Abstract
Dendritic cells (DCs) initiate immune responses as well as tolerance. We showed previously that the neuropeptide vasoactive intestinal peptide (VIP) suppresses innate immune responses, modulates adaptive responses by generating regulatory T cells (Treg) through the induction of tolerogenic DCs (tDCs), and has therapeutic effects in models of autoimmune/inflammatory disorders. Systemic VIP administration is limited by its short biological half-life and by its pleiotropic effects on the cardiovascular system and gastrointestinal tract. Therefore, we used lentiviral vectors to genetically engineer VIP-expressing bone marrow–derived DC (BMDC) and characterized the transduced LentiVIP-DC in terms of phenotype and therapeutic effects in models of experimental autoimmune encephalomyelitis (EAE) and cecal ligation and puncture (CLP) sepsis. LentiVIP-DCs secrete VIP, and resemble tDCs through lack of co-stimulatory molecule upregulation, lack of proinflammatory cytokine secretion, increased interleukin (IL)-10 production, and poor stimulation of allogeneic T cells. A single inoculation of LentiVIP-DC in EAE or CLP mice had therapeutic effects, which correlated with reduced expression of proinflammatory cytokines and increased IL-10 production in spinal cord and peritoneal fluid, respectively. In contrast to systemic VIP administration that requires repeated, high-dose inoculations, local delivery of VIP by LentiVIP-DC may represent a promising therapeutic tool for the treatment of autoimmune diseases and inflammatory disorders.
Introduction
Autoimmune and severe inflammatory diseases are being treated with repeated systemic administrations of high doses of immunosuppressive drugs, which usually mount an incomplete therapeutic response and lead to generalized immunosuppression.1 Gene–cell therapy may become an important alternative treatment, with the advantage of delivering immunosuppressive therapeutic molecules to targeted areas.2
The migratory capability of dendritic cells (DCs) and the fact that they play an essential role in maintaining tolerance are attractive characteristics for their use as vehicles for immunosuppressive therapeutic factors. In response to inflammatory chemokines, immature DCs migrate to inflammatory sites where they capture and process antigens (Ags), and undergo maturation including changes in chemokine receptors. Subsequently mature DCs migrate to secondary lymphoid organs where they activate Ag-specific T cells.3,4 Also, DC subsets, usually characterized by low expression of co-stimulatory molecules and low production of proinflammatory cytokines, display tolerogenic functions secreting anti-inflammatory cytokines like interleukin (IL)-10 and transforming growth factor-β and generating regulatory T cells (Tregs).5,6,7,8
Several studies reported on the expression of the immunosuppressive cytokines IL-10, transforming growth factor-β1, and IL-4 by genetically engineered DCs.9,10,11 DCs secreting IL-4 were actually shown to be therapeutic in collagen-induced arthritis.11 Vasoactive intestinal peptide (VIP) is a potent anti-inflammatory agent, which inhibits both innate and adaptive immune responses12 and has a strong therapeutic effect in the treatment of autoimmune/inflammatory diseases.13,14,15,16,17 We showed previously that bone marrow–derived DC (BMDC) differentiated in the presence of exogenous VIP become tolerogenic DC (tDC),18 do not upregulate co-stimulatory molecules and proinflammatory cytokine expression following Toll-like receptor signaling, but produce significant levels of IL-10, and induce the generation of Ag-specific Treg in vivo and in vitro.17,18 The involvement of VIP in immune tolerance through the induction of Foxp3+ Treg was confirmed recently.19,20
Autoimmune diseases are characterized by inflammation resulting from both innate and adaptive immunity. As VIP inhibits the proinflammatory activities of cells involved in innate immunity and also reduces the adaptive immune response through the generation of Ag-specific Treg, it has a definite therapeutic advantage over agents directed against only one type of immunity. However, systemic VIP administration is limited by its short biological half-life, which requires repeated inoculations and by its pleiotropic effects on the cardiovascular and gastrointestinal systems, including vasodilation, bronchodilation, smooth muscle relaxation, gastric motility, hyperglycemia, analgesia, control of cardiac output, and hormonal regulation.21 Therefore, cell-mediated VIP delivery to both inflammatory sites and secondary lymphoid organs could provide Ag-specific Treg induction and sustained therapeutic VIP levels at the inflammatory site without the side effects resulting from systemic administration.
To control vector distribution and to target its biological effect, we decided upon the use of DC as cellular vehicles for a LentiVIP vector. To our knowledge, this is the first report of VIP-expressing DC following transduction with a LentiVIP vector. In this study, we show that LentiVIP transduction of murine DC during differentiation from bone marrow cells results in VIP-secreting DCs, which share phenotypic and functional characteristics with tDCs. A single administration of LentiVIP-DC proved to be therapeutic in experimental autoimmune encephalomyelitis (EAE) models as well as in the cecal ligation and puncture (CLP) sepsis model.
