Abstract
The therapeutic potential of oncolytic adenoviruses is limited by the rate of adenovirus release. Based on the observation that several viruses induce cell death and progeny release by disrupting intracellular calcium homeostasis, we hypothesized that the alteration in intracellular calcium concentration induced by verapamil could improve the rate of virus release and spread, eventually enhancing the antitumoral activity of oncolytic adenoviruses. Our results indicate that verapamil substantially enhanced the release of adenovirus from a variety of cell types resulting in an improved cell-to-cell spread and cytotoxicity. Furthermore, the combination of the systemic administration of an oncolytic adenovirus (ICOVIR-5) with verapamil in vivo greatly improved its antitumoral activity in two different tumor xenograft models without affecting the selectivity of this virus. Overall, our findings indicate that verapamil provides a new, safe, and versatile way to improve the antitumoral potency of oncolytic adenoviruses in the clinical setting.
Introduction
Conditionally replicative adenoviruses hold promise for the treatment of cancer.1 Their selective replication in tumor cells and consequent lysis and progeny release allows the amplification of the virus and lateral spread to neighboring tumor cells. However, certain limitations encountered by adenovirus during systemic administration and in the tumor make the achievement of systemic antitumoral efficacy challenging. Adenovirus is quickly eliminated from the bloodstream following systemic administration,2 and once in the tumor, oncolytic adenoviruses face physical barriers imposed by the tumor stroma and the recruitment of an antiviral immune response, which may hinder the spread of the antitumor activity.3 In this particular environment, the improvement of the rate of adenovirus spread is critical to allow the progression of the oncolytic effect.4
Strategies to increase systemic oncolytic adenovirus therapy by improving their spread include the expression of proteases,5 which disrupt connective tissue, or fusogenic proteins.6 However, the insertion of transgenes into the adenovirus genome is limited by their size and requires transgene compatibility with the adenovirus replication cycle. Alternatively, specific point mutations or deletions in E1B-19K,7 overexpression of the adenovirus death protein (ADP),8 or c-truncating mutations in the i-leader protein9,10 have been described to improve the cell-to-cell spread in vitro and enhance its therapeutic potential. Despite the advantages of these approaches, the insertion of these modifications still requires the genetic manipulation of the adenovirus genome. In addition, several side effects of some spread-enhancing mutations, such as virus yield reduction11 or partial loss of the E3 immunomodulatory functions,12 may be undesirable in the context of oncolytic adenoviruses. Therefore, a drug capable of increasing the spread of adenovirus without affecting other viral functions would be an attractive alternative.
Using an in vivo bioselection approach, we recently isolated a c-terminal mutation in the E3/19K protein, which enhanced the release of adenovirus from the infected cell and improved its antitumoral efficacy,13 suggesting that the intratumoral spread of adenovirus is limited by the natural rate of adenovirus release. This process is rather inefficient and it does not take place until late times after infection when ADP accumulates to actively promote progeny release.14 Although the mechanism of adenovirus cell lysis and progeny release is not well understood, several hypothesis suggest that ADP may function by modifying intracellular calcium pools.14,15 Moreover, a growing body of evidence suggests that different viruses induce cell death and progeny release by altering intracellular calcium concentration.16,17 Indeed, the c-terminal E3/19K mutation we had previously identified enhanced virus release by disrupting intracellular calcium homeostasis.13 Based on these observations, we hypothesized that verapamil, a calcium channel blocker, could improve the rate of virus release and spread, eventually enhancing the antitumoral activity of oncolytic adenoviruses.
This study was designed to determine the effect of verapamil on adenovirus spread in vitro and to study the selectivity and antitumoral potency of an oncolytic adenovirus, such as ICOVIR-5 (refs. 18,19), in combination with verapamil in vivo. Our results demonstrate that verapamil substantially enhances the release of adenovirus in a variety of cell types, resulting in an improved cell-to-cell spread and cytotoxicity without affecting adenovirus replication or native gene expression. In vivo, the selectivity profile of ICOVIR-5 was maintained following treatment with verapamil, and the antitumoral potency of this oncolytic virus was greatly enhanced in different tumor models. Overall, our findings indicate that verapamil provides a new, safe, and versatile way to improve the antitumoral potency of oncolytic adenoviruses in the clinical setting.
Results
Verapamil enhances virus release and cytotoxicity of adenovirus type 5 in vitro
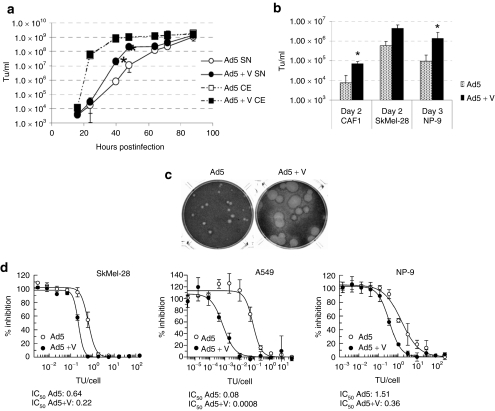
Due to the importance of Ca2+ modulation during virus-induced cell death and progeny release,17,20 and in order to evaluate whether verapamil, a calcium blocking agent, was able to increase the release of adenovirus serotype 5 (Ad5), we assessed the effect of verapamil on the kinetics of virus release and production in lung adenocarcinoma cell line A549. Despite the total yield was the same both in the presence and the absence of verapamil, there was a 30-fold increase in Ad5 release at 40 hours postinfection (p.i.) when combined with verapamil (Figure 1a). Addition of verapamil also resulted in an accelerated rate of Ad5 release from human carcinoma–associated fibroblasts CAF1, SkMel-28 melanoma, and NP-9 pancreatic tumor cell lines (Figure 1b). The early release improved the cell-to-cell spread of Ad5 as demonstrated by the large plaque size in A549 monolayers. In the presence of verapamil, the plaques of Ad5 appeared earlier and were bigger than the control plaques (Figure 1c). In addition, the combination with verapamil rendered Ad5 more cytotoxic because the amount of Ad5 required to cause a reduction of 50% in cell viability (IC50) was 4 times, 3 times, and up to 100 times lower in SkMel-28, NP-9, and A549 cells, respectively, when combined with verapamil (Figure 1d).
Figure 1.
