Abstract
Sanfilippo syndrome type B (MPS IIIB) is a lysosomal storage disease resulting from a deficiency of N-acetyl-glucosaminidase (NAGLU) activity. In an attempt to correct the disease in the murine model of MPS IIIB, neonatal mice were treated with intracranial AAV2/5-NAGLU (AAV), syngeneic bone marrow transplant (BMT), or both (AAV/BMT). All treatments resulted in some improvement in clinical phenotype. Adeno-associated viral (AAV) treatment resulted in improvements in lifespan, motor function, hearing, time to activity onset, and daytime activity level, but no reduction of lysosomal storage. BMT resulted in improved hearing by 9 months, and improved circadian measures, but had no effect on lifespan, motor function, or central nervous system (CNS) lysosomal storage. AAV/BMT treatment resulted in improvements in hearing, time to activity onset, motor function, and reduced CNS lysosomal storage, but had no effect on lifespan. Combination therapy compared to either therapy alone resulted in synergistic effects on hearing and CNS lysosomal inclusions but antagonistic effects on motor function and lifespan. AAV alone is more efficacious than BMT or AAV/BMT treatment for lifespan. BMT was the least efficacious treatment by all measures. CNS-directed AAV treatment alone appears to be the preferred treatment, combining the most efficacy with the least toxicity of the approaches assessed.
Introduction
Mucopolysaccharidosis IIIB is a lysosomal storage disease resulting from a deficiency in N-acetyl-glucosaminidase activity. Typically, the disease manifests clinically around 5 years of age and results in progressive deterioration of motor function and intellectual ability, with eventual early death by ages 15–20. Subsequent to the identification of the gene responsible for MPS IIIB,1,2 a mouse model was created.3 Previously, we quantified the abnormalities in circadian activity, vision, hearing, and motor function, and showed that the MPS IIIB mouse mimicked the human disease phenotype. This functional analysis also facilitated the evaluation of various therapeutic approaches.4 The disease pathology is multifaceted with some aspects seemingly related to inflammation, which may, in part, be mediated by accumulation of heparan sulfate,5 and direct effects of lysosomal inclusion formation, which appears to affect autophagy,6 as well as secondary elevations of alternative enzymes, which may induce localized tau formation.7 Previous studies have demonstrated an increase in immune activation in the brain of MPS IIIB mice,8,9 which may be a target for therapy. Bone marrow transplant (BMT) with N-acetyl-glucosaminidase (NAGLU)-transduced cells reduced the vacuolization of neurons, liver and spleen cells, and resulted in reduction of activated astrocytes in the brain of treated mice.10 Intracranial human umbilical cord blood transplant resulted in reduced cell loss and reduced glycosaminoglycan accumulation in the brain and liver.11 Umbilical cord blood transplant of pregnant female mice stimulated stem cell migration into the developing fetus and resulted in increased NAGLU activity in the offspring.12 Interestingly, treatment with the immunomodulating agent prednisolone has thus far demonstrated the most significant prolongation of lifespan in this model.8
Central nervous system (CNS)–directed gene therapy with adeno-associated viral (AAV) vectors has resulted in sustained increased NAGLU activity, reduced glycosaminoglycan accumulation,13 improvement in the activity level,14 and increased lifespan15 of MPS IIIB mice. Intravenous delivery of a lentiviral vector increased NAGLU activity that persisted for at least 6 months and reduced intracytoplasmic inclusions in the liver, spleen, and lung.16 Combining intracisternal with intravenous AAV-NAGLU demonstrated both improvement in storage and increased lifespan in a select subset of treated mice.15
Unfortunately, none of these approaches has attained normal enzyme levels, eradicated intracytoplasmic inclusions, or corrected the clinical signs of MPS IIIB. This may not be surprising because a myriad of cell types are affected resulting in numerous clinical signs. In fact, it has been shown previously in another mouse lysosomal storage disease model of (globoid-cell leukodystrophy) that simply supplying supraphysiologic levels of enzyme (up to 25-fold greater than normal) only modestly improved the clinical outcome.17,18 However, combining CNS-directed, AAV-mediated gene therapy with BMT resulted in dramatic synergy and significant improvement in several physiologic parameters.19
Systemic therapy with periodic intravenous enzyme replacement has been evaluated previously in other lysosomal disease models and has proven to be successful for the visceral manifestations; however, the neurologic manifestations were not corrected. In MPS VII, initial studies with bolus enzyme replacement performed in adult animals demonstrated no correction of disease in the brain, whereas more continuous therapy resulted in some reduction in brain lysosomal distention.20 The reduction in storage in the MPS VII model only occurred if the therapy with native enzyme was initiated in the neonatal period20,21 or the replacement was with sustained weekly administration of very high enzyme amounts.22 Interestingly, systemic gene therapy resulting in sustained low levels of circulating enzyme provided some reduction of brain lysosomal storage.19,23,24 Alternatively, chemical modification of β-glucuronidase to increase circulating half-life also results in decreased brain storage.25 These studies indicate that attaining continuous rather than episodic systemic enzyme levels may allow some benefit in the brain.
Several groups have shown that combining disparate therapeutic approaches results in a more complete clinical and histopathologic correction.19,26,27,28 We hypothesized that combining CNS-directed gene therapy with BMT in the neonatal period would result in increased enzyme activity levels and reduced inflammation in the brain, and result in correction of the disease. Here, we describe the results of combining intracranial gene therapy and BMT compared with either approach alone.