Results
BMDCs secrete VIP following lentiviral vector transduction
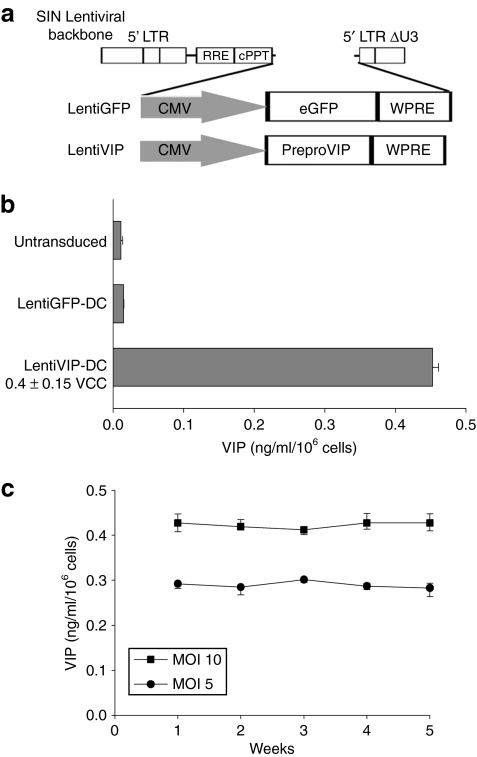
Second generation lentiviral vectors expressing human preproVIP complementary DNA (LentiVIP) or green fluorescent protein (GFP) (LentiGFP) (Figure 1a) were used to transduce BMDC. The expression cassette includes the woodchuck postregulatory element placed behind the preproVIP or GFP complementary DNA to enhance gene expression. BMDCs were differentiated for 3 days in the presence of rmGM-CSF (recombinant murine granulocyte macrophage colony–stimulating factor), nonadherent cells were transduced on day 4, followed by reculture in the presence of rmGM-CSF for another 3 days. BMDCs were efficiently transduced by the LentiGFP (Supplementary Figure S1) and the LentiVIP vector. As the preproVIP complementary DNA expresses a polypeptide with an exportation signal, which allows the processing and secretion of VIP,22 we used competitive enzyme-linked immunosorbent assay to quantify the amount of secreted VIP, and quantitative PCR (qPCR) to determine the vector copy number per cell. LentiVIP-transduced DC (multiplicity of infection 10) had an average of 0.4 vector copy per cell (Figure 1b), and secreted an average of 450 pg VIP/ml/106 cells (Figure 1b). No detectable VIP levels were observed in untransduced or LentiGFP-DC. In addition, we observed that LentiVIP-DC secreted consistent amounts of VIP during a 5-week period (Figure 1c).
Figure 1.
Bone marrow–derived dendritic cells are efficiently transduced with lentiviral vectors expressing vasoactive intestinal peptide (VIP) and green fluorescent protein (GFP). (a) Schematic representation of lentiviral vectors LentiVIP and LentiGFP. Cytomegalovirus (CMV) promoter drives the expression of preproVIP complementary DNA in LentiVIP and of GFP in LentiGFP in a self-inactivating lentiviral vector backbone. (b) VIP secretion by LentiVIP-DC. LentiVIP-DC were cultured for 24 hours at a concentration of 1 × 106 cells/ml. Supernatants were collected and assayed for VIP by competitive enzyme-linked immunosorbent assay. Undetectable levels of VIP in untransduced- and LentiGFP-DC. (c) Stable secretion of VIP over time by LentiVIP-DC at multiplicity of infection (MOI) 5 and 10. Once a week, cells were washed, resuspended in fresh medium, supernatants were collected 24 hours later and VIP levels were determined as described in b. One representative experiment out of three is shown. cPPT, polypurine track; LTR, long terminal repeat; RRE, Rev responsive element; WPRE, woodchuck postregulatory element.
Phenotypic characterization of LentiVIP-DC
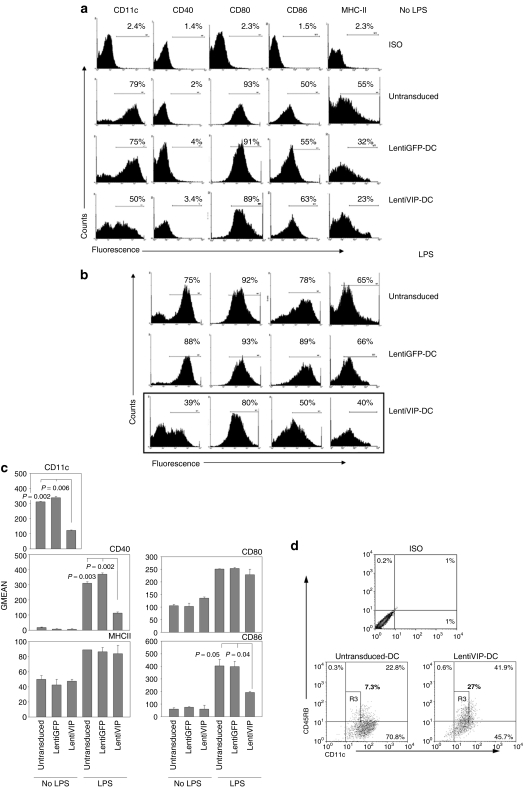
To determine whether lentiviral vector transduction and subsequent VIP secretion by differentiating DC results in phenotypical and/or functional changes, we compared untransduced-DC, LentiGFP-DC, and LentiVIP-DC in terms of CD11c, major histocompatibility complex-II, CD40, CD80, and CD86 expression. All three types of unstimulated DCs expressed similar levels of co-stimulatory molecules (CD40, CD80, and CD86) (Figure 2a,c). In contrast, LentiVIP-DCs expressed significantly lower levels of CD11c both in terms of percentage positive cells and mean fluorescence intensity (Figure 2a,c). Lipopolysaccharide (LPS) stimulation induced increases in CD40, CD86, and to a lesser degree in major histocompatibility complex-II in untransduced and LentiGFP-DCs (Figure 2b,c). In contrast, LentiVIP-DCs only slightly upregulated CD40 and CD86 surface expression following LPS stimulation (Figure 2b,c). Our results are in agreement with the characterization of most tDCs, including tDCs generated previously in our laboratory in the presence of exogenous VIP.18,23,24
Figure 2.