Verapamil enhances the release, spread, and cytotoxicity of Ad5 in vitro. (a) Viral production and release kinetics of Ad5 combined with 40 µmol/l verapamil in A549 cells. Viral content of the total (CE) and extracellular (SN) fractions were analyzed at the indicated time points. Mean values (n = 3) ± SD are plotted.*Significant (P = 8.1 × 10−5 and P = 0.03 at 48 and 64 hours postinfection, respectively) compared to SN of Ad5. (b) Viral release of Ad5 in combination with verapamil in CAF1, SkMel-28, and NP-9 cells. The time point at which the biggest difference in virus release was observed is shown. *Significant (P = 0.015 for CAF1 and P = 0.04 for NP-9) compared to the release of Ad5. (c) Plaque size of Ad5 in the presence of verapamil (30 µmol/l final concentration) in A549 cells at day 7 postinfection. (d) Comparative cytotoxicity of Ad5 ± verapamil in SkMel-28, A549, and NP-9 tumor cell lines. IC50 values (TU/cell of Ad5 required to cause a reduction of 50% in cell viability) for each condition are shown. TU, transducing units.
Verapamil does not alter viral gene expression and enhances the release regardless of ADP levels
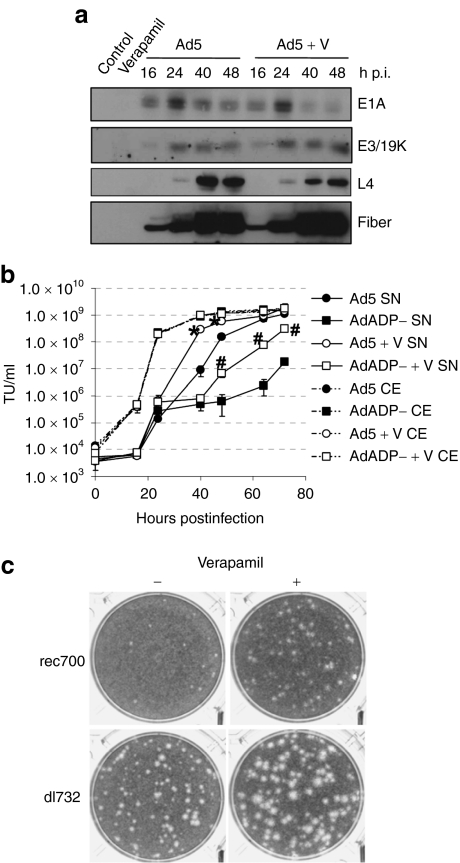
In order to study the effects of verapamil on the viral cycle of Ad5, we analyzed adenovirus early and late protein expression in the presence of verapamil. There were no differences in the pattern of E1A, E3/19K, or late L4, and fiber protein expression when combining Ad5 with verapamil, proving that verapamil had no effect on adenovirus protein expression (Figure 2a).
Figure 2.
Effect of verapamil on viral protein expression and dependence on ADP. (a) Verapamil treatment does not modify adenovirus early and late protein expression pattern. A549 cells were infected with Ad5 with or without verapamil in the extracellular medium, and expression of E1A, E3/19K, L4, and fiber proteins was analyzed at 16, 24, 40, and 48 hours postinfection. (b) Viral release kinetics of Ad5 and AdADP− in A549 cells treated with verapamil. Supernatant viral content was quantified at the indicated time points. Mean values (n = 3) ± SD are plotted. *Significant (P = 0.005 and P = 0.001 at 40 and 48 hours p.i., respectively) compared to the release of Ad5 and #significant (P = 0.03, P = 0.003, and P = 0.02 at 48, 64, and 72 hours p.i., respectively) compared to the release of AdADP−. (c) Comparative plaque size of rec700 and dl732 in the presence and absence of verapamil in A549 cells at day 6 p.i. ADP, adenovirus death protein; p.i., postinfection.
Although the exact mechanism that triggers adenovirus release is unknown, ADP plays a major role in this process because ADP mutant viruses display a defect in virus release without affecting the total viral production.14 In order to evaluate the ADP dependence of the phenotype of Ad5 in the presence of verapamil, we analyzed the virus release of AdADP− (a virus that expresses a truncated form of the ADP protein that partially retains certain functions of the native form but is defective in promoting cell lysis), alone or combined with verapamil. As expected, AdADP− displayed an impaired release compared to Ad5 due to the deletion of residues near the NH2 terminus of the protein that have been suggested to be important for its transport and stability21 (Figure 2b). Interestingly, incubation of AdADP−-infected cells with verapamil also enhanced the release of this defective mutant (Figure 2b) as well as its plaque size (data not shown). Although the increase in the release of AdADP− in combination with verapamil was delayed compared to Ad5 (64 hours p.i. for AdADP− versus 40 hours p.i. for Ad5), the presence of the calcium blocking agent increased the release of both viruses to the same extent (~30-fold) (Figure 2b). This suggests that verapamil triggers a new pathway that results in the improved release of adenovirus and does not require the cell lysis–promoting function of ADP.
ADP overexpression has also been described to improve the spread of adenovirus and render it more cytotoxic.15 Because the effect of verapamil on adenovirus release was independent of ADP, we combined a mutant with ADP overexpression, dl732, with verapamil in order to evaluate whether it could further enhance the release of this virus. The addition of verapamil in a plaque assay in A549 cells resulted in an even greater plaque size of this mutant that demonstrated that the effects of ADP overexpression and verapamil on adenovirus release were additive (Figure 2c).
The early release of Ad5 depends on the calcium channel blocking activity of verapamil
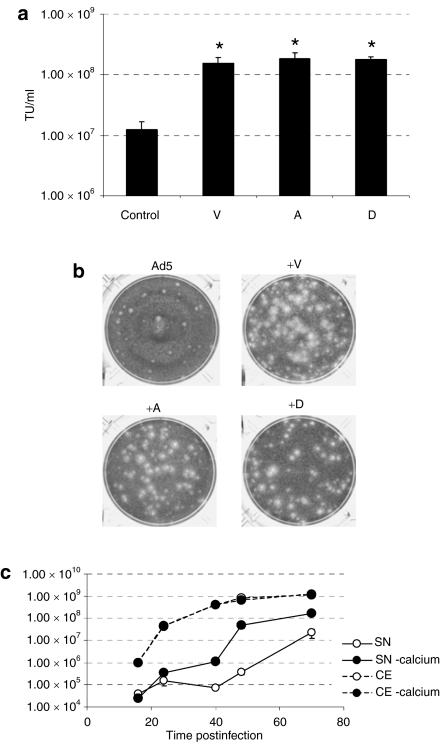
Verapamil is a calcium channel blocker that belongs to the family of phenylalkylamines which as well as the two other types of calcium channel blockers (dihydropyridines and benzothiazepines) inhibits the influx of calcium through passive “slow” channels.22 In order to study whether the calcium blocking activity of verapamil was causing the fast rate of virus release, we assessed extracellular Ad5 levels in the presence of different calcium channel blocking agents: amlodipine (dihydropyridine) and diltiazem (benzothiazepine). Similar to the effect observed with verapamil, Ad5 displayed a 15-fold increase in virus release at 40 hours p.i. and a large plaque size when combined with both amlodipine and diltiazem in A549 cells (Figure 3a,b). Viral release and production in the absence of calcium in the extracellular medium provided further evidence concerning the dependence on the calcium blocking activity of this phenotype. Extracellular calcium deprivation improved the release of Ad5 to a degree similar to that obtained with verapamil (up to 120-fold increase at 48 hours p.i.) without affecting the total viral production (Figure 3c) indicating, again, that the calcium blocking activity of verapamil is triggering the observed release enhancement.