Results
NAGLU activity
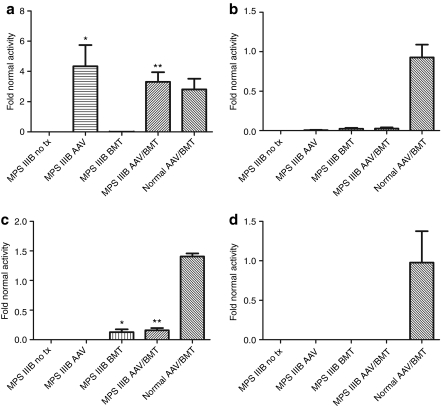
Mice from each group were killed at ~1 year of age for biochemical and histological analyses. Blood from BMT-treated animals was analyzed by FACS to assess the donor chimerism from a syngeneic GFP+ donor. The mean engraftment in both transplanted groups (BMT, AAV/BMT) was found to be 24.7% (data not shown). BMT treatment alone resulted in NAGLU activity levels relative to normals of 0.93% in brain (Figure 1a), 2.3–2.6% in liver (Figure 1b), 13–16% in spleen (Figure 1c), and 0% in heart (Figure 1d). Intracranial AAV-treated animals had high NAGLU activity in the brain, ranging from 2.8- to 4.3-fold greater than normal (Figure 1a), no activity in spleen or heart, and very low activity in the liver (0.6% of normal). Combined treatment animals had systemic activity levels similar to BMT alone and brain activity similar to AAV-treated MPS IIIB animals. The normal animals treated with AAV/BMT demonstrated a significant increase in brain and spleen activity, but no difference in heart or liver NAGLU activity. This is consistent with supranormal enzyme levels produced by the AAV vector in the brain and the contribution of normal donor marrow activity in the spleen.
Figure 1.
Tissue NAGLU enzyme activity in treated and untreated mice. (a) Brain, (b) liver, (c) spleen, and (d) heart from 4 to 6 animals in each group were homogenized and N-acetyl-glucosaminidase activity was determined. Activity values ± SEM for each MPS IIIB group are graphed relative to the mean of the normal untreated group. Significant differences of treated from untreated MPS IIIB groups are indicated with *P < 0.05 and **P < 0.01. AAV, adeno-associated virus; BMT, bone marrow transplant; NAGLU, N-acetyl-glucosaminidase.
Secondary elevations
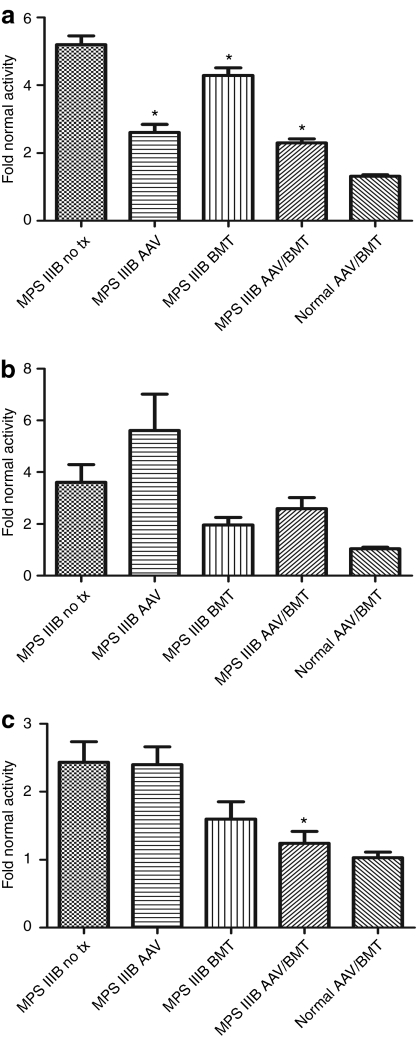
Other lysosomal enzymes, such as β-glucuronidase, are secondarily elevated in MPS IIIB mouse tissues.3 A reduction in secondary elevations has been used as a surrogate for therapeutic response.29,30 β-glucuronidase activities were measured in brain, liver, and spleen for all groups, and specific activities were normalized to the untreated normal group. Not surprisingly, there is no difference between the untreated normal and normal-AAV/BMT groups in any organ measured. Conversely, there was a significant reduction in brain β-glucuronidase activity in all MPS IIIB treatment groups compared to the untreated group (Figure 2a). Untreated MPS IIIB brains had 5.2-fold greater than normal activity. BMT alone reduced this to 4.3-fold (P < 0.05) despite a very low level of NAGLU activity. Despite supranormal NAGLU activity, AAV and AAV/BMT reduced brain β-glucuronidase activity to 2.6- and 2.3-fold normal (each P < 0.001). The liver had no significant reduction in β-glucuronidase activity with any treatment (Figure 2b). In contrast, the spleen revealed a trend toward improvement with BMT alone at 1.6-fold normal β-glucuronidase activity and a significant improvement with AAV/BMT to 1.24-fold normal (P < 0.01) compared to the 2.43-fold activity in the untreated group (Figure 2c).
Figure 2.
Tissue β-glucuronidase activity in treated and untreated mice. (a) Brain, (b) liver, and (c) spleen from 4 to 6 animals in each group were homogenized and β-glucuronidase activity was determined. Activity values ± SEM for each MPS IIIB group are graphed relative to the mean of the normal untreated group. Significant differences of treated from untreated MPS IIIB groups are indicated with *P < 0.05. AAV, adeno-associated virus; BMT, bone marrow transplant.
Histology
Comparison of lysosomal distention in liver, spleen, brain, and kidney revealed less storage in liver and spleen for the MPS IIIB-BMT and MPS IIIB-AAV/BMT animals compared to untreated MPS IIIB mice. The MPS IIIB-AAV animals had no reduction in lysosomal distention in liver or spleen. None of the treated MPS IIIB animals had a reduction in storage in the kidney.
Quantitative comparison in the brain of the number of lysosomal inclusions per parietal cortical neuron confirmed that only the MPS IIIB-AAV/BMT group had a modest but significant reduction (P = 0.0014) in total storage from 480 inclusions/100 cells to 352 inclusions/100 cells.
