Abstract
Duchenne muscular dystrophy (DMD) is characterized by the absence of dystrophin. Several previous studies demonstrated the feasibility of delivering microdystrophin complementary DNA (cDNA) into mouse and normal nonhuman primate muscles by ex vivo gene therapy. However, these animal models do not reproduce completely the human DMD phenotype, while the dystrophic dog model does. To progress toward the use of the best animal model of DMD, a dog microdystrophin was transduced into human and dystrophic dog muscle precursor cells (MPCs) with a lentivirus before their transplantation into mouse muscles. One month following MPC transplantation, myofibers expressing the dog microdystrophin were observed. We also used another approach to introduce this transgene into myofibers, i.e., the electrotransfer of a plasmid coding for the dog microdystrophin. The plasmid was injected into mouse and dog muscles, and brief electric pulses were applied in the region of injection. Two weeks later, the transgene was detected in both animals. Therefore, ex vivo gene therapy and electrotransfer are two possible methods to introduce a truncated version of dystrophin into myofibers of animal models and eventually into myofibers of DMD patients.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked genetic disease characterized by the absence of dystrophin. This large protein of 427 kd is encoded by a 14 kb mRNA.1 This protein, interacting with other membrane-associated proteins, would to be needed to insure mechanical stress resistance of the sarcolemma during muscle contraction. The lack of dystrophin weakens the sarcolemma and thus makes myofibers less resistant to mechanical stress.2,3
There is currently no efficient treatment for DMD. Several groups have however obtained promising results with a variety of approaches in clinical and preclinical experiments such as the exon skipping4 or the use of different types of stem cells.5 The transplantation of normal allogeneic muscle precursor cells (MPCs) has been proven effective to restore the expression of dystrophin, but requires immunosuppression to avoid rejection of the allogeneic cells and myofibers.6,7 Cossu's laboratory reported good expression of dystrophin after intra-arterial delivery of normal mesoangioblasts in dystrophic dogs, but this therapeutic approach also needed immunosuppression because of the allogeneic context of the grafts.8 The systemic delivery of mesoangioblasts seems very promising, but it is very important to verify the potential side effects of the accumulation of these cells in different vital organs. Two recent experiments in dogs demonstrated good expression of dystrophin after intramuscular, regional limb delivery approach or intravenous delivery with an adenovirus-associated virus (AAV) in dystrophic dogs but they also needed immunosuppression to prevent an immune reaction against the delivered vector.9,10
Immunosuppressive drugs induce several adverse effects such as increased risks of cancer, infections, nephrotoxicity, neurotoxicity, and so on. One way to eventually avoid the immune problems associated with allogeneic cell transplantation or viral vector injections is to transplant autologous cells, which have been genetically modified ex vivo. This technique has been proven effective with a truncated version of dystrophin in the mouse11 and in the nonhuman primate models.12 However, the best animal model to study the effects of experimental therapies for DMD is the muscular dystrophy dog, because its dystrophic phenotype is closer to the DMD patient than the dystrophic mouse. Indeed, while research with nonhuman primates is of importance, there is no monkey with muscular dystrophy available for experimentation. Therefore, the present study will focus on the use of the dog microdystrophin. A recent study showed expression of this truncated version of dog dystrophin into dystrophic dog muscles after its intramuscular delivery with an AAV, but immunosuppression would be required to obtain a long-term expression. With this therapeutic approach there is not only a requirement for immunosuppression but also some potential toxicity following the dissemination of the virus throughout the body.13 Another approach to avoid these problems is to directly electrotransfer a complementary DNA (cDNA) coding for the gene of interest into muscles. A clinical trial performed on nine DMD patients has shown weak expression of human dystrophin in up to 6% of the myofibers following intramuscular injection of a plasmid containing the full-length dystrophin gene.14 This technique was safe, fast, and easy to use, although it needs to be substantially improved, as could be the case using electrotransfer to increase the incorporation of the plasmid into more myofibers.15 Moreover, this electrotransfer technique is already in clinical trials.16
In the present study, a lentivirus vector was used to introduce a dog microdystrophin cDNA into human and dystrophic dog MPCs. This vector was selected for its property to integrate into the genome and to be able to carry a microdystrophin. The genetically modified MPCs were transplanted into immunodeficient mouse muscles and myofibers expressing the dog microdystrophin were observed. Moreover, the electrotransfer of the same cDNA was tested with success in mouse and dog muscles. Afterwards, we plan to introduce the microdystrophin into dystrophic dog muscles with these two techniques.
Results
In vitro expression of the dog microdystrophin
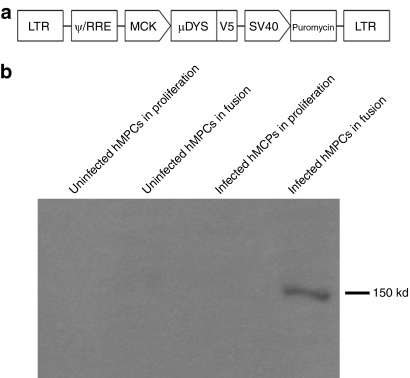
Since dystrophin is normally expressed in myotubes and muscle fibers but not in MPCs, we have chosen to clone the dog microdystrophin cDNA (µDys) under the control of a muscle creatine kinase (MCK) promoter in a lentiviral backbone. To track its expression, this transgene was fused with the V5 tag (µDysV5). A puromycin resistance gene was also included in the backbone in order to allow cell selection. This plasmid (pLeMCK.µDysV5) (Figure 1a) was first transduced in human MPCs (hMPCs) using the packaging cell supernatant. Transduced cells were selected with 2 days exposure to puromycin. Puromycin-resistant cells were proliferated to confluence and placed 3 days in differentiation medium to form myotubes. Proteins were harvested from cells grown in proliferation and differentiation media to verify that the transgene was only expressed in myotubes and not in MPCs. A western blot with an antibody against the V5 tag was performed to verify whether the µDysV5 protein was expressed in these cells. As expected, only the cultures of transduced hMPCs in differentiation medium expressed the dog microdystrophin (Figure 1b).