Figure 3.
Dependence of the enhanced release phenotype on the calcium blocking activity of verapamil. (a) Ad5 release at 40 hours postinfection in the presence of calcium channel blockers. Mean values (n = 3) ± SD are plotted.*Significant compared to the release of Ad5 (P = 0.025, P = 0.023, and P = 0.003 for verapamil, amlodipine, and diltiazem, respectively). (b) Plaque size of Ad5 in the presence of calcium channel blockers: verapamil (V), amlodipine (A), and diltiazem (D) at 30 µmol/l. Pictures of representative plaques at 7 days postinfection (p.i.) are shown. (c) Virus production and release kinetics in the presence or absence of calcium in the extracellular medium. Mean values (n = 3) ± SD are plotted.
Cell death mechanisms triggered by verapamil that may result in enhanced release
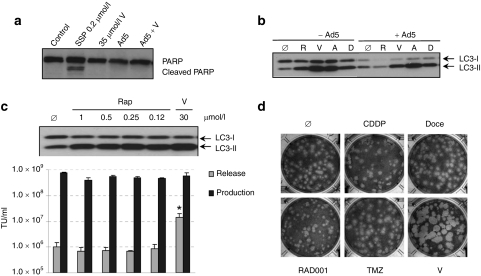
The importance of intracellular calcium in the regulation of apoptosis23 and the fact that apoptosis activation can confer a large plaque size to Ad5 similar to that conferred by verapamil24 lead us to evaluate apoptosis activation in the presence of the drug. As Figure 4a displays, the levels of PARP cleavage, indicative of apoptosis activation, during infection with Ad5 alone or in the presence of verapamil were similar. This demonstrates that verapamil was not enhancing apoptosis and that the verapamil-induced large plaque size of Ad5 was not apoptosis-mediated.
Figure 4.
Potential death mode triggered by verapamil and comparison of the effect of verapamil with that of other drugs that synergize with adenovirus. (a) Apoptosis activation status in Ad5-infected A549 cells in the presence or absence of verapamil. PARP cleavage was detected by western blot at 40 hours postinfection. Cells incubated with Staurosporine (SSP, 0.2 µmol/l) are used as positive control of apoptosis induction. (b) Anti-LC3 western blot in A549 cells in the presence of calcium channel blockers. A549 cells were infected with Ad5 and incubated with normal medium or medium containing rapamycin (positive control of autophagy induction), verapamil (V), amlodipine (A), or diltiazem (D). (c) Virus release in the presence of rapamycin. A549 cells were incubated with increasing concentrations of rapamycin or verapamil, and LC3 expression was assessed by western blot 40 hours p.i. At the same conditions, the extracellular and total virus produced were quantified at 40 hours p.i. Mean values (n = 3) ± SD are plotted. *Significant compared to the release of Ad5 (P = 0.04). (d) Plaque size of Ad5 in the presence of 30 µmol/l verapamil (V) or 5 µmol/l cisplatin (CDDP), 200 pg/ml docetaxel (Doce), 10 nmol/l RAD001, or 10 µmol/l temozolomide (TMZ) (concentration that gave 10% growth inhibition). Cells were stained 7 days postinfection and pictures of representative plaques are shown. p.i., postinfection.
The calcium channel blocking activity of verapamil has recently been reported to enhance autophagic vesicle formation,25 and prominent autophagy induction at late stages of adenovirus infection has led to speculation that autophagy induction during adenovirus infection may facilitate adenovirus release.26 In order to study whether the enhanced release in the presence of verapamil associates to a more pronounced induction of autophagy in combination with this calcium channel blocking agent, we performed a western blot anti-LC3 in A549 cells infected with Ad5 alone or in combination with different calcium channel blocking agents. At a time point at which there was a marked increase in adenovirus release (40 hours p.i.), verapamil substantially enhanced the ratio LC3-II/LC3-I both in control A549 cells and in Ad5-infected cells (Figure 4b). Indeed, all the calcium channel blockers tested were able to increase the ratio LC3-II/LC3-I (Figure 4b). This established an association between increased autophagosome formation or accumulation, and the enhanced progeny release triggered by different calcium channel blocking agents.
The synergistic antitumoral effect of verapamil and adenovirus differs from the effect of other drugs
To further study the association of autophagy and verapamil, we tested virus release in the presence of increasing concentrations of rapamycin. Previously, several drugs capable of inducing autophagy, such as rapamycin, its analogue RAD001 (everolimus) or temozolomide, have been found to improve the antitumoral effect of a telomerase-selective oncolytic adenovirus by inducing autophagy.27 Although rapamycin was able to induce LC3-I to LC3-II cleavage, which is indicative of autophagy induction, combination of adenovirus with rapamycin displayed wild-type levels of extracellular virus (Figure 4c) and small plaque size (data not shown). This indicated that the ability of verapamil to enhance adenovirus release was autophagy-independent. In addition, verapamil-induced large plaque size was not observed in the presence of other drugs that have previously demonstrated synergistic antitumoral effect with oncolytic adenoviruses,28,29 such as cisplatin, docetaxel, RAD001, and temozolomide (Figure 4d). This indicated that the early release observed with verapamil was unique for the combination with the calcium channel blocker.