Circadian activity
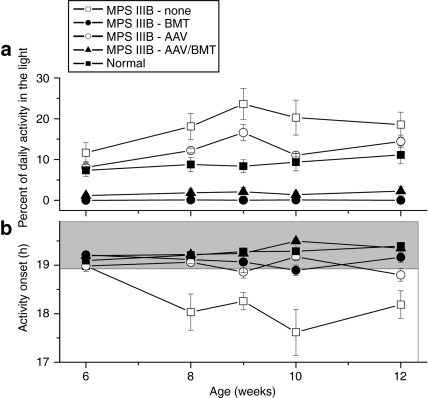
Long-term running wheel recordings from MPS IIIB and wild-type mice from 40 to 84 days of age showed that they both entrain to light–dark cycles and free run in constant darkness. Consistent with our previous observations,4 MPS IIIB mice started their daily activity ~1 hour earlier than wild-type mice (P = 0.003, t = 3.4). The MPS IIIB mice also ran more in the light phase of a 12-hour:12-hour light–dark cycle (Figure 3a; P = 0.05, t = −2.09). Time of activity onset for MPS IIIB-AAV, MPS IIIB-BMT, or MPS IIIB-AAV/BMT animals was not significantly different than untreated normal controls (all P > 0.40). In contrast, all of the treatment groups were significantly different from the untreated MPS IIIB mice (P = 0.04, t = 2.2 for AAV treated; P = 0.02, t = 2.6 for BMT; and P = 0.003, t = 3.4 for AAV/BMT) (Figure 3b). The percentage of daily activity that occurred during the light portion of the light–dark cycle was significantly reduced in the MPS IIIB mice by BMT (P = 0.002, t = −3.6), or AAV and BMT (P = 0.003, t = −3.5), but not by AAV alone (P = 0.3, t = −1.2) (Figure 3b). MPS IIIB mice did not differ significantly from wild-type mice in period, cycle-to-cycle period variation, rhythm amplitude, total daily activity, or duration of daily activity.
Figure 3.
Circadian assessment of treated and untreated mice. Male mice from each group (n = 5 except for n = 3 for MPS IIIB-BMT) were individually housed in cages, and wheel running activity was recorded from ages 8 to 12 weeks. (a) Proportion of activity occurring while lights were on, and (b) mean time from lights off to activity onset are plotted ± SEM. AAV, adeno-associated virus; BMT, bone marrow transplant.
Interestingly, the normal and MPS IIIB animals treated with conditioning radiation and BMT had a near complete cessation of light time activity, indicating the radiation may have damaged the brain or retina resulting in increased circadian drive on activity.
Auditory function and histology
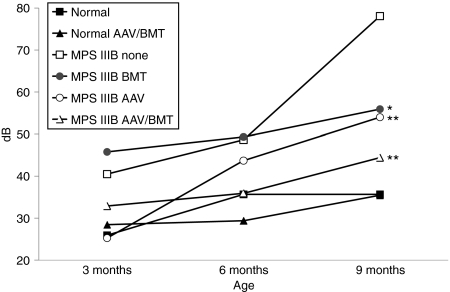
We previously described the diminished auditory function at 5–40 kHz beginning at 4–5 months of age for MPS IIIB mice compared to normals.4 Untreated MPS IIIB mice had significantly diminished auditory-evoked brainstem response (ABR) thresholds compared to normal at all frequencies. Globally, all MPS IIIB treatment groups had a significant improvement (P < 0.001) in hearing when compared by group, as represented by the data at 10 kHz (Figure 4). When compared at each frequency to untreated MPS IIIB animals, the AAV/BMT group had significant improvements at all frequencies tested. The MPS IIIB-AAV group had significant improvements in hearing at 10, 28.3, and 40 kHz. The MPS IIIB-BMT group had significant improvements only at 9 months of age. The improvement of the combined group would indicate some synergy between AAV and BMT on auditory function. The normal AAV/BMT group was not significantly different than untreated normals at all frequencies indicating no adverse effect of the treatments on hearing.
Figure 4.
Hearing sensitivity in decibels of treated and untreated mice at 10 kHz. Auditory-evoked brainstem responses (ABR) recording was performed at 5, 10, 20, 28.3, and 40 kHz on 13–17 animals per group at 3, 6, and 9 months of age. The 10 kHz data are representative of the trends observed at each of the frequencies tested. Each group's mean decibel threshold to detect the audible stimuli is shown at 3, 6, and 9 months of age. Significant differences of treated from untreated MPS IIIB groups are indicated with *P < 0.05 and **P < 0.01. AAV, adeno-associated virus; BMT, bone marrow transplant.
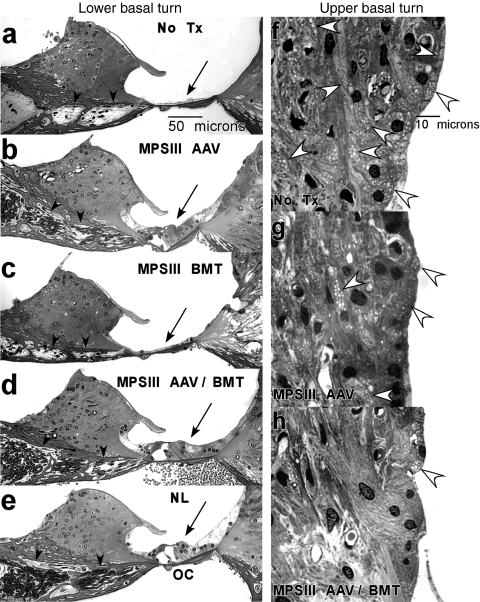
To determine why treated MPS IIIB mice showed more rapid deterioration of hearing with age than WT or Het mice, representative cochleas were examined from each group at 9 months of age. Examination of middle ears at dissection (data not shown) revealed a thickened mucosal lining and thick exudate within the middle ear cavity of all treated and untreated MPS IIIB mice. Middle ear cavities of WT and Het mice appeared normal. Within the inner ear, untreated MPS IIIB mice showed lysosomal storage in a wide range of cochlear cell types, including glial cells of the spiral ganglion, pillar cells of the organ of Corti, and fibrocytes of the spiral limbus and spiral ligament (Figure 5f). We previously noted potentially unique (among MPS forms) degenerative changes in the cochlea of MPS IIIB mice whereby loss of hair cells and afferent neurons are greatly accelerated.4 This was also observed in untreated MPS IIIB mice in the present study (compare Figure 5a,e). Both AAV treatment and AAV/BMT combined appeared to attenuate this sensory cell loss (Figure 5b,d). There was some suggestion that AAV treatment alone may be more effective in preserving hair cells and neurons than is BMT alone (compare Figure 5b,c), so that AAV treatment may account for cell protection provided by combined AAV/BMT.