Figure 1.
In vitro experiments with the lentivirus coding for the dog microdystrophin fused with a V5 tag and for the puromycin resistance gene. (a) Schematic representation of the pLeMCK.µDysV5. The dog microdystrophin (µDys) is fused with the V5 tag and is under the control of the MCK promoter. The puromycin resistance gene is under the ubiquitous promoter SV40. (b) Western blot made with uninfected and infected hMPCs cultured in proliferation or in fusion media. The V5 tag was detected only in infected cells in fusion (150 kd). hMPC, human muscle precursor cell; LTR, long-terminal repeat; MCK, muscle creatine kinase.
Electrotransfer of the µDysV5 plasmid into mouse muscles
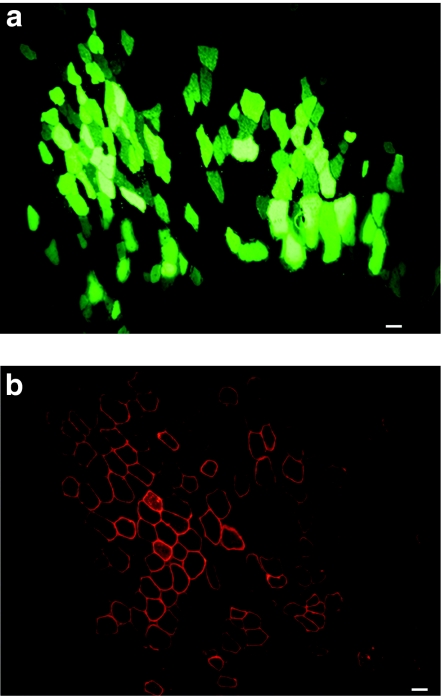
In the aim to introduce the µDysV5 transgene by electrotransfer in dog muscles, a pilot study was first made in mouse muscles. A plasmid coding for green fluorescent protein (pLeGFP) was initially injected into the tibialis anterior (TA) to determine the efficiency of this method. Forty microgram of pLeGFP were electrotransferred into the TAs of immunodeficient (Rag−/−) mice (n = 4). Two weeks later, the TAs were harvested and GFP was detected in cryostat sections up to 40% in the best cases (Figure 2a). After this proof of principle, the pLeMCK.µDysV5 (40 µg) was electrotransferred into Rag−/− mouse TAs (n = 8). Mice were sacrificed after 2 weeks and the TAs were analyzed. Immunofluorescence with an antibody recognizing the V5 tag was performed revealing myofibers-expressing V5, and thus the dog microdystrophin. In the best cases, up to 20% of the muscle fibers expressed the transgene (Figure 2b).
Figure 2.
Cross-sections of mouse muscles electrotransferred with the pLeGFP and pLeMCK.µDysV5. (a) The electrotransfer of the pLeGFP induced the expression of GFP in several myofibers. (b) The fluorescent immunodetection of V5 in a muscle electrotransferred with the pLeMCK.µDysV5 shows several myofibers-expressing V5 in the typical subsarcolemmal location of dystrophin. Bar = 50 µm. The cross-sections represent the global result from the electrotransfer with n = 4 for pLeGFP and n = 8 for pLeMCK.µDysV5. MCK, muscle creatine kinase; pLeGFP, plasmid coding for green fluorescent protein.
LeMCK.µDysV5-transduced hMPCs grafted in mouse muscles
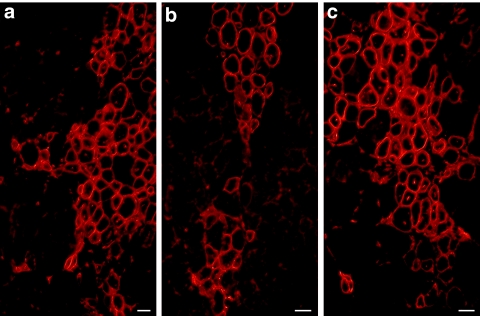
After this first positive in vivo result, hMPCs were transduced with the LeMCK.µDysV5 lentivirus. Cells were selected with puromycin and 106 puromycin-resistant hMPCs were transplanted in the TAs of Rag−/− mice (n = 8). Two days before, the TAs of these mice were irradiated to inhibit the proliferation of the endogenous satellite cells17 and injected with 1 µg of cardiotoxin to damage the muscle.18 One month after the transplantation, the TAs were harvested and immunohistochemical staining of V5 tag was used to detect the expression of the dog µDys. Roughly 30–40% of the myofibers expressed V5 and thus the dog microdystrophin (Figure 3a–c). The myofibers expressing this transgene, observed following the cell transplantation, were more abundant than those observed following electrotransfer.
Figure 3.