Verapamil enhances the cytotoxicity of ICOVIR-5 in vitro and maintains the selectivity of oncolytic adenovirus ICOVIR-5 in vivo
To evaluate whether verapamil was also able to increase the cytotoxicity of tumor-selective oncolytic adenoviruses, we tested its combination with ICOVIR-5. Previously, we have demonstrated that ICOVIR-5 displays a safe toxicity profile after systemic virus administration based on E2F-1 promoter–regulated E1AΔ24 expression.18,19 As expected, verapamil increased ICOVIR-5 plaque size (data not shown) and enhanced its cytotoxicity in vitro. The IC50 value of ICOVIR-5 in A549 and NP-9 cells was 0.69 and 4.96 transducing units (TU)/cell, respectively, whereas in the presence of verapamil, the IC50 of ICOVIR-5 was reduced to 0.067 and 3.2.
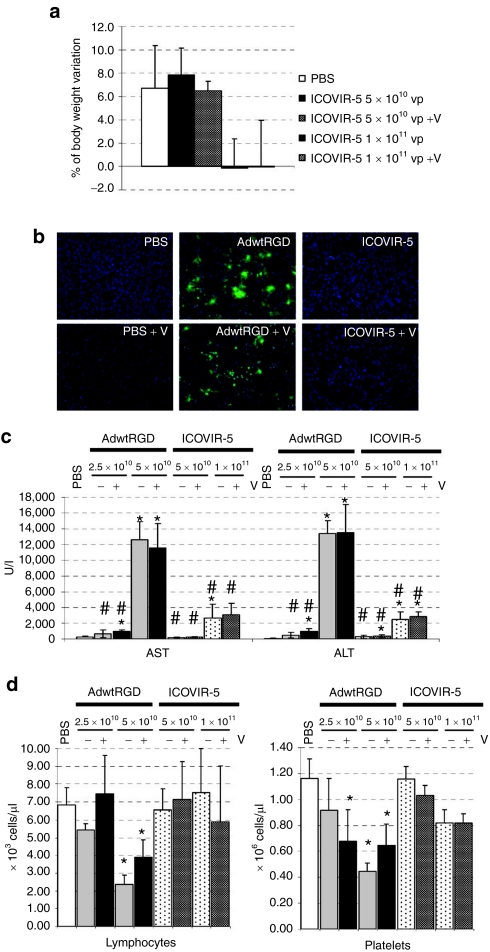
We also evaluated the effects of verapamil on the selectivity of ICOVIR-5 in an immunocompetent model in vivo. The toxicity of a single intravenous dose of ICOVIR-5 alone or combined with daily intraperitoneal administration of 20 mg/kg verapamil (a dose chosen based on studies of the combination of verapamil with chemotherapy in vivo30) was assessed in Balb/C immunocompetent mice and compared to the toxicity of a nonselective control (AdwtRGD). Mice treated with 5 × 1010 viral particles (vp) or 1 × 1011 vp of ICOVIR-5 combined with daily verapamil administration showed a similar body weight variation as the groups treated with ICOVIR-5 alone at day 5 postinjection (Figure 5a). Anti-E1A immunostaining of frozen liver sections from mice treated with 5 × 1010 vp of AdwtRGD alone or in combination with verapamil displayed the same levels of E1A expression (Figure 5b), whereas ICOVIR-5 alone or combined with verapamil both efficiently abrogated E1A expression compared to AdwtRGD (Figure 5b). Furthermore, transaminase levels in AdwtRGD- or ICOVIR-5-treated mice were not affected by daily verapamil administration (Figure 5c), and verapamil administration was still able to prevent the reduction in platelet count and lymphopenia in ICOVIR-5-treated mice compared to the AdwtRGD-injected groups (Figure 5d). Overall, these data indicated that daily verapamil administration did not increase the toxicity of AdwtRGD and maintained the selectivity profile of ICOVIR-5 regardless of the dose.
Figure 5.
The selectivity of ICOVIR-5 is maintained in the presence of verapamil in an immunocompetent model in vivo. (a) Percent of body weight variation after systemic administration of PBS or ICOVIR-5 alone or combined with daily i.p. injection of 20 mg/kg of verapamil. (b) Liver E1A expression analyzed by immunohistochemistry of representative frozen liver sections of mice treated with PBS or 5 × 1010 vp of AdwtRGD, or ICOVIR-5 + daily verapamil (V) at day 5 postinfection (AdwtRGD groups were killed at day 3 due to toxicity). (c) Mean values of AST (aspartate animotransferase) and ALT (alanine aminotransferase) in serum and (d) lymphocyte and platelet concentrations in peripheral blood at day 5 postinjection (day 3 postinjection for the groups injected with AdwtRGD at 5 × 1010 vp) of the doses indicated. Mean values of five animals per group ± SD are plotted. *Significant (P < 0.05) compared to PBS and #significant compared to AdwtRGD. PBS, phosphate-buffered saline.
Verapamil improves the antitumoral efficacy of ICOVIR-5 in vivo
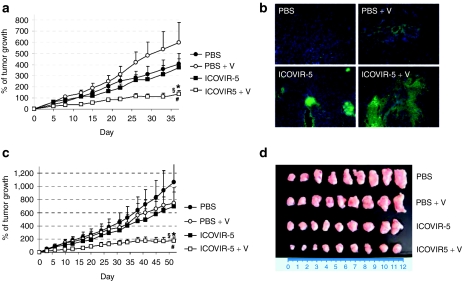
Once we had demonstrated the improved cytotoxicity in vitro and safety of ICOVIR-5 combined with verapamil, we sought to determine whether it conferred an advantage in antitumoral efficacy in vivo compared to ICOVIR-5 alone. A single injection of phosphate-buffered saline (PBS) or 5 × 1010 vp of ICOVIR-5 was injected systemically into mice bearing subcutaneous A549 (lung) or SkMel-28 (melanoma) tumor xenograft models. Starting at day 1 postinjection, half of the mice in each group received daily intraperitoneal injections of 20 mg/kg of verapamil. As Figure 6a displays ICOVIR-5 combined with verapamil was more efficient at delaying the growth of A549 subcutaneous tumors in vivo. Moreover, four tumors treated with ICOVIR-5 and verapamil completely regressed and maintained the regression status by 4 months after treatment (time of killing), indicating that tumor cells had been completely eradicated. This enhanced antitumoral effect correlated with a more diffuse distribution observed by antiadenovirus immunostaining on tumor sections at day 13 postinjection (Figure 6b), indicating an improved intratumoral spread of ICOVIR-5 in the presence of verapamil. Similarly, whereas only a discrete effect on tumor growth was observed in the ICOVIR-5-treated SkMel-28 subcutaneous tumor xenografts at this dose, the combination with verapamil showed a marked control of tumor growth in this model (sixfold reduction in tumor growth compared to PBS) (Figure 6c,d). The enhanced therapeutic effect of the combination of ICOVIR-5 with verapamil in two different tumor xenograft models confirms the benefits of the use of this drug to improve the therapeutic potential of oncolytic adenoviruses.