Phenotypic characterization of LentiVIP-DC. (a) Untransduced-DC, LentiGFP-DC, and LentiVIP-DC harvested on day 7 were washed and stained with antibodies to CD11c, I-Ek, CD40, CD80, and CD86 and analyzed by flow cytometry. (b) Dendritic cells were treated with 1 µg/ml lipopolysaccharide (LPS) for 24 hours. Histograms are representative of three experiments performed in duplicate. (c) Results of fluorescence-activated cell-sorting analysis expressed as geometric mean of fluorescence (GMEAN) ± SD of three experiments performed in duplicate. Degree of significance P < 0.05 compared with untransduced-DC and LentiGFP-DC. Final GMEAN values are the result of GMEAN subtraction from isotype control. (d) LentiVIP-DC are CD11low and CD45RBhigh. Untransduced-DC and LentiVIP-DC were double stained for CD11c and CD45RB expression and analyzed by flow cytometry. CD11clowCD45RBhigh population is gated as R3. One representative experiment of three is shown.
Previous reports indicated a decrease in CD11c and an increase in CD45RB expression in the tDCs differentiated in the presence of exogenous galectin-1 or VIP.18,25 We observed a similar pattern in LentiVIP-DC (Figure 2d).
Functional characterization of LentiVIP-DC
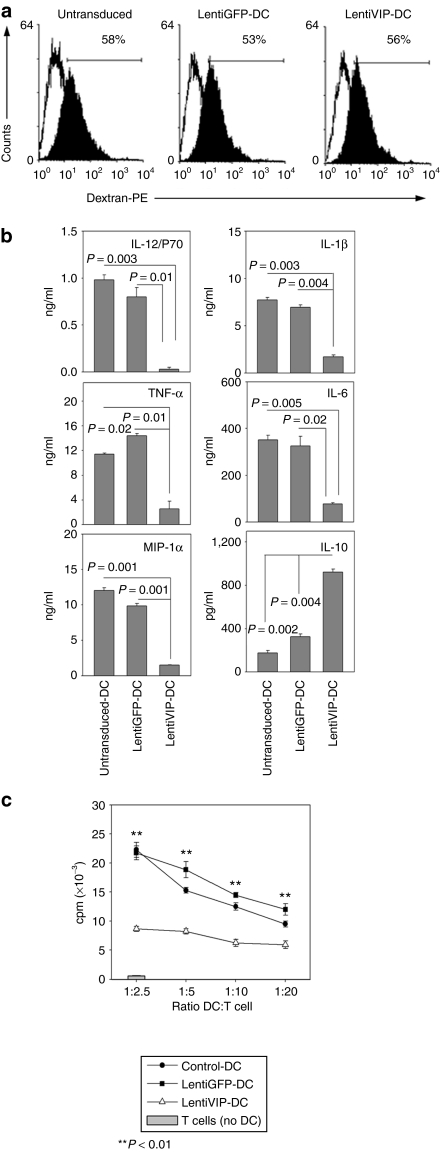
Immature DCs exhibit high endocytic capacity. We evaluated the endocytic capacity of LentiVIP-DC and of control untransduced and LentiGFP-DC using fluorescent dextran. The endocytic capacity of LentiVIP-DC was similar to that of LentiGFP-DC and of untransduced immature DC (Figure 3a).
Figure 3.
Functional characteristics of LentiVIP-DC. (a) Untransduced-, LentiGFP-, and LentiVIP-DC were incubated in medium containing 1 mg/ml of dextran-PE (40 kd) for 2 hours at 4 °C (control; open histograms) or 37 °C (filled histograms), extensively washed and analyzed by flow cytometry. One representative experiment of two performed in duplicate is shown. (b) Untransduced-, LentiGFP-, and LentiVIP-DC were cultured with LPS (1 µg/ml) for 24 hours. Culture supernatants were assayed for IL-12p70, IL-1β, TNF-α, IL-6, MIP-1α, and IL-10. Results are the mean ± SD of three experiments performed in duplicate. Degree of significance P < 0.05. (c) Untransduced-, LentiGFP-, and LentiVIP-DC were cocultured at different ratios with allogeneic CD4+ T cells (5 × 104) in a 4-day mixed lymphocyte reaction. Proliferation was measured by [3H]-thymidine incorporation. A representative experiment of three is shown.