Cross-sections of mouse muscles grafted with hMPCs transduced with the pLeMCK.µDysV5. The sections (a–c) of three different mice illustrate V5 expression in several myofibers in the typical sarcolemmal location of dystrophin. Bar = 50 µm. The cross-sections represent the global result from the transplantation with n = 8. hMPC, human muscle precursor cell; MCK, muscle creatine kinase.
Influence of donor's age in the success of dog MPC transplantation in mice
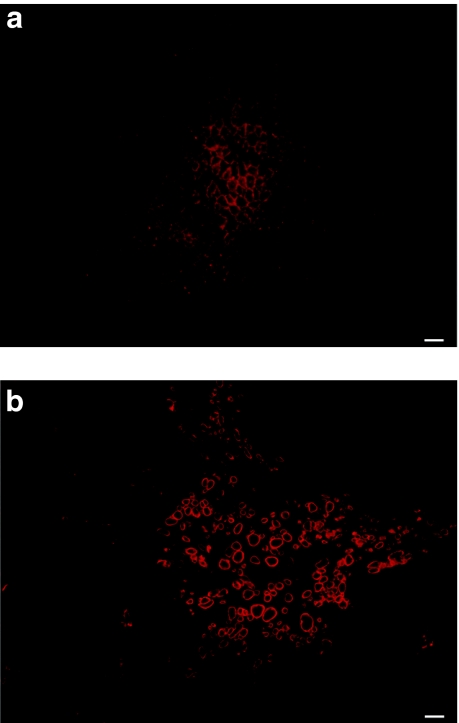
Muscle biopsies from a young (3 month old) and a mature (3 years old) dog were taken and their MPCs isolated and proliferated in vitro. Preliminary experiments showed that dog myoblasts had a tendency to fuse at confluence as low as 50% (even in high-serum medium) and that their proliferation was not as good as that of hMPCs. The low proliferation and early fusion were even more important when MPCs were obtained from the older dog. Indeed, no myofiber expression of dog dystrophin was observed 1 month after transplantation of nondystrophic MPCs (n = 4) from the older donor in Rag−/− mouse muscles (Figure 4a) whereas transplantation of nondystrophic MPCs (n = 4) from the young donor showed many myofibers-expressing dog dystrophin (Figure 4b).
Figure 4.
Cross-sections of mouse muscles grafted with dog MPCs. An antibody that recognizes dog dystrophin but not mouse dystrophin was used in immunohistochemistry. The transplantation of (a) mature dog MPCs did not induce expression of dog dystrophin whereas many fibers expressed dog dystrophin after the transplantation of (b) young dog MPCs. Bar = 100 µm. The cross-sections represent the global result from the transplantation with n = 4 for each group. MPC, muscle precursor cell.
Immortalization and LeMCK.µDysV5 transduction of dystrophic dog MPCs
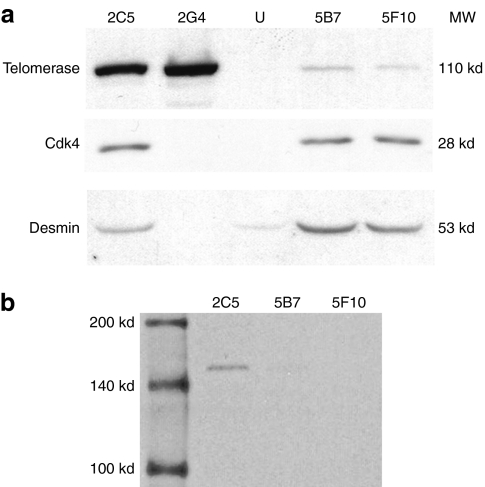
Since, we initially had just biopsies from a 2-year-old dystrophic dog, its MPCs were transduced with lentivirus coding for cyclin-dependent kinase 4 (Cdk4) and telomerase to immortalize them. After 2 months, several clones were obtained from these immortalized dog MPCs, and some were selected for their high-desmin expression and their capacity to fuse in vitro. Western blot against telomerase, Cdk4 and desmin were done to confirm the two transductions and the myogenic potential of the transduced cells (Figure 5a). The 2G4 clone was discarded because no expression of Cdk4 nor desmin was detected. The selected clones, 2C5, 5B7, and 5F10, were transduced with the LeMCK.µDysV5 virus. As these clones already contained a puromycin resistance gene (included in the telomerase plasmid), puromycin selection of cells containing the MCK.µdysV5 transgene was not possible. In consequence, the modified MPCs were transferred to a differentiation medium to activate the MCK promoter and proteins were harvested to make a western blot to detect V5 (Figure 5b). The 2C5 clone expressed the dog microdystrophin whereas only a very weak band was observed in the 5B7 clone and no expression was detected for the 5F10 clone. Thus, the 2C5 clone expressed the MCK.µDysV5 transgene.
Figure 5.
Western blots on immortalized dog MPC clones transduced with LeMCK.µDysV5. (a) Western blots to detect telomerase, Cdk4, and desmin in uninfected dog MPCs (U) and four different clones infected with the telomerase and the Cdk4 viruses, namely 2C5, 2G4, 5B7, and 5F10. Telomerase, Cdk4, and desmin were detected only in clones 2C5, 5B7, and 5F10. (b) Western blot to detect V5 in the three clones selected for their expression of telomerase, Cdk4, and desmin, transduced with the LeMCK.µDysV5 virus and transferred in differentiation medium. A clear expression of V5 was detected (150 kd) in clone 2C5, a very weak expression of V5 was distinguished in the 5B7 clone and no V5 expression was noticed in the 5F10 clone. Cdk4, cyclin-dependent kinase 4; MPC, muscle precursor cell; MW, molecular weight.