Figure 5.
Light photomicrographs of cochlea in representative mice from each treatment group. Within the lower base: (a) untreated MPS IIIB mouse (no Tx) shows loss of hair cell receptors (along with the entire organ of Corti, arrow), and loss of afferent neuronal processes (arrowheads). (b–d) AAV alone and AAV/BMT appear more protective of receptor cells and neurons than BMT alone. (e) Untreated normal (NL) mouse. Within the upper base: (f) untreated MPS IIIB mouse shows abundant storage in fibrocytes of the spiral ligament (white arrowheads). Storage is modestly reduced in mouse receiving (g) AAV alone and more apparently reduced by (h) AAV/BMT in combination. AAV, adeno-associated virus; BMT, bone marrow transplant; OC, organ of Corti.
Our sample further suggested that superior ABR threshold preservation in MPS IIIB mice receiving combined AAV/BMT (Figure 4) may have its basis in the cochlear lateral wall. Figure 5 shows reduced lysosomal storage within the spiral ligament (indicated by arrows) of a mouse receiving both AAV and BMT, compared to AAV alone (compare Figure 5g,h).
In summary, progressive threshold elevation in treated MPS IIIB mice may primarily reflect middle ear lesions that are delayed, but not eliminated. Based on our threshold data, AAV and AAV/BMT may be more effective than BMT alone in slowing conductive hearing loss. We speculate that the benefits of combined AAV/BMT therapy derive from (i) preservation of sensory cells by AAV, and (ii) enhanced control of lysosomal storage in connective tissue cells by AAV and BMT together. Because cochlear extracellular matrix plays an important role in fluid homeostasis,31 improved retention of sensory cells and improved function of regulatory cells would be expected to yield superior results versus either benefit alone.
Motor function
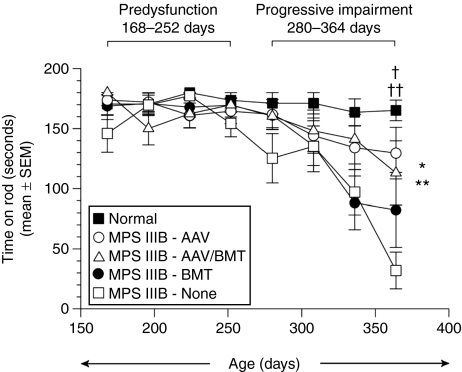
All treatment and control groups were evaluated on the rocking (reversing) rotarod test every 4 weeks beginning at ~168 days of age (Figure 6). In a previous study, we showed that MPS IIIB mice gradually developed deficits on the rocking rotarod as a function of age, with performance decrements appearing after 250–300 days of age. There is no difference in performance from wild type using an accelerating paradigm indicating there is little or no contribution of the bone and joint disease to impaired rotarod function.4 In the present study, we therefore separated the rocking rotarod data into two age intervals for analysis to maximize test sensitivity. The first age interval (168–252 days) represents a predysfunction period. The second interval (280–364 days) represents a period of progressive impairment. As expected, the results of an analysis of variance and subsequent pairwise comparisons of the data from the predysfunction period did not reveal significant impairment for any group compared to normal controls. In contrast, statistical analysis of the groups during the second age interval indicated group performance differences were age dependent Figure 6. Pairwise comparisons showed that the untreated MPS IIIB mice had a shorter latency to fall off of the rod than the normal control mice (P = 0.004). Compared to the untreated MPS IIIB animals, the MPS IIIB-AAV group had a large improvement in fall latency over the 280–364 days of age interval that reached statistical significance (P = 0.040). The MPS IIIB-AAV/BMT group had a similar improvement in mean performance as the MPS IIIB-AAV group. However, due to intragroup variability in the MPS IIIB-AAV/BMT group, statistical significance was not reached. The MPS IIIB-BMT group had no improvement in rotarod performance. The normal and normal-AAV/BMT groups showed no difference in ability to stay on the rocking rotarod over the entire duration of testing and both remained significantly longer than any MPS IIIB group.
Figure 6.
Rocking rotarod performance as a function of age in each treatment group. Mean duration of the time spent on a rocking (reversing) rotarod as a function of increasing age for all treated and normal control groups is depicted. No performance deficits were evident in the untreated MPS IIIB mice (or in the other treated groups) compared to normal controls during the predysfunction period (168, 196, 224, and 252 days). However, a significant main effect of group (*P = 0.048) and a significant group by age interaction (**P = 0.008) suggest that age-dependent group performance differences occurred during the progressive impairment period (280, 308, 336, and 364 days). Pairwise comparisons showed that on average across the ages at testing, the MPS IIIB mice were significantly impaired relative to normal control mice (P = 0.004) and that MPS IIB-AAV mice showed improved performance compared to MPS IIB mice (P = 0.040). Subsequent one-way analysis of variances conducted at each age showed that these effects were mostly due to differences observed at 364 days (P = 0.005). At this age, the untreated MPS IIIB mice showed performance deficits compared to the normal controls (†P = 0.0006), and MPS IIIB-AAV mice exhibited significantly improved performance levels relative to the untreated MPS IIIB mice (††P = 0.010). AAV, adeno-associated virus; BMT, bone marrow transplant.
Cerebellar Purkinje cell counts were determined as previously described.4 The results confirmed a diminished total Purkinje cell count in the MPS IIIB untreated group compared to normals. In contrast to the clinical improvement in performance on the rotarod for the AAV-treated groups, there was no significant improvement in cerebellar Purkinje cell counts in any treatment group compared to the MPS IIIB animals (data not shown). There was no difference in counts between the untreated normal and the normal-AAV/BMT groups.