Upon Toll-like receptor stimulation, DCs mature and secrete proinflammatory cytokines/chemokines, which attract and activate monocytes, macrophages, neutrophils, and T cells. Untransduced-DCs and LentiGFP-DCs treated with LPS secreted high levels of IL-12p70, IL-6, tumor necrosis factor (TNF)-α, IL-1β, and macrophage inflammatory protein (MIP)-1α and low levels of IL-10 (Figure 3b). LPS-treated LentiVIP-DC secreted little if any IL-12p70, IL-1β, TNF-α, IL-6, and MIP-1α. In contrast, they produced high levels of IL-10 (Figure 3b).
Both soluble VIP and tDC generated in the presence of exogenous VIP have been previously shown to inhibit CD4+ T-cell proliferation.18 We analyzed the stimulatory capacity of LentiVIP-DC for CD4+ T cells in cocultures with alloreactive CD4+ T cells. In contrast to untransduced-DC and LentiGFP-DC, which promoted strong T-cell proliferation, LentiVIP-DC induced much lower levels of T-cell proliferation (Figure 3c).
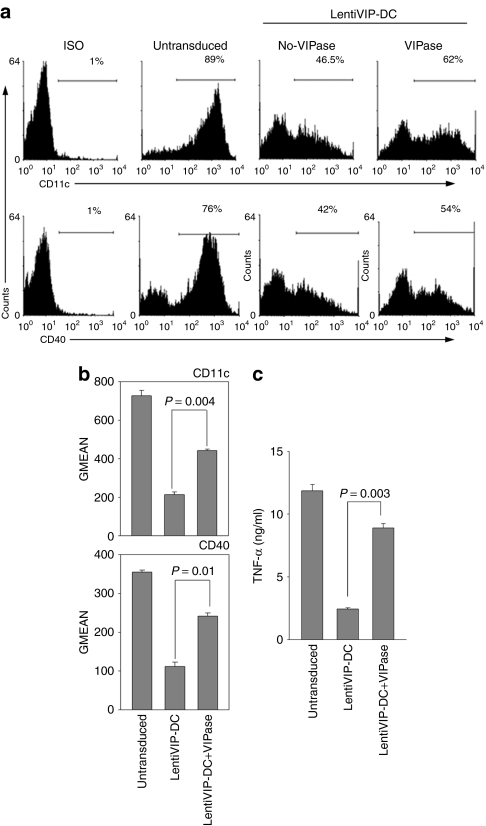
Catalytic VIPase antibody partially reverses the effect of transduction with LentiVIP
To address whether the altered function of LentiVIP-DC was the result of endogenous VIP secreted by the transduced DC, we treated differentiating DCs with a catalytic VIPase antibody daily, starting immediately after lentiviral vector transduction. The catalytic VIPase antibody has enzymatic activity catalyzing the cleavage of soluble VIP.26 The resulting LentiVIP-DCs were analyzed in terms of CD11c and CD40 expression and TNF-α secretion following LPS stimulation. The VIPase-treated LentiVIP-DCs expressed higher levels of CD11c and CD40, and secreted higher levels of TNF-α compared to LentiVIP-DCs treated with control IgG (Figure 4). The fact that we did not observe a complete reversal of the downregulation of CD11c, CD40, and TNF-α is probably due to the difficulty in neutralizing the VIP continuously secreted by LentiVIP-DCs.
Figure 4.
VIPase catalytic antibody partially reverses the characteristics of LentiVIP-DC. LentiVIP-DC were grown in the presence of daily added 180 µg of VIPase or control antibody (UPC10 IgG2a) as described in Materials and Methods. (a) LentiVIP-DC treated with or without VIPase were stained with anti-CD11c, or treated for 24 hours with lipopolysaccharide (LPS) and stained for CD40 expression. (b) Results are expressed as geometric mean of fluorescence (GMEAN) ± SD of three experiments performed in duplicate. Degree of significance P < 0.05. (c) TNF-α levels were determined by enzyme-linked immunosorbent assay in supernatants of LPS-treated cells. Three experiments were performed in duplicate. Degree of significance P < 0.05.