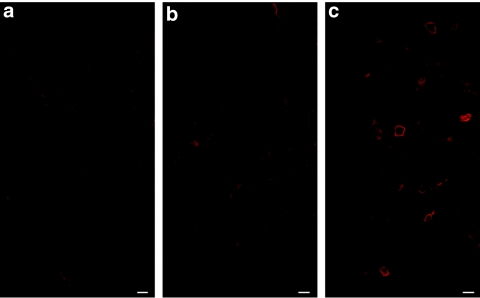
Transplantation of LeMCK.µDysV5-transduced dystrophic dog MPCs in mice
The three clones transduced with the LeMCK.µDysV5 virus (5 × 105 cells each) were transplanted into the TA of Rag−/− mice (n = 4 for each clone) before obtaining the western blot results for V5. In this case, the TAs did not receive irradiation nor cardiotoxin to be closer to the clinical situation of cell transplantation. One month after the graft, the TAs were harvested and immunohistochemical staining for V5 was used to detect the expression of dog µDysV5 in cryostat sections of the grafted muscles. As expected, no V5 expression was detected in muscles grafted with the 5F10 clone (Figure 6a) nor with the 5B7 clone (Figure 6b). Nevertheless, some myofibers-expressing V5 were observed in muscles grafted with the 2C5 clone (Figure 6c). Thus the transplantation of transduced dystrophic dog MPCs with the LeMCK.µDysV5 virus allowed the expression of the dog microdystrophin in myofibers of the grafted muscles.
Figure 6.
Cross-sections of mouse muscles transplanted with the three immortalized dog MPC clones infected with the LeMCK.µDysV5 virus, treated for fluorescent immunodetection of V5. No V5 tag was detected in muscles grafted with the (a) 5F10 and (b) 5B7 clones. On the contrary, some myofibers expressed the V5 tag at the sarcolemmal position in the muscles grafted with the (c) 2C5 clone. Bar = 50 µm. The cross-sections represent the global results from the transplantation with n = 4 for each group. MCK, muscle creatine kinase; MPC, muscle precursor cell.
Electrotransfer of the pLeMCK.µDysV5 into dog muscles
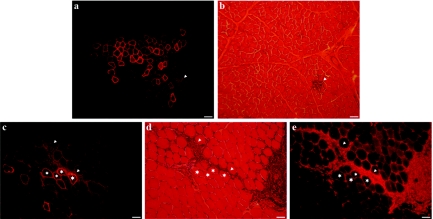
Finally, the pLeMCK.µDysV5 was electrotransferred into dog muscles. Two hundred microgram of this plasmid were injected in the brachialis anterior of three nondystrophic 2-year-old dogs. Two weeks after the electrotransfer, myofibers expressing the transgenic dog microdystrophin were observed in cryosections of the three dogs using an antibody against the V5 flag (Figure 7a,c), but twice less myofibers expressed this transgene than following electrotransfer into mouse muscles. To verify whether µDysV5 induced an immune response in these animals, the muscle sections were stained with hematoxylin–eosin. Accumulations of mononuclear cells with a lymphocyte aspect were observed in the muscle sections. These lymphocyte accumulations colocalized (Figure 7c,d) or did not colocalized (Figure 7a,b) with myofibers-expressing V5. An immunohistochemical staining for CD8+ confirmed that many of these cells were cytotoxic T-lymphocytes (Figure 7e).
Figure 7.
Cross-sections of dog muscles electrotransferred with the pLeMCK.µDysV5. Two regions (from two different muscles) are shown through serial sections respectively in a, b, and c, d, e. (a,c) Fluorescent immunodetection of V5 shows the typical sarcolemmal location of dystrophin in several myofibers. (b,d) The hematoxylin–eosin staining shows infiltrates of mononuclear cells with the aspect of lymphocytes (arrowheads) in the proximity of the V5-positive myofibers. (e) The fluorescent immunodetection of CD8+ cells shows that many cells in the mononuclear infiltrates are cytotoxic T-lymphocytes. The asterisks indicate the same myofibers through the sections. Bar = 150 µm (a,b) and 75 µm (c–e). The cross-sections represent the global results from the electrotransfer with n = 3. MCK, muscle creatine kinase.
Discussion
In this study, we have managed to introduce a truncated version of the dog dystrophin with a lentiviral vector into human and dystrophic dog MPCs. We also obtained the expression of the dog microdystrophin into mouse muscles following the transplantation of these genetically modified MPCs. This potential therapeutic approach consisting in the autotransplantation of the patient own cells after their genetic correction in vitro is called “ex vivo gene therapy”. In the case of genetic myopathies, ex vivo gene therapy has already shown promising results, using different techniques of gene modification in human, mouse, and monkey cells, grafted in mice and nonhuman primates.11,12,19
Our first experiment was to insert the dog microdystrophin cDNA into hMPCs. We begin with these cells because they are easy to proliferate in culture. Since dystrophin is usually only expressed in myotubes and muscle fibers but not in MPCs,20 we placed the microdystrophin cDNA under the control of a MCK promoter21 to obtain dystrophin expression in physiological conditions. Using this promoter, the expression of the transgene following cell transplantation is only induced when the genetically modified MPCs fused with the host myofibers. Thus the expression of the dog microdystrophin was only detected in the myotubes formed in culture by transduced MPCs using western blot and in the muscles grafted with these cells by immunohistochemistry.
