once the tumor size reached ~100 mm3, mice were treated with a single intratumoral injection of 2 × 106 pfu of wild-type, ICP27–, A27, or AU27 virus at day 0. For dose–response experiment, mice were treated with one single intravenous injection of 5 × 107 pfu of ICP27–, AU27, or 5 × 105 pfu of AU27 virus at day 0. At the end of the experiment, mice were killed using CO2 asphyxiation, and organs (eyes, brain, heart, kidneys, liver, lung, spleen, stomach, intestines, prostate, bladder, and testes) and the tumors were removed and analyzed by real-time PCR. All experimental procedures were approved by University of British Columbia Animal Care Committee and followed the guidelines and policies of the Canadian Council on Animal Care.
Statistical analysis. Statistical significance (P < 0.05) was determined using Student's t-test and the results are presented as mean ±SD. Kaplan–Meier survival curves were created using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA) and the log-rank (Mantel–Cox) test was used to determine statistical significance.
Acknowledgments
We thank Mary Bowden (The Prostate Centre at Vancouver General Hospital) for her technical assistance with the animal work. This work was supported by the Terry Fox Foundation (P.S.R. and W.W.G.J., grant number 017007). A predoctoral training award for prostate cancer research was provided to C.Y.F.L. by the US Army Department of Defense.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, TAX 327 Investigators et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Petrylak DP. Future directions in the treatment of androgen-independent prostate cancer. Urology. 2005;65 6 Suppl:8–12. doi: 10.1016/j.urology.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Varghese S., and , Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- Yu D, Jia WW, Gleave ME, Nelson CC., and , Rennie PS. Prostate-tumor targeting of gene expression by lentiviral vectors containing elements of the probasin promoter. Prostate. 2004;59:370–382. doi: 10.1002/pros.20010. [DOI] [PubMed] [Google Scholar]
- Kevil C, Carter P, Hu B., and , DeBenedetti A. Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene. 1995;11:2339–2348. [PubMed] [Google Scholar]
- Jiang Y., and , Muschel RJ. Regulation of matrix metalloproteinase-9 (MMP-9) by translational efficiency in murine prostate carcinoma cells. Cancer Res. 2002;62:1910–1914. [PubMed] [Google Scholar]
- De Benedetti A., and , Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- Graff JR., and , Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- Seki N, Takasu T, Mandai K, Nakata M, Saeki H, Heike Y, et al. Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin Cancer Res. 2002;8:3046–3053. [PubMed] [Google Scholar]
- Yu D, Scott C, Jia WW, De Benedetti A, Williams BJ, Fazli L, et al. Targeting and killing of prostate cancer cells using lentiviral constructs containing a sequence recognized by translation factor eIF4E and a prostate-specific promoter. Cancer Gene Ther. 2006;13:32–43. doi: 10.1038/sj.cgt.7700885. [DOI] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Lynch RL, Dumstorf CA, Dowless MS, McNulty AM, et al. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 2009;69:3866–3873. doi: 10.1158/0008-5472.CAN-08-3472. [DOI] [PubMed] [Google Scholar]
- Lee CY, Bu LX, Rennie PS., and , Jia WW. An HSV-1 amplicon system for prostate-specific expression of ICP4 to complement oncolytic viral replication for in vitro and in vivo treatment of prostate cancer cells. Cancer Gene Ther. 2007;14:652–660. doi: 10.1038/sj.cgt.7701052. [DOI] [PubMed] [Google Scholar]
- Beardsley EK., and , Chi KN. Systemic therapy after first-line docetaxel in metastatic castration-resistant prostate cancer. Curr Opin Support Palliat Care. 2008;2:161–166. doi: 10.1097/SPC.0b013e32830c48a3. [DOI] [PubMed] [Google Scholar]
- Lukonis CJ., and , Weller SK. The herpes simplex virus type 1 transactivator ICPO mediates aberrant intracellular localization of the viral helicase/primase complex subunits. Virology. 1996;220:495–501. doi: 10.1006/viro.1996.0338. [DOI] [PubMed] [Google Scholar]