Figure 6.
Verapamil enhances the antitumoral activity of ICOVIR-5 in vivo. (a) Nude mice with A549 tumor xenografts were treated with PBS, PBS combined with daily 20 mg/kg verapamil i.p. injection, or a single dose of 5 × 1010 vp of ICOVIR-5 alone or combined with verapamil. Percent of tumor growth ± SEM is plotted. *Significant (P = 0.0019) compared to PBS; §Significant (P = 0.016) compared to PBS + V; and #Significant (P = 0.019) compared to ICOVIR-5. (b) Antiadenovirus immunostaining of frozen A549 tumor sections treated with PBS, PBS + verapamil, ICOVIR-5, or ICOVIR-5 + daily verapamil at day 13 after virus administration. (c) Nude mice with SkMel-28 tumor xenografts were treated with PBS, PBS combined with daily 20 mg/kg verapamil i.p. injection, or a single dose of 5 × 1010 vp of ICOVIR-5 alone or combined with verapamil. Percent of tumor growth ± SEM is plotted. *Significant (P = 0.0009) compared to PBS; §Significant (P = 0.0015) compared to PBS +V; and #Significant (P = 0.017) compared to ICOVIR-5. (d) Comparative size of SkMel-28 tumor xenografts treated with PBS, PBS + verapamil, ICOVIR-5, or ICOVIR-5 + verapamil at day 52 after virus injection. PBS, phosphate-buffered saline.
Discussion
The clinical use of oncolytic adenoviruses revealed a good toxicological and safety profile but also pointed out the need of an improved oncolytic potency of the candidate viruses. Improvement of intratumoral spread of oncolytic adenoviruses can enhance the therapeutic potential of oncolytic adenoviruses, but this step is limited by the natural rate of adenovirus.13,15 To date, the poor understanding of the mechanism of adenovirus release has restricted the rational approaches to improve progeny release to ADP overexpression8 or to insertion of transgenes that induce early cell death.31 Although the contribution of calcium modulation to adenovirus release has not yet been studied, we recently reported that a c-truncation in the E3/19K glycoprotein could enhance adenovirus release by disrupting intracellular calcium homeostasis.13 Additionally, the modification of the intracellular calcium pools is a widespread mechanism used by viruses to induce cell lysis and progeny release.16,17 Calcium is important in apoptosis regulation23 and several hypotheses suggest that ADP, required to induce cell lysis and progeny release, may act as a calcium channel.14,15 Based on these observations, we hypothesized that calcium channel blocker verapamil could improve the rate of adenovirus release and spread, eventually enhancing the therapeutic activity of oncolytic adenoviruses.
Our results demonstrate that, indeed, the combination of Ad5 with verapamil in vitro substantially enhanced the release of adenovirus from the infected cell without affecting virus production. The calcium channel blocker improved the rate of adenovirus release from a variety of cell types including lung, pancreas, and melanoma adenocarcinomas and cancer-associated fibroblasts, and this resulted in an improved cell-to-cell spread and cytotoxicity. The benefits of the use of verapamil to improve the spread of oncolytic adenoviruses were confirmed with a highly selective candidate, such as ICOVIR-5. Combination of ICOVIR-5 with verapamil enhanced its cytotoxicity in vitro, and most importantly, greatly improved the antitumoral activity of this oncolytic adenovirus in two different human tumor xenograft models in vivo. Despite the enhanced cell killing and antitumoral effect in vivo, combination of systemic ICOVIR-5 administration with verapamil in an immune competent model strictly maintained the E2F promoter–driven E1AΔ24 selectivity of the virus. Thus, by specifically acting on adenovirus release, a late event in the replication cycle, verapamil preserves the selectivity of conditionally replicative adenoviruses regardless of the regulatory elements used.
Such increase in the antitumoral effect without the requirement of genetic modifications, coupled with the safety of the combination with verapamil make this drug an attractive alternative to improve the efficacy of oncolytic adenoviruses. Whereas other genetic-based methods used to enhance the spread of adenoviruses may compromise some of the E3 immunomodulatory functions8,32 or reduce virus production by inducing early cell death,11 verapamil was able to enhance the spread and cytotoxicity without impairing virus production or modifying the expression of adenovirus proteins. The applicability of the combination with verapamil is further augmented by its ability to rescue the impaired progeny release of an ADP-defective adenovirus. The E3 region, considered unnecessary for adenovirus replication in vitro, was universally deleted from Ad5 gene therapy constructs until recent efforts to reduce the immune response.33 Consequently, some oncolytic adenoviruses constructed in an ADP-defective background, which exhibit an impaired spread, could benefit from the combination with verapamil. The calcium channel blocker was also able to further enhance the in vitro spread of an ADP-overexpressing mutant (dl732). Oncolytic adenoviruses that overexpress ADP display an enhanced antitumoral activity in preclinical models8,34 and are currently proposed as clinical candidates. Although the additive effect of verapamil and ADP overexpression has not yet been confirmed in vivo, our results suggest that the intratumoral spread of these viruses could still be further increased by verapamil. Furthermore, the fast rate of virus release and enhanced intratumoral spread observed in combination with verapamil is also desirable with oncolytic adenoviruses expressing pro-drug converting enzymes, fusogenic proteins, extracellular matrix metalloproteases, or other transgenes to improve the extent of their antitumoral effect.
Combination therapy of oncolytic adenoviruses with other drugs has already shown promising results. Administration of ONYX-015 together with cisplatin and 5-FU led to improved antitumor response in a phase II clinical trial.35 Doxorubicin and paclitaxel have also shown synergistic antitumor effects when combined with oncolytic adenoviruses in vivo,28,36 and autophagy-inducing agents such as temozolomide or RAD001 also enhance their therapeutic potential.27,37 The discovery that verapamil can be used to enhance the therapeutic activity of adenoviruses by an independent mechanism is particularly noteworthy, as it is safer than other chemotherapeutic agents used for this purpose. Verapamil has been used for decades in patients to treat arrhythmias and hypertension with minimal side effects. Other properties associated with verapamil make combination of this drug with oncolytic adenoviruses even more appealing. Verapamil is able to enhance the cytotoxic effect of certain chemotherapies38 and reverse multidrug resistance by competitively inhibiting drug transport through P-glycoprotein.39,40,41,42 The positive effect of verapamil on chemotherapy and on the intratumoral adenovirus spread, separately, could further improve the synergistic antiproliferative response observed when combining chemotherapy with oncolytic adenoviruses and makes a multimodal therapy including chemotherapy, an oncolytic adenovirus, and verapamil an interesting option. Additionally, several reports describe that the calcium blocking activity of verapamil also inhibits T-cell activation.43,44 This activity of verapamil may further enhance the oncolytic effect in the presence of an immune system, where the virus is more exposed to an antiviral immune response.