The V5 expression following transduced hMPC transplantation was more pronounced than following the transplantation of transduced dystrophic dog MPCs. This may be due to the lower proliferating capacity of the dog MPCs compared to hMPCs. Moreover, MPCs isolated from a young dog had more proliferation and fusion capacities than those from a mature dog. In some of our experiments, the transduced MPCs came from a dystrophic dog that was 2 years old and had been previously transduced with a telomerase and a Cdk4 lentivirus to increase their proliferation capacity.22 Even if we selected the best cell clones, their capacity to proliferate and to fuse was inferior to those observed for MPCs derived from a 3-month-old dog. It is also probable that the dystrophic dog MPCs were close to senescence before or between the two transductions and selections. The immortalization induced by the transductions of the Cdk4 and telomerase genes did not restore the low-fusion capacity of these cells compared to the fusion capacity of MPCs isolated from a young dog. What is important, however, is that despite this potential senescence/fusion problem, we obtained some expression of dog microdystrophin after the graft of the MCK.µdysV5-transduced dystrophic dog MPCs into mouse muscles. For future experiments, biopsies from dystrophic dogs should be taken from young dogs so that their proliferation and fusion capacity are better.
In addition to the experiments of ex vivo gene therapy, we also tested with success the electrotransfer of the plasmid coding for the dog microdystrophin both in mouse and dog muscles. However, our transgene was more expressed in the mouse muscles, probably simply due to an easier and more effective electrotransfer technique in the small muscles (i.e., mice) than bigger muscles (i.e., dogs). In addition, the electric parameters for electrotransfer in smaller muscles are already well-established whereas this is not the case for large dog muscles.
This method combining the uses of electrotransfer and gene therapy has been used in muscles, tumors, and skin in large animals such as dogs, pigs, nonhuman primates, and is currently in clinical trials to treat different diseases.16,23 In particular, this technique allows a long-term expression of a plasmid in skeletal muscles because muscle fibers are postmitotic. Compared to ex vivo gene therapy, this is a faster method because there is no cellular culture and this may also be less immunogenic and safer since viral capsids are not introduced in the patients.24 Even whether during the electrotransfer, some injected plasmids may reach the blood circulation, the use of a muscle-specific promoter will insure that transgene expression is restricted to myofibers. In addition, the incorporation of naked plasmids by myofibers, without electrotransfer, is very low. Despite the lower electrotransfer efficiency in our experiments in comparison with the ex vivo gene therapy results, electrotransfer remains a potential method to introduce dystrophin cDNA especially in small muscles. This technique should be further improved in the future.
In our study, to detect the truncated dystrophin in the dogs, it was necessary to distinguish it from the endogenous dystrophin. To do so, we used a V5 tag in fusion with the protein. However, this tag may induce an immune response at long-term.12 In our electrotransfer experiment in dogs, a cellular infiltration, i.e., a sign of a specific immune response, was observed in the regions expressing the transgene at 2 weeks. In the future, we will extend our electrotransfer experiments in dystrophic dogs to verify whether the specific immune response producing the rejection of the myofibers is due to the microdystrophin itself or to the V5 tag. A specific immune response may also be induced by the use of antibiotic-resistance genes used to select the transduced cells; in our case, the electrotransferred plasmid contained the µDysV5 and the puromycin resistance gene. In fact, an autologous graft of dog MPCs genetically modified with a lentivirus, containing only this resistance gene, induced a specific immune response at the injection sites (data not shown). This preliminary result suggests that the puromycin resistance gene can induce an immune response by itself.
We expect that this immune problem will be absent in future experiments of electrotransfer or ex vivo gene therapy in dystrophic dogs. For these experiments, the V5 tag and the puromycin resistance gene will be removed from the plasmid because we will be able to detect the microdystrophin without needing a tag. Obviously, in this case the percentage of revertant fibers will have to be taken into account. However, we cannot exclude that an immune response against microdystrophin may appear. There were contradictory results in previous experiments in dystrophic mice receiving transplantation of syngeneic MPCs expressing normal dystrophin. One study reported the production of antibodies reacting with dystrophin, but without inducing rejection at long-term.25 Another study reported both antibody production and specific T-lymphocyte reaction against dystrophin, leading to rejection.26 In DMD patients, antidystrophin antibodies were also observed following nondystrophic MPC grafts.27
Recent studies using intramuscular injection of AAV2 and AAV6 vectors carrying the canine microdystrophin cDNA in dystrophic dogs showed good expression of dystrophin, but a fast and strong immune response was observed against the capsid.9 Using the AAV8 vector, this immune response was delayed to 8 weeks, however, this delay will not insure a long-term microdystrophin expression if an immunosuppressive treatment is not administrated.10 Another team obtained good results using an AAV carrying the canine microdystrophin without immunosuppression in mdx mice.28 This team also observed canine microdystrophin expression for over 1 year with either direct intramuscular (AAV8) or regional limb or systemic intravenous (both AAV9) injection.29,30 If the AAV vectors are administered systemically by intravascular injection, they will spread throughout the body reaching other organs than skeletal muscles.31 In consequence, if there is an immune response against the viral capsid spread in the whole body, the risks of such a systemic immune response may be extremely severe. One study has shown weak toxicity following in vivo AAV systemic administration, but unfortunately the analyses were only done during 3 days.32 With our ex vivo gene therapy approach, we avoid the problem of immune reactions against the viral capsid because the cells are genetically modified in vitro. The electrotransfer also avoid the immune response against the viral capsid because a naked plasmid is injected. Keeping in mind the considerations discussed above, it maybe be possible to avoid immunosuppression with these two methods. Toxicity, the need of immunosuppression and the spreading of the viral particles are also problems observed following direct in vivo injections of adenovirus.33,34 and for probably all viral vectors.35 There is, however, currently no evidence in the literature of these problems with the use of a lentivirus following ex vivo gene therapy. For these reasons, the ex vivo gene therapy may be a safer method than the direct injection of viral vectors to introduce a microdystrophin cDNA into dystrophic muscles and, eventually, into muscles of DMD patients.