Lifespan
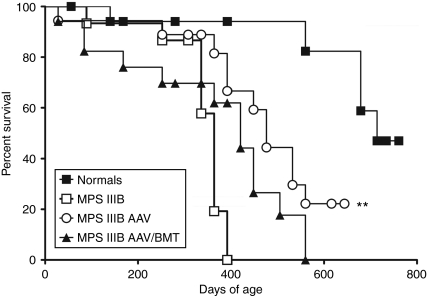
MPS IIIB mice have a significantly shortened lifespan compared to normal mice.3,4,15 The Kaplan–Meier survival analysis was carried out for all groups until median survival had been reached (Figure 7). Similar to the effects on motor function, the MPS IIIB-AAV group had significantly prolonged survival by ~112 days (median = 476 days). The MPS IIIB-AAV/BMT group had early mortalities mixed with some long-lived mice but had no significant increase in survival (median = 420 days) compared to untreated MPS IIIB mice. Untreated MPS IIIB and MPS IIIB-BMT animals had identical median survivals (364 days). In contrast to the other parameters described above, BMT appears to be deleterious to survival in the MPS IIIB mice when combined with AAV. There was no significant decrease in the normals treated with AAV/BMT (median survival = 736 days) compared to untreated normals (median survival = 714 days).
Figure 7.
Survival curves by Kaplan–Meier (KM) analysis. All treated mice were analyzed for median survival by KM analysis and plots are shown. Normal-AAV/BMT did not differ from untreated normals and MPS IIIB-BMT did not differ from MPS IIIB, so these two groups are not shown for clarity. The MPS IIIB-AAV group had a significantly prolonged median survival compared to MPS IIIB (**P = 0.0054). AAV, adeno-associated virus; BMT, bone marrow transplant.
Discussion
Two important measures of success for any therapy are prolongation of life and improvement in physical ability. We have demonstrated improvements in median lifespan, motor function, hearing, and correction of altered circadian function by neonatal treatment with intracranial AAV2/5-hNAGLU. This treatment results in persistent, supraphysiologic expression of NAGLU activity in the brain and corresponding reduction in secondary elevation of β-glucuronidase activity. This is consistent with previously reported studies using rAAV vectors in the MPS IIIB mouse.13,14,15
We hypothesized that BMT performed in the neonatal period would be more effective at preventing the disease rather than reversing existing disease if BMT were performed later in life. We have demonstrated that BMT alone results in a very minor improvement in auditory function, improvements in visceral histology, increased systemic NAGLU activity, and a reduction in secondary elevation of β-glucuronidase activity. The persistent activity associated with BMT did not, however, result in significant brain NAGLU activity or any improvement in CNS pathology, despite a significant reduction in secondary elevation of β-glucuronidase activity. In addition, BMT did not have any effect on lifespan or motor function. This is consistent with the observation of Zheng et al.,10 who demonstrated minimal effect of nontransduced BMT on the CNS. They conjectured that the level of circulating NAGLU may not be adequate to allow CNS effects. This could contribute to the disappointing findings in this study as well. We were able to achieve only 25% donor chimerism due to the sublethal conditioning used. Unfortunately, higher conditioning doses in newborn animals result in CNS and skeletal toxicity.
Interestingly, BMT does synergize with AAV treatment with respect to hearing function and causes reduced accumulation of CNS lysosomal inclusions. However, this combination also resulted in a decrease in median lifespan, relative to AAV alone, due to an increase in early mortality and no improvement in motor function. We speculate that the radiation conditioning may possibly stimulate immune sensitivity in the already immune-activated MPS IIIB animals, particularly in neonatal animals that are more susceptible to radiation.32 Thus, it appears that although CNS-directed AAV treatment is beneficial but not sufficient to completely correct the disease, BMT with radiation conditioning is not the preferred supplemental treatment. It is possible that BMT with cells expressing very high levels of NAGLU, which has been shown to improve CNS histology,10 may provide sufficient benefit to warrant combining this with CNS-directed gene therapy. Alternatively, higher levels of engraftment may also increase efficacy when combined with AAV. Interestingly, despite high levels of CNS NAGLU activity and obvious benefit of the therapy on lifespan and physical performance, we observed little or no improvement in histology. CNS-directed AAV-mediated therapy in this model has previously shown histologic correction in areas close to the injection sites with very little improvement at distant sites.13,14,15 The discordance between phenotypic and histologic improvement would argue that the pathologic effect of the enzyme deficiency is not primarily due to the lysosomal distention but secondary effects of either heparan sulfate accumulation, lack of recycling of HS components for cell use, or incitement of an inflammatory response.
Others have shown both an increase in inflammatory mediators and astrocyte and glial activation in the CNS of the MPS IIIB mouse.9,33 DiRosario et al.8 also demonstrated that MPS IIIB mice have activation of T cells and increases in inflammatory mediators as would be expected with increased inflammation. Additionally, they showed that MPS IIIB mice produce unidentified, CNS-specific autoantibodies. Supporting the significant role of inflammation on the disease, they found that prolonged treatment with prednisolone alone improved lifespan and physical performance while having no effect on lysosomal storage. Immunomodulation with BMT did not have a similar effect, but BMT would not necessarily be expected to suppress immune activation. Thus, it seems complete correction of disease will require either early treatment to prevent initiation of the inflammatory process or substantial suppression of the process once it is started. We intend to assess the effect of combining gene therapy with immunomodulatory agents in subsequent studies.
In conclusion, we have demonstrated that CNS-directed AAV-NAGLU therapy initiated during the newborn period provides substantial therapeutic benefit to the MPS IIIB mouse and that the addition of nontransduced BMT does not augment this benefit. Further correction of the disease will require additional interventions, but CNS-directed therapy may be a reasonable starting point for human interventions.
Materials and Methods
Viral construct. The AAV vector was constructed by inserting the huNAGLU cDNA (kind gift of Elizabeth Neufeld) into an AAV2 vector containing the CMV enhancer, chicken β-actin promoter, SV40 poly-A, and the 3′ untranslated region from the rabbit β-globin gene.34 The vector was pseudotyped with the AAV5 capsid proteins and produced at the University of Florida Vector Core. The virus was diluted to 1.5 × 1012 viral particles/ml with lactated Ringer's and aliquoted prior to freezing at −85 °C.
Mice. The congenic C57BL/6 NAGLU-deficient mouse strain was obtained from The Jackson Laboratory, Bar Harbor, ME3 and maintained by strict sibling mating at Washington University by M.S.S. Genotypes were determined by PCR of NAGLU exon 6 and the neomycin insertion cassette, or by enzyme assay35 on tissue samples from newborn mice.