LentiVIP-DC's migration pattern
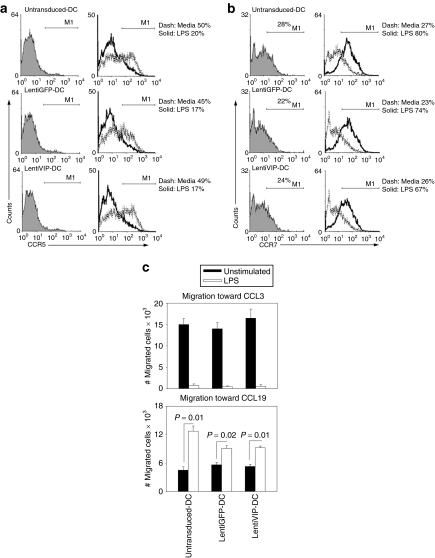
Immature DCs express high levels of CCR5 and migrate toward chemokines released within inflammatory sites such as CCL3, 4, and 5. In contrast, Toll-like receptor–stimulated mature DCs upregulate CCR7 expression and migrate in response to CCL19 and 21, chemokines constitutively expressed in secondary lymphoid organs.27 We compared first CCR5/CCR7 expression in immature and LPS-stimulated DC, LentiGFP-DC, and LentiVIP-DC, respectively. In all three types of DCs, surface CCR5 is expressed before stimulation and downregulated following LPS stimulation by similar percentages (50–60%) (Figure 5a). Surface CCR7 expression is increased following LPS stimulation in a similar manner in all three types of DCs (Figure 5b). mRNA analysis (Supplementary Figure S2) confirmed the similar patterns of CCR5/CCR7 regulation in control DC, LentiGFP-DC, and LentiVIP-DC.
Figure 5.
CCR5 and CCR7 patterns in LentiVIP-DC. (a) CCR5 and (b) CCR7 expression was analyzed by flow cytometry in untransduced-, LentiGFP-, and LentiVIP-DC before and after lipopolysaccharide (LPS) activation. Cells were treated with LPS (0.1 µg/ml) for 24 hours before detection of CCR5 and 48 hours before detection of CCR7. Indirect immunostaining was performed using a primary purified CCR5 or 7 antibody, a secondary biotin-conjugated antibody, and APC-conjugated streptavidin. Filled: isotype. Dashed line: not activated. Solid line: LPS-activated. (c) Migration toward CCL3 (100 ng/ml) and CCL19 (100 ng/ml) was tested for untransduced-, LentiVIP-, and LentiGFP-DC, before and after LPS treatment. A volume of 2 × 105 cells were plated and allowed to migrate for 4 hours. Absolute number of cells determined by FACS (120-second counts) are presented. Number of migrated cells in control wells without chemokines were <1,000 cells in all of the cases (data not shown). Two independent experiments in triplicate were performed. Degree of significance P < 0.05.
In agreement with the observed regulation of CCR5/CCR7 expression, transwell migration toward CCL3 and 19 confirmed that all three types of unstimulated DCs responded to CCL3 and that LPS stimulation induced a migratory response to CCL19 (Figure 5c).
Therapeutic effect of LentiVIP-DC in EAE models
We assessed the potential therapeutic role of LentiVIP-DC in two models of EAE, the relapsing–remitting model in SJL/J mice and the chronic model in C57BL/6 mice. Both models exhibit an adaptive immune response driven by Th1 and Th17 cells and a local inflammatory response in the central nervous system mediated primarily by activated microglia.28
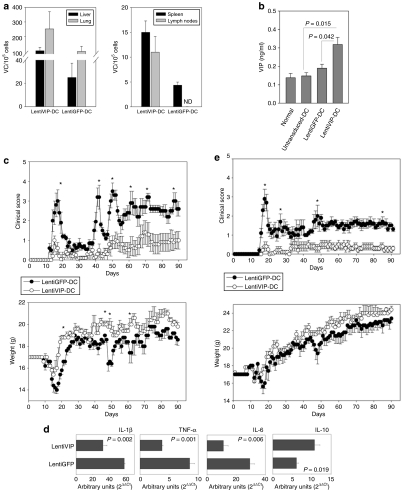
In the relapsing–remitting model, we injected 3 × 106 proteolipid protein (PLP)139–151-pulsed LentiVIP-DC (secreting 0.45 ng/ml VIP) or LentiGFP-DC cells intravenously 6 days after the immunization with PLP139–151 (efferent phase of the disease). After 48 hours LentiVIP-DC and LentiGFP-DC were detected by qPCR in liver, lung, spleen, and lymph nodes of injected animals (Figure 6a) with similar distribution patterns. Mice that received LentiVIP-DC also showed an increase in VIP levels in serum 48 hours after DC inoculation (Figure 6b). Both clinical score and weight were monitored for 90 days. The mice in the LentiVIP-DC group had delayed disease onset (day 14 versus day 10 for the LentiGFP-DC group) and the clinical symptoms were less severe (score at peak of disease: LentiGFP-DC: 3; LentiVIP-DC: 1) (Figure 6c—upper panel). The weight loss observed during the first peak of disease was much less severe in the LentiVIP-DC group (Figure 6c—lower panel). The protective effect of LentiVIP-DC was supported by the cytokine profile in spinal cord at the peak of disease. A significant decrease in inflammatory cytokines (IL-1β, TNF-α, IL-6) and an increase in IL-10 was observed in the LentiVIP-DC group (Figure 6d). The protective effect of LentiVIP-DC was long-term, reducing not only the initial peak of disease, but also the subsequent relapses.
Figure 6.