In a clinical setup with our two methods, the dystrophin cDNA must be under the control of a muscle-specific promoter to avoid toxicity due to dystrophin expression in unfused MPCs or due to the dispersion of the plasmid to other organs. The absence of an antibiotic-resistance gene in the plasmids will be needed to avoid immune reactions against its product. However, since the random integration of the vector may induce tumorigenicity,35 samples of the cells transduced with lentivirus should be tested in vitro and in vivo, as previously done by our team for clinical trials of nondystrophic MPC transplantation.36
In conclusion, ex vivo gene therapy and plasmid electrotransfer must be improved to be used as potential treatments of muscular dystrophies such as DMD.
Material and Methods
Animals. Rag−/− mice were provided by Charles River (Willington, MA). Nondystrophic dogs were provided by La ferme aux toits oranges (Neuville, Quebec, Canada). All the experiments made on these animals were approved by the Animal Protection Committee of Laval University. The dystrophic dog came from a colony founded from one carrier female donated by J.N.K. The genotypes of the wild-type dogs and the dystrophic dog in pedigrees segregating golden retriever muscular dystrophy were confirmed by exon 7 specific genomic PCR followed by Sau96I digestion and analyzed by restriction fragments length polymorphism as described.37 The dystrophic dog was housed at the Muscular Dystrophy Research Center (AADM/UNAERP, Ribeirão Preto-SP, Brazil) according the guidelines set by the Institutional Laboratory Animal Care Committee of the University of Ribeirão Preto.
Cell culture. 293T cells were cultured in Dulbecco's modified Eagle's medium high glucose (Gibco, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% penicillin–streptomycin (Gibco). The hMPCs were obtained from a postmortem muscle sample of a normal 13-month-old male. These cells were proliferated in MCBD120 medium (Hyclone, Mississauga, Ontario, Canada) supplemented with 15% fetal bovine serum (Gibco), 1% penicillin–streptomycin (Gibco), 10 µg/l of bFGF (Feldan, Saint-Laurent, Quebec, Canada), 0.4 mg/l of dexamethasone (Sigma, Oakville, Ontario, Canada), and 5 mg/l of insulin (Sigma). To obtain the activation of the MCK promoter, cells were cultured in differentiation medium [DMEM (Gibco) supplemented with 2% fetal bovine serum (Invitrogen)]. The dystrophic dog MPCs were isolated from a muscle biopsy of a 2-year-old dystrophic animal. Nondystrophic dog MPCs were obtained from muscle biopsies of a 3-month-old dog and a 3-year-old dog. Dystrophic and nondystrophic dog MPCs were cultured in the same medium as the hMPCs. All the cells were maintained at 37 °C under 5% CO2.
Lentiviral plasmid construction. Dog microdystrophin cDNA contained in an AAV vector28 was amplified with Turbo Pfu DNA polymerase with sense and reverse primers containing respectively at each ends BamH1 and Spe1 sites. Amplified product was purified from agarose gel and cloned by fusion (BD in Fusion; Clontech, Mountain View, CA) in BamH1/Spe1 sites of lentiviral vector L6/V5 (Invitrogen) in which Xho1 site was eliminated by cutting with Spe1/Xho1, followed by Klenow (GE Healthcare, Mississauga, Ontario, Canada) and ligated with T4 DNA ligase (NEB, Pickering, Ontario, Canada). Moreover, blasticidin contained in the lentiviral plasmid L6/V5 has been substituted by a puromycin resistance gene between the sites of Sma1/Kpn1 in the viral vector. The original cytomegalovirus promoter has been also substituted by the MCK promoter38 making the final construct LeMCK.µDysV5 (Figure 1a). Constructs used to immortalize dog MPCs are described in reference.22 Both cDNAs coding for telomerase and Cdk4 were cloned each in L6/V5 in which the original resistant gene blasticidin was respectively substituted by puromycin and hygromycin. Finally, pLeGFP construct is the result of cloning of eGFP cDNA39 cloned in L6/V5 and expressed with a cytomegalovirus promoter.
Virus production. Self-inactivating lentiviral vectors were produced with the third packaging system40 and the co-transfection method allowed their production as previously described.41 Briefly, psPAX2, pMD2G, and the vector plasmid were transfected in 293T cells using CaCl2 precipitation. The medium was removed 16 hours post-transfection and the supernatant was harvested at 12, 24, and 36 hours. Average titers were around 106 transduction units/ml. Cells were transduced with the lentivirus with a multiplicity of infection of 100 in the presence of 8 µg/ml of polybrene. The medium was removed after 12 hours. Cells transduced with a lentivirus coding for the puromycin resistance or the hygromycin resistance gene were respectively selected during 2 days with the puromycin (Clontech) at 2 µg/ml or during 1 week with the hygromycin (MultiCell Technologies, Woonsocket, RI) at 200 µg/ml, starting 2 days after the transduction.