The exact mechanism of how verapamil promotes adenovirus release remains unknown. The low levels of PARP cleavage indicate that the enhanced release phenotype and spread observed were independent of apoptosis. Moreover, verapamil also enhances the release of an ADP-defective mutant (AdADP−), a mutant that is unable to promote cell lysis. Although this virus still expresses a smaller form of the protein that only partially retains its plaque-development function, the extent of the effect of verapamil on its release is very similar to that observed in Ad5, which suggests that mechanism induced by verapamil is independent of ADP expression. Therefore, the calcium channel blocker verapamil triggers a new, not previously described, pathway that promotes the release of adenovirus from the infected cell. Other calcium channel blocking agents (phenylalkylamines, dihydropyridines, and benzothiazepines) and calcium deprivation had a similar effect on adenovirus release, suggesting that the calcium channel blocking activity could be responsible for the enhanced release observed. Interestingly, Williams and collaborators have recently reported that the calcium blocking activity of verapamil induces autophagy in certain cell types,25 and autophagy has been proposed as a new pathway induced by adenovirus that leads to cell death45,46 and progeny release.26 We found that the ability of calcium channel blockers to enhance Ad5 release correlated with their ability to enhance autophagic vesicle formation or accumulation. However, other autophagy-inducing agents and other drugs that have demonstrated synergies with oncolytic adenoviruses do not reproduce the enhanced release observed with verapamil, which makes the effect of this drug unique. Because different autophagic pathways are currently being characterized, further studies are required to understand the role of autophagy in the process of virus release in verapamil-treated cells.
Our data demonstrate that inhibition of calcium influx can be used as a new rational approach to induce adenovirus release and improve the spread and antitumoral potency of oncolytic adenoviruses. Verapamil and other calcium channel blockers are able to enhance the release, spread, and cytotoxicity of Ad5 in vitro, and combination of verapamil with oncolytic adenovirus ICOVIR-5 in vivo has demonstrated enhanced antitumor activity and safety. The versatility, broad applicability, and good tolerability of verapamil make the combination of this drug and oncolytic adenoviruses very promising and, as such, they deserve further clinical validation.
Materials and Methods
Cell lines, virus, and reagents. Human HEK 293, A549, and SkMel-28 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). NP-9 and CAF1 (human carcinoma–associated fibroblasts) cell lines were established in our laboratory.13,47 All cell lines were routinely tested by mycoplasma presence and authenticated by morphology and growth curve analysis. To obtain >80% infection, A549, SkMel-28, NP-9, and CAF1 cells were infected with 25, 35, 30, and 45 TU/cell, respectively. For extracellular calcium deprivation, a calcium-free medium containing 200 mg/l MgSO4, 400 mg/l KCl, 6,400 mg/l NaCl, 3,700 mg/l NaHCO3, 141 mg/l NaH2PO42H20, 4,500 mg/l D-glucose (Sigma-Aldrich, St Louis, MO), MEM amino acid, MEM vitamin, MEM nonessential amino acids, and L-glutamine solutions (Invitrogen, Paisley, UK), and 5% FBS were prepared. This medium was supplemented with 1.8 mmol/l CaCl2 when used as a complete medium. Verapamil, amlodipine, and diltiazem were purchased from Abbott Laboratories (Abbott Park, IL), Laboratorios Almirall (Barcelona, Spain), and Pfizer (New York, NY), respectively. RAD001 (everolimus) was supplied by Novartis (Basel, Switzerland), and rapamycin, cisplatin, docetaxel (Taxotere), and temozolomide (Temodal) were purchased from Calbiochem (Darmstadt, Germany), Sigma-Aldrich, Aventis Pharma (Dagenham, UK), and Schering-Plough (Madrid, Spain), respectively.
Human Ad5 was obtained from ATCC. Adenovirus mutant dl732, which overexpresses ADP,48 and its wild-type counterpart, rec700, were kindly provided by WSM Wold (St Louis University, St Louis, MO). To construct an ADP-defective mutant (AdADP−), a T was introduced at position 7 of the ADP coding sequence in pAd5CAU13 to generate a STOP codon at position 3 of amino acid sequence. Resulting plasmid (pAdADP−) was PacI-digested and transfected into 293 cells. AdADP− virus expresses a truncated form of ADP from Met41 in the native protein sequence and lacks the entire luminal sequence of ADP (data not shown). ICOVIR-5 (Ad-DM-E2F-K-Δ24RGD) has been previously described.18 All the viruses were propagated in A549 cells, and TU were quantified using an antihexon staining–based method in 293 cells.49
Virus production and release kinetics. Preliminary experiments were carried out to evaluate the effect of increasing doses of calcium channel blockers on Ad5 release and cell viability. For each cell type, the concentration of verapamil that gave the lowest toxicity and highest viral release was chosen (40 µmol/l verapamil for A549, NP-9, and CAF1 cells; 25 µmol/l for SkMel-28 cells; 30 µmol/l amlodipine or 30 µmol/l diltiazem for A549 cells) to carry out the virus production and release kinetics experiments. Briefly, A549, SkMel-28, NP-9, and CAF1 cells were seeded in 24-well plates and infected to allow >80% infection. Two hours after infection, cells were washed twice and incubated with fresh medium or medium containing verapamil, other calcium channel blockers, or rapamycin. The virus released into the supernatant and the total virus produced (cell + media suspension) were collected in triplicate at different time points after infection and quantified using an antihexon staining–based method. The supernatant was centrifuged at 5,000 rpm before titration of the extracellular virus in order to eliminate detached cells and debris.