The CAG-GFP hematopoietic stem cell donor mouse strain was obtained from The Jackson Laboratory, and GFP+ animals were identified by UV excitation and emission. All animal procedures were in accordance with guidelines established by the Institutional Animal Care and Use Committee at Washington University in St Louis.
Treatments. All treatments were performed at 2–4 days of age. Affected animal groups were either untreated (MPS IIIB-no tx, n = 15), treated with intracranial AAV-NAGLU (MPS IIIB-AAV, n = 18), BMT alone (MPS IIIB-BMT, n = 18), or both intracranial AAV-NAGLU and BMT (MPS IIIB-AAV/BMT, n = 17). Untreated normal control group [normal-no tx, n = 18 (8 heterozygous, 10 homozygous normal)] and combination intracranial AAV-NAGLU and BMT treated normals (normal-AAV/BMT, n = 14, 12 heterozygous, 2 homozygous normal) were identified at birth and treated on days 2–4. We showed previously that there are no significant differences between heterozygous and homozygous normal animals.
Intracranial AAV-NAGLU was administered by six direct injections per mouse of 2 µl each into the bilateral frontal (from bregma: 2 mm lateral and 1 mm posterior, 1.5 mm deep), temporal (from bregma: 3 mm lateral and 3 mm posterior, 1.5 mm deep) and cerebellar (from lambda: 1 mm lateral and posterior, 2.5 mm deep) regions using a 32-gauge needle. BMTs were performed by injection of ~1 × 106 unfractionated GFP+ bone marrow cells in 100 µl of normal saline via the superficial temporal vein36 after conditioning with 200 rad of gamma irradiation from a 137Cs source.
Daily activity and response to light. These experiments were performed essentially as described previously.4 Briefly, male mice (n = 5 in all groups but MPS IIIB/BMT with n = 3) from 8 to 12 weeks of age were individually housed in cages outfitted with a running wheel in light-tight, ventilated chambers illuminated internally by fluorescent bulbs (F30T12-SP41-RS; 3.9 × 1017 to 6.9 × 1018 photons/s/m2 at the bottom of the cages; General Electric, Fairfield, CT). We recorded wheel running activity in 1-minute bins (Clocklab; Actimetrics, Evanston, IL) while mice were exposed to a light–dark schedule (lights on at 7 AM and off at 7 PM). Water and food were changed weekly. One week of entrainment/adaptation to the cage and lighting conditions was given prior to assessments. One untreated MPS IIIB mouse died prior to the end of the first week of assessment.
Wheel running records were analyzed for three parameters: phase angle of entrainment (delay between daily light offset and onset of activity), total daily activity, and proportion of daily activity in the light phase of the photocycle. Clocklab was used to determine the onset for each circadian cycle as described previously.37 Statistical analysis entailed linear contrast following mixed-model repeated-measures analysis of variance treating each mouse as the subject nested within genotype (MPS IIIB or wild type) and treatment (AAV, BMT, AAV, and BMT), modeling covariance as unstructured.
Motor function assessment. The rocking (reversing) rotarod test was performed essentially as described previously.4 Mice were trained on the rocking rotarod (rotarod for mice; UGO Basile, Varese, Italy) for two consecutive days with three attempts each day prior to the first testing on the third consecutive day. The speed of the rocking rod was 10 rpm with a change in direction after each full rotation and up to 180 seconds at each attempt. Animals were tested every 4 weeks starting from 168 days of age until death (MPS IIIB-no tx n = 14, MPS IIIB-AAV n = 17, MPS IIIB-BMT n = 16, MPS IIIB-AAV/BMT n = 13, normal-no tx n = 15, normal-AAV/BMT n = 13). Mice were timed for how long they could remain on the rod for each of three attempts, and the longest time was used. To increase test sensitivity for detecting deficits and treatment effects, performance on the rocking rotarod was evaluated within two different age intervals determined from prior studies of untreated MPS IIIB animals: a predysfunction period and a progressive impairment period. The data from each period were subjected to repeated-measures analysis of variance with a between-subjects variable (group) and a within-subjects variable (age at testing) and Huynh–Feldt adjustment for repeated-measures variables containing more than two levels.
Cerebellar Purkinje cell assessment. Cerebella from mice between 336 and 399 days of age were immersion fixed in 4% paraformaldehyde in PBS followed by 10% neutral buffered formalin. Each cerebellum was embedded in paraffin and 5 micron thick sagittal sections were hematoxylin and eosin stained. Purkinje cells in the cerebellum were counted at the vermis by light microscopy as described previously.4 The cell count data were analyzed by repeated-measures analysis of variance (MPS IIIB-no tx n = 3, MPS IIIB-AAV n = 3, MPS IIIB-BMT n = 4, MPS IIIB-AAV/BMT n = 4, normal-no tx n = 4, normal-AAV/BMT n = 3).
ABR recording. ABRs were performed at 3, 6, and 9 months of age using Tucker–Davis Technologies System 3 Complete ABR/OAE Workstation (Tucker–Davis Technologies, Alachua, FL) as described previously.4,38 Mice were anesthetized with 80 mg/kg ketamine and 15 mg/kg xylazine (i.p.). Core temperature was maintained at 37.5 ± 1.0 °C using a thermostatically controlled heating pad in conjunction with a rectal probe (Yellow Springs Instruments Model 73A; Yellow Springs Instruments, Yellow Springs, OH). Platinum needle electrodes (Grass, West Warwick, RI) were inserted subcutaneously just behind the right ear, at the vertex, and in the back (ground). Electrodes were led to a Grass P15 differential amplifier (100–10,000 Hz, 100×), then to a custom amplifier providing another 1,000× gain, and digitized at 30 kHz using a Cambridge Electronic Design Micro1401 (Cambridge Electronic Design, Cambridge, England) in conjunction with SIGNAL and custom signal averaging software operating on a 120 MHz Pentium PC. Sine-wave stimuli generated by a HP 3445 oscillator (Hewlett-Packard, Palo Alto, CA) were shaped by a custom electronic switch to 5 ms total duration, including 1 ms rise/fall times. The stimulus was amplified by a Crown D150A amplifier (Crown, Rockwall, TX) and output to an Alpine SPS-OEOA coaxial speaker (Crutchfield, Charlottesville, VA) located 10 cm directly lateral to the right ear. Toneburst stimuli at each frequency and level were presented 1,000 times at 20/second. The minimum sound pressure level required for visual detection of wave I was determined at each frequency using a 5 dB minimum step size. To calibrate sound stimuli, a B&K 4135 1/4 inch microphone (B&K, Norcross, GA) was placed where the ear would normally be located. The mean response, standard deviation, and standard error of the mean were calculated for each treatment group. Statistical analysis by two-way analysis of variance followed by Tukey pairwise multiple comparisons were performed using SigmaStat software (SPSS, Chicago, IL). (MPS IIIB-no tx n = 14, MPS IIIB-AAV n = 17, MPS IIIB-BMT n = 15, MPS IIIB-AAV/BMT n = 13, normal-no tx n = 15, and normal-AAV/BMT n = 13).