LentiVIP-DC reduces severity in two experimental autoimmune encephalomyelitis (EAE) models. (a) Liver, lung, spleen, and lymph nodes (axillary and inguinal) were collected 48 hours after intravenous inoculation of transduced cells and genomic DNA was isolated. The presence of LentiVIP-DC and LentiGFP-DC was analyzed by quantitative PCR using primers specific for sequences for each vector. Presented data are after subtraction of background from tissues of control mice. (b) Serum from LentiVIP-DC, LentiGFP-DC, and untransduced-DC SJL-treated mice was obtained 48 hours after the dendritic cell (DC) inoculation and vasoactive intestinal peptide (VIP) levels were measured by enzyme-linked immunosorbent assay and compared with endogenous levels of VIP in serum of healthy mice. (c) SJL/J mice (5 mice/group) immunized with PLP139–151 as described in Methods were inoculated intravenous (tail vein) on day 6 with LentiVIP-DC or LentiGFP-DC (3 × 106 cells) pulsed with 50 µg/ml of PLP (3 × 106). Clinical score and weight was followed daily for >90 days. (d) Total RNA was extracted from spinal cord of LentiVIP-DC and LentiGFP-DC SJL-treated animals at the peak of disease and analyzed for cytokine expression by real-time qPCR. The expression of cytokines was normalized to GAPDH and is relative to healthy controls (no EAE). Degree of significance P < 0.05. (e) C57BL/6 mice (6 mice/group) were immunized with MOG35–55 as described in Materials and Methods. On day 8, the mice were inoculated intravenous with 3 × 106 LentiGFP-DC or LentiVIP-DC pulsed with 50 µg/ml of MOG. Clinical score and weight was followed daily for >90 days. *P < 0.05. IL, interleukin; TNF, tumor necrosis factor.
In the C57BL/6 model of chronic EAE, mice were injected intravenously with 3 × 106 myelin oligodendrocyte glycoprotein (MOG)35–55-pulsed LentiVIP-DC or LentiGFP-DC at 24 hours after the second immunization with MOG35–55. LentiVIP-DC prevented the development of the disease, with only 40% of mice showing a score of 0.5 at the peak of disease. In contrast, 100% of the mice injected with LentiGFP-DC developed clinical disease with a score of 3 at the peak of disease (Figure 6e). Both the LentiVIP-DC and LentiGFP-DC groups lost weight, although the LentiVIP-DC group exhibited less weight loss throughout the experiment (statistically not significant).
Therapeutic effect of LentiVIP-DC in the CLP sepsis model
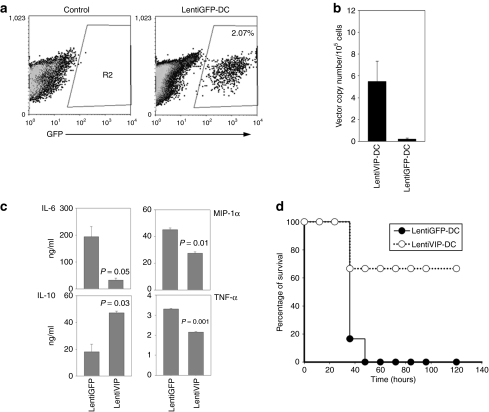
As LentiVIP-DC could release VIP locally in vivo, we evaluated the therapeutic potential of LentiVIP-DC in CLP, a clinically relevant model for human sepsis that involves lethal peritonitis due to polymicrobial infections. BALB/c mice were injected intraperitoneally with 2 × 106 LentiVIP-DC or LentiGFP-DC immediately following surgery. At 20 hours after DC inoculation, we detected GFP+ cells in the peritoneal exudate cell population from mice injected with LentiGFP-DC, and LentiVIP vector presence by qPCR in peritoneal exudate cells from mice injected with LentiVIP-DC (Figure 7a,b). Analysis of peritoneal fluid indicated reductions in proinflammatory molecules (IL-6, TNF-α, and MIP-1α) and an increase in IL-10 in mice inoculated with LentiVIP-DC (Figure 7c). Lethality for mice injected with LentiGFP-DC was 100% at 48 hours, whereas the group inoculated with LentiVIP-DC had a long-term survival rate (up to 120 hours) of 66% (Figure 7d). The surviving animals were monitored for 4 more months with no clinical symptoms.
Figure 7.
LentiVIP-DC increases survival rate in the cecal ligation and puncture model. Male BALB/c mice (6–8 weeks old; 6/group) were subjected to cecal ligation and puncture as described in Materials and Methods. Immediately after the surgical procedure LentiVIP-DC and LentiGFP-DC (2 × 106 cells/animal) were injected intraperitoneal and 20 hours after the surgical procedure peritoneal exudate cells and fluid were collected. (a) LentiGFP-DC cells were detected by flow cytometry in the peritoneal cavity. (b) LentiVIP was detected by quantitative PCR after DNA extraction from peritoneal exudate cells. Primers to detect LentiVIP were used; LentiGFP-DC was used as negative control. (c) Peritoneal fluid was analyzed by enzyme-linked immunosorbent assay for cytokine production. Degree of significance P < 0.05. (d) The therapeutic effect of LentiVIP-DC was evaluated by survival rate over time. GFP, green fluorescent protein; IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor.
Discussion
The multifactorial immune components involved in autoimmune diseases together with limited access to specific areas such as the central nervous system raise significant therapeutic difficulties. In most cases, therapy must face the challenge of locally inhibiting innate immunity without generalized immunosuppression, and at the same time of regulating the antigen-specific response.