Plaque assay. A549 monolayers were seeded in six-well plates and infected with serial dilutions of Ad5, rec700, or dl732. Four hours after infection, the medium was removed and cells were washed twice with PBS. A 1:1 (DMEM 10% FBS: 1% agarose) solution was added to the cells and, once the agarose overlay had solidified, another layer was added of fresh DMEM 5% FBS alone or containing verapamil (30 µmol/l final concentration), other calcium channel blockers (amlodipine 30 µmol/l and diltiazem 30 µmol/l) or other drugs (5 µmol/l cisplatin, 200 pg/ml docetaxel, 10 nmol/l RAD001, and 10 µmol/l temozolomide; concentration that gave 10% growth inhibition). The plaque assay was stained at the day indicated by incubation with 0.5 mg/ml thiazolyl blue tetrazolium bromide during 3 hours at 37 °C and 5% CO2. Pictures of representative plaques were taken.
In vitro cytotoxicity assay. 2 × 104 (SkMel-28), 3 × 104 (A549), or 1 × 104 (NP-9) cells were seeded in 96-well plates in the absence or presence of 20 µmol/l (SkMel-28 cells) or 35 µmol/l (A549, NP-9) of verapamil and infected with serial dilutions starting at 150, 85, and 260 TU/cell, respectively. At day 6 postinfection, plates were processed as previously described.13 IC50 value was calculated from dose–response curves by standard nonlinear regression (GraFit; Erithacus Software, Horley, UK) using an adapted Hill equation.
Western blot analysis. A549 cells (1.5 × 106 cells/well of six-well plate) were infected with Ad5 to allow >80% infection. Two hours after infection, cells were washed twice and incubated in fresh media or media containing calcium channel blockers. At the indicated time points postinfection, cell extracts were obtained with Iso-Hi-pH buffer.13 Protein samples (20 µg/lane) were separated electrophoretically on SDS-PAGE and transferred to membranes. Blots were probed with primary antibody anti-Ad2 E1A (clone 13 S-5; Santa Cruz Biotechnology, Santa Cruz, CA), anti-E3/19K [Tw1.3 (ref. 50) kindly provided by Jonathan W Yewdell, NIAID/NIH, Bethesda, MD], anti-L4/100K (clone 7/199 kindly provided by WC Russell, St Andrews University, St Andrews, UK), antiadenovirus fiber A-4 mAb (clone 4D2; Fitzgerald Industries International, Concord, MA), antihuman PARP [poly-(ADP ribose) polymerase] (clone #551024; Becton Dickinson, Erembodegem, Belgium) or anti-LC3 (Novus Biologicals, Littleton, CO).
Measurement of autophagy. For measurement of autophagy, cells were seeded in six-well plates, infected with 25 TU/cell of Ad5, and incubated with fresh medium or medium containing verapamil (40 µmol/l), amlodipine (30 µmol/l), diltiazem (30 µmol/l), or increasing concentrations of rapamycin (positive control). Forty hours postinfection, protein cell extracts were collected and LC3-II/LC3-I ratio was calculated after densitometry of the corresponding bands detected by western blot with anti-LC3 antibody (Novus Biologicals).
In vivo toxicity assay in immune competent mice. All animal studies were carried out in the facility of IDIBELL (AAALAC Unit 1,155) after approval by IDIBELL's Ethical Committee of Animal Experimentation. Six-week-old immunocompetent Balb/C male mice were injected intravenously by the tail vein with PBS, 2.5 × 1010 vp, or 5 × 1010 vp of AdwtRGD, or 5 × 1010 vp or 1 × 1011 vp of ICOVIR-5 in a final volume of 200 µl (n = 10 animals per group). Starting at day 1 postinjection, 20 mg/kg of verapamil was daily injected intraperitoneally into five animals from each experimental group. Animals were monitored for signs of morbidity and body weight recorded. At day 5 after virus administration, animals were killed and blood was drawn by intracardiac puncture. Complete clinical biochemistry and hematology assessment were performed at the Clinical Biochemistry or Hematology Service of the Veterinary Faculty at the Autonomous University of Barcelona. Mice livers were collected and fixed in 4% formaldehyde (for paraffin embedding and hematoxylin/eosin staining) or frozen in OCT for anti-E1A immunofluorescence staining, as previously described.49
In vivo antitumoral efficacy studies. Subcutaneous A549 or SkMel-28 tumor xenografts were established by injection of 1 × 107 cells into the flanks of 6-week-old Balb/C nu/nu mice. Once the mean tumor volume reached 100 mm3, mice were tail vein-injected with PBS or 5 × 1010 vp of ICOVIR-5 (n = 24) in a final volume of 200 µl. Starting at day 1 after virus administration, half of the mice from each group (n = 12) were injected with 20 mg/kg of verapamil intraperitoneally daily until the end of the experiment. Tumor size and mouse body weight were recorded at 3- to 4-day intervals. Tumor volume and tumor growth were calculated as previously described.18 Tumor samples were OCT-included to assess virus replication by antiadenovirus immunostaining of A549 tumor xenografts at day 13 postinjection, as previously described.13
Statistical analysis. Data from in vitro studies were tested for significance by means of the Student's t-test. The t-test was also used for comparing the toxicity and tumor progression in mice in the different treatment groups. All P values are two-tailed. P value <0.05 was considered to be statistically significant in Student's t-test.
Acknowledgments
We thank Eduard Serra and Blanca Luena for their technical assistance, and Lynda Coughlan for extensive revision of this manuscript. We also thank Jordi Martínez-Quintanilla, Francisca Alcaya, Raúl Gil, Miguel Camacho, Marta Giménez, and Eduardo Laborda. We also acknowledge WC Russell (St Andrews University, St Andrews, UK), WSM Wold (St Louis University), and Jonathan W Yewdell (Laboratory of Viral Diseases, NIAID, NIH, Bethesda, MD) for providing reagents. This work was supported by the Mutua Madrileña Medical Research Foundation (Spain), an Instituto de Salud Carlos III (Spanish Ministry of Health) grant (PI08/1661), a “Ministerio de Educación y Ciencia” grant (BIO2008-04692-C03-01), and by a European Council 6th Framework Research contract (18700; Theradpox). RA belongs to the Network of Cooperative Research on Cancer (C03-10), Instituto de Salud Carlos III of the Ministerio de Sanidad y Consumo, Government of Spain.