- Yang CT, Song J, Bu X, Cong YS, Bacchetti S, Rennie P, et al. Herpes simplex virus type-1 infection upregulates cellular promoters and telomerase activity in both tumor and nontumor human cells. Gene Ther. 2003;10:1494–1502. doi: 10.1038/sj.gt.3302005. [DOI] [PubMed] [Google Scholar]
- Cheng H, Snoek R, Ghaidi F, Cox ME., and , Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Cancer and Leukemia Group B Study 9663 et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Taplin ME., and , Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- Albertsen PC, Hanley JA., and , Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- Mathis JM, Williams BJ, Sibley DA, Carroll JL, Li J, Odaka Y, et al. Cancer-specific targeting of an adenovirus-delivered herpes simplex virus thymidine kinase suicide gene using translational control. J Gene Med. 2006;8:1105–1120. doi: 10.1002/jgm.935. [DOI] [PubMed] [Google Scholar]
- Bell JC., and , Kirn D. MicroRNAs fine-tune oncolytic viruses. Nat Biotechnol. 2008;26:1346–1348. doi: 10.1038/nbt1208-1346. [DOI] [PubMed] [Google Scholar]
- Lee CY, Rennie PS., and , Jia WW. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res. 15:5126–5135. doi: 10.1158/1078-0432.CCR-09-0051. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Nedeljkovic-Kurepa A, DeBenedetti A, Li XL, Odaka Y, et al. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res Treat. 2008;108:43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG., and , Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Emoto N, Koba A, Mercado M, Shibata F, Cooksey K, et al. Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem Biophys Res Commun. 1988;157:256–263. doi: 10.1016/s0006-291x(88)80041-x. [DOI] [PubMed] [Google Scholar]
- Horsburgh BC, Hubinette MM, Qiang D, MacDonald ML., and , Tufaro F. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 1999;6:922–930. doi: 10.1038/sj.gt.3300887. [DOI] [PubMed] [Google Scholar]
- Morris DJ., and , Robinson TJ. Thymidine kinase (Tk-1) maps below the T42H breakpoint on mouse chromosome 11. Mamm Genome. 1991;1:263–264. doi: 10.1007/BF00352335. [DOI] [PubMed] [Google Scholar]
- Rhim JS, Webber MM, Bello D, Lee MS, Arnstein P, Chen LS, et al. Stepwise immortalization and transformation of adult human prostate epithelial cells by a combination of HPV-18 and v-Ki-ras. Proc Natl Acad Sci USA. 1994;91:11874–11878. doi: 10.1073/pnas.91.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, et al. The LNCaP cell line–a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- Stone KR, Mickey DD, Wunderli H, Mickey GH., and , Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF., and , Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Bepler G, Guccion JG, Marangos PJ, Moody TW, et al. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985;45:2913–2923. [PubMed] [Google Scholar]
- Bacus SS, Kiguchi K, Chin D, King CR., and , Huberman E. Differentiation of cultured human breast cancer cells (AU-565 and MCF-7) associated with loss of cell surface HER-2/neu antigen. Mol Carcinog. 1990;3:350–362. doi: 10.1002/mc.2940030607. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N., and , Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro. Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Degeorges A, Hoffschir F, Cussenot O, Gauville C, Le Duc A, Dutrillaux B, et al. Recurrent cytogenetic alterations of prostate carcinoma and amplification of c-myc or epidermal growth factor receptor in subclones of immortalized PNT1 human prostate epithelial cell line. Int J Cancer. 1995;62:724–731. doi: 10.1002/ijc.2910620613. [DOI] [PubMed] [Google Scholar]
- Lee CY, Rennie PS., and , Jia WW. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res. 2009;15:5126–5135. doi: 10.1158/1078-0432.CCR-09-0051. [DOI] [PubMed] [Google Scholar]
- Hubenthal-Voss J, Houghten RA, Pereira L., and , Roizman B. Mapping of functional and antigenic domains of the alpha 4 protein of herpes simplex virus 1. J Virol. 1988;62:454–462. doi: 10.1128/jvi.62.2.454-462.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Braun DK, Pereira L., and , Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]