Cell transplantation in mice. For human cell transplantation, Rag−/− mice were γ-ray irradiated (12Gy) at the leg level to inhibit the proliferation of the recipient satellite cells, a condition that favors the participation of grafted cells in recipient muscle regeneration.42 Two days before the graft, 1 µg of cardiotoxin (Sigma) was injected into the TAs to damage the muscle fibers inducing a process of muscle regeneration to induce the fusion of the transplanted cells.43 For the transplantations of nondystrophic and dystrophic dog MPCs, Rag−/− mice did not receive cardiotoxin nor irradiation.
Ten intramuscular injections were done for each MPC transplantation in the TA muscle. A total of 106 cells (for hMPCs) or 5 × 105 cells (for dog MPCs) resuspended in 8 µl of Hank's buffered salt solution (Gibco) in the TA were injected in each muscle. The grafted TAs were harvested 1 month after transplantation and were snap frozen in liquid nitrogen. Serial 12-µm cryostat sections were obtained throughout the muscle.
Electrotransfer. Forty microgram of the pLeMCK.µDysV5 were injected percutaneously at a final volume of 40 µl of Tyrode's buffer 1× (Sigma) in the mouse muscle. A single-longitudinal injection was made into the TA and the “Electrode Electrolyte” cream (Teca, Pleasantville, NY) was applied on the skin to favor the passage of the electric current between the two metal plaques. The generator used in this experiment delivers square wave pulses with the following parameters: 10 pulses of 200 V/cm, duration of 25 ms, and delay of 300 ms. The electrotransferred muscles were harvested 2 weeks after the experiment and were processed as the cell-grafted muscles.
For dogs, 200 µg of the pLeMCK.µDysV5 were injected at a final volume of 200 µl of Tyrode's buffer 1× (Sigma) into the brachialis anterior. Two close injections of 100 µl were directly made into the muscle previously exposed by cutting the skin. Afterwards, two needles separated by 4 mm were implanted around the plasmid injection area and the electric pulses were applied. The electrical parameters for dogs were the same as for mice: 10 pulses of 200 V/cm, duration of 25 ms, and delay of 300 ms. Suture points were placed in the muscle to distinguish the area of electrotransfer. The electrotransferred regions were biopsied 2 weeks later and the biopsies were prepared similar to that of the mouse muscles, except that the cryostat sections were 18-µm thick.
Western blot and histological analysis. Proteins from MPCs or myotubes (3 days in differentiation medium) were extracted by a methanol–chloroform technique to perform western blots. Thirty microgram of proteins were loaded per well. The V5 tag was detected with a mouse anti-V5 antibody (1:5,000; Invitrogen). The Cdk4 was detected with a rabbit anti-Cdk4 antibody (1:500; eBioscience, San Diego, CA). The telomerase was detected with a rabbit anti-telomerase antibody (1:500; Acris, Herford, Germany) and a goat antidesmin antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect desmin.
Hematoxylin–eosin staining was used for a morphological analysis of the grafted TAs. Immunohistochemistry to detect V5 was performed with a mouse anti-V5 antibody (1:200; Invitrogen) and dog dystrophin was detected with a mouse antidystrophin antibody MANDYS104 (1:8; CIND, Oswestry, UK), which reacts with dog but not mouse dystrophin. To detect the presence of CD8+ cells, a rat anti-CD8 antibody (1:80; AbD Serotec, Oxford, UK) was used. These primary antibodies were followed by incubation with a biotinylated anti-mouse or anti-rat antibody (1:300; Dako, Mississauga, Ontario, Canada) and with the Streptavidin-Cy3 (1:300; Sigma). Afterwards, the sections were mounted in phosphate-buffered saline-glycerol (1:1).
GFP and histological analyses were observed under fluorescence using an Axiophot microscope (Zeiss, Oberkochen, Germany).
Acknowledgments
We thank Glenn E. Morris and Le Thanh Lam (MRIC Biochemistry Group, Wrexham, UK) for providing the MANDYS104 antibody and Guylaine Jalbert for her technical assistance during the experiments performed in dogs. J.P.T. is the president and hold shares in CellGene Inc., a biotech company involved in the development of cell and gene therapies.