Transfection or transduction of DC with genes encoding immunoregulatory molecules represents an attractive strategy for generating immunomodulatory DC. Presently, few studies used genetically modified DC in autoimmune diseases. Immature DC transduced with adenoviral vectors expressing TRAIL or IL-4 were shown to reduce disease incidence and severity and suppress established Th1 responses in CIA mice.11,29,30 As VIP acts as an anti-inflammatory agent in innate immunity and also promotes Th2/Treg differentiation, it represents an attractive target for the genetic engineering of immunosuppressive DCs.
An important decision pertains to the nature of the viral vectors. Although adenoviral vectors have been used successfully to transduce DCs, their use has been limited by nuclear factor (NF)-κB activation leading to DC maturation and cytokine production, and by the induction of host immunity to immunodominant viral epitopes.31,32 Oncoretroviral vectors integrate into the host genome, allowing stable gene transfer. However, oncoretroviral vectors require cell division33 and therefore are inefficient at transducing DCs. In contrast, lentiviruses stably integrate in nondividing cells, do not encode viral proteins, and therefore do not stimulate the host immune system and do not affect DC's viability, phenotype, or function.34 In addition, long-term in vivo studies after direct injection of lentiviral vectors in strains with high tumor incidence did not report tumorigenesis.35
The lentiviral-transduced DCs represent a rather new field in immunotherapy. The focus until now has been on lentiviral-transduced DC as immunostimulants, for possible use in cancer therapy.36,37 To our knowledge, with the exception of one recent report on IL-10-expressing DC inducing Ag-specific tolerance in a model of asthma,38 there are no published reports on the ex vivo lentiviral transduction of DC with immunosuppressive genes, and their potential use in autoimmune diseases.
In a previous study, we developed a lentiviral vector (LentiVIP) expressing the potent immunosuppressive agent VIP. LentiVIP administration to CIA mice resulted in remission of clinical symptoms of arthritis, even when inoculated at the most severe stage of the disease.39 The study demonstrated high therapeutic value, but future clinical use raises serious concerns regarding the lack of control over the cellular targets of vector integration and the potential side effects due to systemic constitutive VIP expression.
In this study, we used LentiVIP for transduction of BMDC as a strategy to deliver VIP locally to inflammatory sites and/or secondary lymphoid organs. We developed a transduction DC protocol that allows for high transduction efficiency at a multiplicity of infection of 10 without activating transduced DC. No upregulation of major histocompatibility complex-II or co-stimulatory molecules and no cytokine expression were observed in unstimulated transduced DC.
Following Toll-like receptor stimulation, LentiGFP-transduced DCs behave similar to untransduced-DC in terms of upregulation of co-stimulatory molecules and proinflammatory cytokine expression. In contrast, similar to some of the tDC described in the literature,18,25 LPS-stimulated LentiVIP-DC were CD11clowCD45RBhigh and did not upregulate the co-stimulatory molecules CD40 and CD86. Their cytokine profile (reduced proinflammatory cytokines and increased IL-10 secretion) and their weak stimulatory activity for allogeneic CD4+ T cells are also in agreement with previous reports regarding tDC, and in particular with our previous studies with BMDC differentiated in the presence of VIP.18 Although we showed that LentiVIP-DCs exhibit weak stimulatory capacity for T-cell activation, future experiments will have to ascertain whether LentiVIP-DC induce Ag-specific T-cell anergy and/or Treg in vitro and in vivo. We expect this to be the case, based on our previous studies with DC differentiated in the presence of exogenous VIP.17
The transduction of BMDC on day 3–4 of differentiation was based on the fact that transductions of BM cells at earlier stages are more restrictive40 and on our previous observation that induction of tDC required exogenous VIP at earlier, rather than later, differentiation stages.18 We hypothesized that endogenous VIP secreted by the LentiVIP-DC acted in an autocrine and paracrine manner promoting the development of tDC. Indeed, the increase in CD40 and CD11c expression and in TNF-α expression in LentiVIP-DC cultured in the presence of the VIPase antibody supports the autocrine/paracrine role of the endogenous VIP released by LentiVIP-DC.
Immature DCs express CCR5, allowing migration to inflammatory sites, where they endocytose Ag. Following maturation, DCs upregulate CCR7 that drives them to CCL19/21 constitutively produced in lymph nodes. tDCs share the same chemokine receptor and migration pattern with immunogenic DC.41,42 We observed a similar pattern of CCR5/7 expression and migration toward CCL3/19 in our LentiVIP-DC. We hypothesize that LentiVIP-DC administered under inflammatory conditions migrate initially to local inflammatory sites. At the inflammatory site LentiVIP-DCs release VIP that inhibits the innate immune response, followed by migration of LentiVIP-DC that take up Ag to draining lymph nodes where they act as tDCs inhibiting T-cell activation. The consistent release of VIP by LentiVIP-DC could provide a local environment rich in endogenous VIP. This might play an important role in reducing local inflammation through direct effects on activated innate immune cells, in addition to inhibition of T-cell proliferation and changes in T-cell differentiation.