Processing of cochleas for histology. At the final ABR recording, animals were overdosed with sodium pentobarbital and perfused transcardially with cold 2.0% paraformaldehyde/2.0% glutaraldehyde in 0.1 mol/l phosphate buffer (pH 7.4). Each cochlea was rapidly isolated, immersed in the same fixative, and the stapes was quickly removed. Complete infiltration of the cochlea by fixative was facilitated by drilling a small hole at the apex of the cochlear capsule, and gently circulating the fixative over the cochlea using a transfer pipette. After decalcification in sodium EDTA for 72 hours, cochleas were post-fixed in buffered 1% osmium tetroxide, dehydrated in an ascending acetone series, and embedded in Epon. Cochleas were sectioned in the mid-modiolar plane at 4.0 µm, then stained with toluidine blue for bright field viewing with a Nikon Optiphot light microscope (Nikon, Melville, NY). Typically 50 sections were obtained from each cochlea, spanning 200 µm centered on the modiolar “core.”
Histology and biochemistry. Experimental and control mice were killed by CO2 asphyxiation between 330 and 390 days of age (all groups n = 6 but normal AAV/BMT n = 4). Parts of liver, spleen, kidneys, heart, lungs, and brain were dissected and one-half of each organ sample was immersion fixed in 2% glutaraldehyde/4% paraformaldehyde in phosphate-buffered saline. Liver, kidney, spleen, and brain were evaluated for lysosomal storage and the number of vacuoles in 100 parietal cortical neurons was counted as described previously.25
The other half of each organ sample was flash-frozen in liquid nitrogen and stored at −85 °C until mechanical homogenization in 10 mmol/l Tris (pH 7.5), 150 mmol/l NaCl, 1 mmol/l DTT. Homogenates were centrifuged at 14,000 rpm for 30 seconds to pellet debris and supernatants were transferred to a new tube. Supernatants were assayed in duplicate by enzyme assay,35 briefly 25 µl of supernatant was added to 25 µl of 0.2 mmol/l 4-methylumbelliferone-N-acetyl-α-D-glucopyranoside (Sigma, St Louis, MO), 0.1 mol/l NaC2H3O2, 0.5 mg/ml bovine serum albumin and incubated at 37 °C for 2 hours for brain and liver or 4 hours for heart and spleen. Reactions were stopped with 1 ml of 0.2 mol/l Na2CO3, 0.32 mol/l glycine. Substrate cleavage was determined at excitation 365 nm and emission 448 nm using a Hitachi F-2000 fluorescence spectrophotometer (Hitachi, Pleasanton, CA) using a standard curve of 0.5–5 nm/ml. Specific activity was corrected for protein concentration. Secondary lysosomal enzyme elevation was determined for β-glucuronidase using the same liver and spleen supernatants in duplicate using the 4-methylumbelliferone enzyme assay method previously described.32
Lifespan. All weaned animals were analyzed by intention to treat using Kaplan–Meier curves with a log-rank test to assess for treatment on lifespan. Data analysis and curves were generated using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
Acknowledgments
Special thanks to Shannon Macauley-Rambach for assistance with histologic evaluation and Patty Gagnon for assistance with figures. This work was funded in part by NIH grants NS043205 (M.S.S.), HD055461 (M.S.S.), P30 DC004665 (R. Chole), P30 NS057105 (D. Holtzman), WUSM Department of Otolaryngology, and a grant from the National MPS Society (M.S.S.).