REFERENCES
- Hoffman EP, Brown RH., Jr, and , Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Campbell KP., and , Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338:259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Ervasti JM., and , Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A., and , van Ommen GJ. Progress in therapeutic antisense applications for neuromuscular disorders. Eur J Hum Genet. 2010;18:146–153. doi: 10.1038/ejhg.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard JP, Roy R, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Côté CH, Chapdelaine P, et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisset PA, Skuk D, Asselin I, Goulet M, Roy B, Karpati G, et al. Successful transplantation of genetically corrected DMD myoblasts following ex vivo transduction with the dystrophin minigene. Biochem Biophys Res Commun. 1998;247:94–99. doi: 10.1006/bbrc.1998.8739. [DOI] [PubMed] [Google Scholar]
- Quenneville SP, Chapdelaine P, Skuk D, Paradis M, Goulet M, Rousseau J, et al. Autologous transplantation of muscle precursor cells modified with a lentivirus for muscular dystrophy: human cells and primate models. Mol Ther. 2007;15:431–438. doi: 10.1038/sj.mt.6300047. [DOI] [PubMed] [Google Scholar]
- Zaiss AK., and , Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Romero NB, Braun S, Benveniste O, Leturcq F, Hogrel JY, Morris GE, et al. Phase I study of dystrophin plasmid-based gene therapy in Duchenne/Becker muscular dystrophy. Hum Gene Ther. 2004;15:1065–1076. doi: 10.1089/hum.2004.15.1065. [DOI] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodles-Brakhop AM, Heller R., and , Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009;17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Guérette B, Asselin I, Roy R., and , Tremblay JP. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve. 1994;17:1407–1415. doi: 10.1002/mus.880171210. [DOI] [PubMed] [Google Scholar]
- Harris JB., and , Johnson MA. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol. 1978;5:587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Floyd SS, Jr, Clemens PR, Ontell MR, Kochanek S, Day CS, Yang J, et al. Ex vivo gene transfer using adenovirus-mediated full-length dystrophin delivery to dystrophic muscles. Gene Ther. 1998;5:19–30. doi: 10.1038/sj.gt.3300549. [DOI] [PubMed] [Google Scholar]
- Miranda AF, Bonilla E, Martucci G, Moraes CT, Hays AP., and , Dimauro S. Immunocytochemical study of dystrophin in muscle cultures from patients with Duchenne muscular dystrophy and unaffected control patients. Am J Pathol. 1988;132:410–416. [PMC free article] [PubMed] [Google Scholar]
- Jaynes JB, Johnson JE, Buskin JN, Gartside CL., and , Hauschka SD. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988;8:62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, et al. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 2007;6:515–523. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Mir LM. Nucleic acids electrotransfer-based gene therapy (electrogenetherapy): past, current, and future. Mol Biotechnol. 2009;43:167–176. doi: 10.1007/s12033-009-9192-6. [DOI] [PubMed] [Google Scholar]
- Trollet C, Scherman D., and , Bigey P. Delivery of DNA into muscle for treating systemic diseases: advantages and challenges. Methods Mol Biol. 2008;423:199–214. doi: 10.1007/978-1-59745-194-9_14. [DOI] [PubMed] [Google Scholar]
- Vilquin JT, Wagner E, Kinoshita I, Roy R., and , Tremblay JP. Successful histocompatible myoblast transplantation in dystrophin-deficient mdx mouse despite the production of antibodies against dystrophin. J Cell Biol. 1995;131:975–988. doi: 10.1083/jcb.131.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y, Udaka K, Yamashiro Y, Yagita H., and , Okumura K. Dystrophin acts as a transplantation rejection antigen in dystrophin-deficient mice: implication for gene therapy. J Immunol. 1998;160:4635–4640. [PubMed] [Google Scholar]
- Roy R, Tremblay JP, Huard J, Richards C, Malouin F., and , Bouchard JP. Antibody formation after myoblast transplantation in Duchenne-dystrophic patients, donor HLA compatible. Transplant Proc. 1993;25 1 Pt 2:995–997. [PubMed] [Google Scholar]
- Wang B, Li J, Qiao C, Chen C, Hu P, Zhu X, et al. A canine minidystrophin is functional and therapeutic in mdx mice. Gene Ther. 2008;15:1099–1106. doi: 10.1038/gt.2008.70. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Qiao C, et al. 2009Widespread muscle expression of an AAV-9 human mini-dystrophin construct after systemic intravenous injection in golden retriever muscular dystrophy (GRMD) neonatal dogs Mol Ther 17suppl 1): S152–S153. [Google Scholar]
- Li J, Bogan J, Chen C, Bogan D, Wang B, Yuan Z, et al. 2009Hydrodynamic limb vein injection of AAV9 results in regional and systemic long-term expression of minidystrophin in young adult GRMD dogs Mol Ther 17supp 1): S278 [Google Scholar]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D, Liu Y, Li ZY, Strauss R, Finn EE, Allen JM, et al. Biodistribution and safety profile of recombinant adeno-associated virus serotype 6 vectors following intravenous delivery. J Virol. 2008;82:7711–7715. doi: 10.1128/JVI.00542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JT. Adenoviral vectors for gene therapy. Mol Biotechnol. 2007;36:71–80. doi: 10.1007/s12033-007-0021-5. [DOI] [PubMed] [Google Scholar]
- Stolberg SG.1999F.D.A. Officials Fault Penn Team in Gene Therapy DeathNew York Times (Print): p. A22. [PubMed]
- Porteus MH, Connelly JP., and , Pruett SM. A look to future directions in gene therapy research for monogenic diseases. PLoS Genet. 2006;2:e133. doi: 10.1371/journal.pgen.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Bartlett RJ, Winand NJ, Secore SL, Singer JT, Fletcher S, Wilton S, et al. Mutation segregation and rapid carrier detection of X-linked muscular dystrophy in dogs. Am J Vet Res. 1996;57:650–654. [PubMed] [Google Scholar]
- Wang B, Li J., and , Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine P, Moisset PA, Campeau P, Asselin I, Vilquin JT., and , Tremblay JP. Functional EGFP-dystrophin fusion proteins for gene therapy vector development. Protein Eng. 2000;13:611–615. doi: 10.1093/protein/13.9.611. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P., and , Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci. 2006;Chapter 4:Unit 4.21. doi: 10.1002/0471142301.ns0421s37. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP., and , Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111 6 Pt 1:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeford S, Watt DJ., and , Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]