REFERENCES
- Liu TC, Galanis E., and , Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Alemany R, Suzuki K., and , Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81 Pt 11:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wein LM, Wu JT., and , Kirn DH. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 2003;63:1317–1324. [PubMed] [Google Scholar]
- Kim JH, Lee YS, Kim H, Huang JH, Yoon AR., and , Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- Guedan S, Gros A, Cascallo M, Vile R, Mercade E., and , Alemany R. Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication. Gene Ther. 2008;15:1240–1245. doi: 10.1038/gt.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Hallden G, Wang Y, Brooks G, Francis J, Lemoine N, et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther. 2004;9:786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Toth K, Djeha H, Ying B, Tollefson AE, Kuppuswamy M, Doronin K, et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated wnt signaling. Cancer Res. 2004;64:3638–3644. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- Yan W, Kitzes G, Dormishian F, Hawkins L, Sampson-Johannes A, Watanabe J, et al. Developing novel oncolytic adenoviruses through bioselection. J Virol. 2003;77:2640–2650. doi: 10.1128/JVI.77.4.2640-2650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S., and , Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80:2000–2012. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S, Logan J., and , Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984;52:664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- Gros A, Martínez-Quintanilla J, Puig C, Guedan S, Molleví DG, Alemany R, et al. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res. 2008;68:8928–8937. doi: 10.1158/0008-5472.CAN-08-1145. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ., and , Wold WS. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE., and , Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- Carrasco L. Modification of membrane permeability by animal viruses. Adv Virus Res. 1995;45:61–112. doi: 10.1016/S0065-3527(08)60058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MC, Cohen J., and , Michelangeli F. Role of Ca2+in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium. 2000;28:137–149. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- Cascallo M, Alonso MM, Rojas JJ, Perez-Gimenez A, Fueyo J., and , Alemany R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Cascallo M, Gomez-Manzano C, Jiang H, Bekele BN, Perez-Gimenez A, et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- Chami M, Oulès B., and , Paterlini-Bréchot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Ying B., and , Wold WS. Mutations within the ADP (E3-11.6K) protein alter processing and localization of ADP and the kinetics of cell lysis of adenovirus-infected cells. J Virol. 2003;77:7764–7778. doi: 10.1128/JVI.77.14.7764-7778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deedwania PC. Calcium channel blockers. West J Med. 1982;137:24–31. [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- White E, Grodzicker T., and , Stillman BW. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984;52:410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, White EJ, Gomez-Manzano C., and , Fueyo J. Adenovirus's last trick: you say lysis, we say autophagy. Autophagy. 2008;4:118–120. doi: 10.4161/auto.5260. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Iwado E, Kondo Y, Aoki H, Hayashi Y, Georgescu MM, et al. Autophagy-inducing agents augment the antitumor effect of telerase-selve oncolytic adenovirus OBP-405 on glioblastoma cells. Gene Ther. 2008;15:1233–1239. doi: 10.1038/gt.2008.98. [DOI] [PubMed] [Google Scholar]
- Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H, et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Nakano G, Nagashima K, Sakamoto K, Harasawa N, Kitamura T, et al. Verapamil enhancement of antitumor effect of cis-diamminedichloroplatinum(II) in nude mouse-grown human neuroblastoma. Cancer Res. 1987;47:231–234. [PubMed] [Google Scholar]
- van Beusechem VW, van den Doel PB, Grill J, Pinedo HM., and , Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE., and , Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Droguett G, Chowdhury NR, Li Y, Sengupta K, Thummala NR, et al. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS., and , Toth K. Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Ther. 2005;12:1608–1617. doi: 10.1038/sj.gt.3302581. [DOI] [PubMed] [Google Scholar]
- Lamont JP, Nemunaitis J, Kuhn JA, Landers SA., and , McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7:588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- Li Y, Yu DC, Chen Y, Amin P, Zhang H, Nguyen N, et al. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61:6428–6436. [PubMed] [Google Scholar]
- Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF, et al. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16:487–493. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- Mason RP. Calcium channel blockers, apoptosis and cancer: is there a biologic relationship. J Am Coll Cardiol. 1999;34:1857–1866. doi: 10.1016/s0735-1097(99)00447-7. [DOI] [PubMed] [Google Scholar]
- Yusa K., and , Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989;49:5002–5006. [PubMed] [Google Scholar]
- Millward MJ, Cantwell BM, Munro NC, Robinson A, Corris PA., and , Harris AL. Oral verapamil with chemotherapy for advanced non-small cell lung cancer: a randomised study. Br J Cancer. 1993;67:1031–1035. doi: 10.1038/bjc.1993.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvelo F, Poupon MF, Bichat F, Grossin F, Bourgeois Y, Jacrot M, et al. Adding a reverser (verapamil) to combined chemotherapy overrides resistance in small cell lung cancer xenografts. Eur J Cancer. 1995;31A:1862–1868. doi: 10.1016/0959-8049(95)00386-w. [DOI] [PubMed] [Google Scholar]
- Belpomme D, Gauthier S, Pujade-Lauraine E, Facchini T, Goudier MJ, Krakowski I, et al. Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann Oncol. 2000;11:1471–1476. doi: 10.1023/a:1026556119020. [DOI] [PubMed] [Google Scholar]
- Birx DL, Berger M., and , Fleisher TA. The interference of T cell activation by calcium channel blocking agents. J Immunol. 1984;133:2904–2909. [PubMed] [Google Scholar]
- Blaheta RA, Hailer NP, Brude N, Wittig B, Leckel K, Oppermann E, et al. In vitro analysis of verapamil-induced immunosuppression: potent inhibition of T cell motility and lymphocytic transmigration through allogeneic endothelial cells. Transplantation. 2000;69:588–597. doi: 10.1097/00007890-200002270-00021. [DOI] [PubMed] [Google Scholar]
- Ito H, Aoki H, Kühnel F, Kondo Y, Kubicka S, Wirth T, et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst. 2006;98:625–636. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- Villanueva A, García C, Paules AB, Vicente M, Megías M, Reyes G, et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- Scaria A., and , Wold WS. Fine-mapping of sequences that suppress splicing in the E3 complex transcription unit of adenovirus. Virology. 1994;205:406–416. doi: 10.1006/viro.1994.1661. [DOI] [PubMed] [Google Scholar]
- Majem M, Cascallo M, Bayo-Puxan N, Mesia R, Germa JR., and , Alemany R. Control of E1A under an E2F-1 promoter insulated with the myotonic dystrophy locus insulator reduces the toxicity of oncolytic adenovirus Ad-Delta24RGD. Cancer Gene Ther. 2006;13:696–705. doi: 10.1038/sj.cgt.7700940. [DOI] [PubMed] [Google Scholar]
- Cox JH, Bennink JR., and , Yewdell JW. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991;174:1629–1637. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]