REFERENCES
- Weber B, Blanch L, Clements PR, Scott HS., and , Hopwood JJ. Cloning and expression of the gene involved in Sanfilippo B syndrome (mucopolysaccharidosis III B) Hum Mol Genet. 1996;5:771–777. doi: 10.1093/hmg/5.6.771. [DOI] [PubMed] [Google Scholar]
- Zhao HG, Li HH, Bach G, Schmidtchen A., and , Neufeld EF. The molecular basis of Sanfilippo syndrome type B. Proc Natl Acad Sci USA. 1996;93:6101–6105. doi: 10.1073/pnas.93.12.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, et al. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase. Proc Natl Acad Sci USA. 1999;96:14505–14510. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldermon CD, Hennig AK, Ohlemiller KK, Ogilvie JM, Herzog ED, Breidenbach A, et al. Development of sensory, motor and behavioral deficits in the murine model of Sanfilippo syndrome type B. PLoS ONE. 2007;2:e772. doi: 10.1371/journal.pone.0000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausseil J, Desmaris N, Bigou S, Attali R, Corbineau S, Vitry S, et al. Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS ONE. 2008;3:e2296. doi: 10.1371/journal.pone.0002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, et al. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Ohmi K, Kudo LC, Ryazantsev S, Zhao HZ, Karsten SL., and , Neufeld EF. Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc Natl Acad Sci USA. 2009;106:8332–8337. doi: 10.1073/pnas.0903223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRosario J, Divers E, Wang C, Etter J, Charrier A, Jukkola P, et al. Innate and adaptive immune activation in the brain of MPS IIIB mouse model. J Neurosci Res. 2009;87:978–990. doi: 10.1002/jnr.21912. [DOI] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH., and , Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ryazantsev S, Ohmi K, Zhao HZ, Rozengurt N, Kohn DB, et al. Retrovirally transduced bone marrow has a therapeutic effect on brain in the mouse model of mucopolysaccharidosis IIIB. Mol Genet Metab. 2004;82:286–295. doi: 10.1016/j.ymgme.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Willing AE, Desjarlais T, Davis Sanberg C., and , Sanberg PR. Transplantation of human umbilical cord blood cells benefits an animal model of Sanfilippo syndrome type B. Stem Cells Dev. 2005;14:384–394. doi: 10.1089/scd.2005.14.384. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Gografe SJ, Sanberg CD, Willing AE, Saporta S, Cameron DF, et al. Maternal transplantation of human umbilical cord blood cells provides prenatal therapy in Sanfilippo type B mouse model. FASEB J. 2006;20:485–487. doi: 10.1096/fj.05-4684fje. [DOI] [PubMed] [Google Scholar]
- Fu H, Samulski RJ, McCown TJ, Picornell YJ, Fletcher D., and , Muenzer J. Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery. Mol Ther. 2002;5:42–49. doi: 10.1006/mthe.2001.0514. [DOI] [PubMed] [Google Scholar]
- Cressant A, Desmaris N, Verot L, Bréjot T, Froissart R, Vanier MT, et al. Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum. J Neurosci. 2004;24:10229–10239. doi: 10.1523/JNEUROSCI.3558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Kang L, Jennings JS, Moy SS, Perez A, Dirosario J, et al. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14:1065–1077. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- Di Natale P, Di Domenico C, Gargiulo N, Castaldo S, Gonzalez Y Reyero E, Mithbaokar P, et al. Treatment of the mouse model of mucopolysaccharidosis type IIIB with lentiviral-NAGLU vector. Biochem J. 2005;388 Pt 2:639–646. doi: 10.1042/BJ20041702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA, et al. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther. 2005;12:422–430. doi: 10.1016/j.ymthe.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M, et al. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Mol Ther. 2005;11:734–744. doi: 10.1016/j.ymthe.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Lin D, Donsante A, Macauley S, Levy B, Vogler C., and , Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- Sands MS, Vogler C, Kyle JW, Grubb JH, Levy B, Galvin N, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler C, Levy B, Galvin NJ, Thorpe C, Sands MS, Barker JE, et al. Enzyme replacement in murine mucopolysaccharidosis type VII: neuronal and glial response to beta-glucuronidase requires early initiation of enzyme replacement therapy. Pediatr Res. 1999;45:838–844. doi: 10.1203/00006450-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferra TJ, Backstrom K, Wang C, Rennard R, Miller M., and , Hu Y. Widespread correction of lysosomal storage following intrahepatic injection of a recombinant adeno-associated virus in the adult MPS VII mouse. Mol Ther. 2004;10:478–491. doi: 10.1016/j.ymthe.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Donsante A, Levy B, Vogler C., and , Sands MS. Clinical response to persistent, low-level beta-glucuronidase expression in the murine model of mucopolysaccharidosis type VII. J Inherit Metab Dis. 2007;30:227–238. doi: 10.1007/s10545-007-0483-4. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Levy B, Galvin N, Tan Y., and , Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MS, Vogler C, Torrey A, Levy B, Gwynn B, Grubb J, et al. Murine mucopolysaccharidosis type VII: long term therapeutic effects of enzyme replacement and enzyme replacement followed by bone marrow transplantation. J Clin Invest. 1997;99:1596–1605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar M, Norflus F, Tifft CJ, Cortina-Borja M, Butters TD, Proia RL, et al. Enhanced survival in Sandhoff disease mice receiving a combination of substrate deprivation therapy and bone marrow transplantation. Blood. 2001;97:327–329. doi: 10.1182/blood.v97.1.327. [DOI] [PubMed] [Google Scholar]
- Biswas S., and , LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Birkenmeier EH. Correction of murine mucopolysaccharidosis type VII (MPS VII) by bone marrow transplantation and gene transfer therapy. Hum Gene Ther. 1991;2:113. doi: 10.1089/hum.1991.2.2-113. [DOI] [PubMed] [Google Scholar]
- Wolfe JH, Sands MS, Barker JE, Gwynn B, Rowe LB, Vogler CA, et al. Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature. 1992;360:749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol (Lond) 2006;576 Pt 1:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N, et al. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest. 1993;68:676–686. [PubMed] [Google Scholar]
- Li HH, Zhao HZ, Neufeld EF, Cai Y., and , Gómez-Pinilla F. Attenuated plasticity in neurons and astrocytes in the mouse model of Sanfilippo syndrome type B. J Neurosci Res. 2002;69:30–38. doi: 10.1002/jnr.10278. [DOI] [PubMed] [Google Scholar]
- Daly TM, Okuyama T, Vogler C, Haskins ME, Muzyczka N., and , Sands MS. Neonatal intramuscular injection with recombinant adeno-associated virus results in prolonged beta-glucuronidase expression in situ and correction of liver pathology in mucopolysaccharidosis type VII mice. Hum Gene Ther. 1999;10:85–94. doi: 10.1089/10430349950019219. [DOI] [PubMed] [Google Scholar]
- Marsh J., and , Fensom AH. 4-Methylumbelliferyl alpha-N-acetylglucosaminidase activity for diagnosis of Sanfilippo B disease. Clin Genet. 1985;27:258–262. doi: 10.1111/j.1399-0004.1985.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Sands MS., and , Barker JE. Percutaneous intravenous injection in neonatal mice. Lab Anim Sci. 1999;49:328–330. [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y., and , Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Daly TM, Ohlemiller KK, Roberts MS, Vogler CA., and , Sands MS. Prevention of systemic clinical disease in MPS VII mice following AAV-mediated neonatal gene transfer. Gene Ther. 2001;8:1291–1298. doi: 10.1038/sj.gt.3301420. [DOI] [PubMed] [Google Scholar]