In vivo, a single administration of LentiVIP-DC significantly decreased clinical symptoms in EAE and increased survival rate in CLP, and these effects correlated with reductions in the expression of proinflammatory cytokines in spinal cord and peritoneal fluid, respectively. CLP, a severe model of peritoneal sepsis, is considered an inflammatory disorder where innate immunity plays the major role. On the other hand, in EAE, a model for multiple sclerosis, both adaptive and innate immunity play essential roles. We chose these two models based on the fact that LentiVIP-DCs were expected to affect both innate immunity (through local release of VIP) and adaptive immunity (through effects on T cells). Although the results reported here show significant effects of LentiVIP-DC in both models, future studies will have to determine the mechanisms by which the VIP-expressing DCs exert their therapeutic action in vivo.
Some other current approaches for the treatment of autoimmune diseases include transplantation of transduced stem cells expressing self-antigens or immunosuppressive molecules.43,44 However, this raises safety issues due to the persistence and/or their potential malignant transformation of genetically modified cells. Our protocol overcomes some of the current issues of gene therapy. The use of low multiplicity of infection together with the transduction of cells already committed to a specific differentiation pathway decreases significantly the risk of genotoxicity. Although genetically modified DCs seem to have a limited life span in vivo, their effects appear to be long lasting11 (and this study). Therefore, in contrast to the systemic administration of VIP or other immunosuppressive factors, which require repeated, high-dose inoculations, local delivery of VIP by LentiVIP-DC may represent a promising therapeutic tool for the treatment of autoimmune diseases and inflammatory disorders.
Materials and Methods
Plasmids and lentiviral vectors. The human immunodeficiency virus–packaging (pCMVΔR8.91) and vesicular stomatitis virus-G (pMD.G) plasmids45 were kindly provided by D. Trono. The LentiVIP and LentiGFP constructs have been previously described.39
Vector production and titration. Lentiviral vectors were produced by co-transfection of 293T kidney cells (ATCC CRL11268) with three plasmids: (i) vector plasmid (SEWP, LentiVIP, or LentiVIP-GFP), (ii) packaging plasmid pCMVΔR8.9, and (iii) envelope plasmid pMD.G, as previously described46 using GenJet Plus (SignaGen Laboratories, Gaithersburg, MD). Vector titration was performed in 293T cells. LentiGFP vector titration was determined by the percentage of eGFP+ cells by fluorescence-activated cell-sorting analysis 7 days post-transduction. Briefly, cells were harvested and washed 2× in cold phosphate-buffered saline (PBS)–0.1% azide. 2 × 105 cells were resuspended in 200 µl of PBS–azide and analyzed by fluorescence-activated cell-sorting. For titration of LentiVIP vectors, cells were lysed and DNA was extracted 7 days after transduction. Vector copy number per cell was determined using qPCR as described later.
Animals. B10.A, SJL/J, and BALBc mice were obtained from Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice were obtained from Taconic (Hudson, NY). All mice used were between 5 and 7 weeks of age. All animal procedures were approved by the Temple University Institutional Animal Care and Use Committee, on accordance with American Association for the Accreditation of Laboratory Animal Care guidelines.
Reagents. Monoclonal anti-mouse anti-CD11c-APC was purchased from BD Pharmingen (San Diego, CA). Monoclonal anti-CD11c-FITC, anti-CD45RB (B220), anti-CD40-APC (clone 1C10), anti-CD80-APC (clone 16-10A1), anti-CD86-APC (GL1), anti-MHCII-PE (I-Ek) (clone 14-4-4S), anti-CCR5 (clone 7A4), anti-CCR7 (clone 4B12) and their isotypes-APC/PE were purchased from eBioscience (San Diego, CA). Recombinant mouse chemokine ligands CCL3/MIP-1α and CCL19/MIP-3β were purchased from R&D Systems (Minneapolis, MN). Capture and biotinylated anti-mouse IL-12p70, TNF-α, IL-6, IL-10, IL-1β, MIP-1α were purchased from BD Biosciences Pharmingen (San Diego, CA).
Preparation and transduction of murine BMDCs. Bone marrow cells were flushed from femur and tibiae of 5–7 weeks old mice and cultured as previously described.47 On day 4, nonadherent cells were harvested and plated (1 × 106 cells/well) in 48-well plates (cat. no. 351178; BD Falcon, San Diego, CA) in 150 µl of RPMI containing 10% fetal calf serum, 20 ng/ml rmGM-CSF (PeproTech, Rocky Hill, NJ), and 50 µg/ml of protamine sulfate. Lentiviral vectors were added at multiplicity of infection 10. Untransduced cells received the same volume of Opti-MEM media (Invitrogen, Carlsbad, CA). The plates were incubated at 37 °C in a CO2 incubator for 3 hours. The cells were harvested and recultured in the presence of rmGM-CSF for 3 additional days.
DNA preparation and real-time qPCR. Genomic DNA from 1 × 106 cells was isolated by adding 1 ml of SNET extraction buffer (20 mmol/l Tris–HCl pH 8, 5 mmol/l EDTA pH 8, 400 mmol/l NaCl, 1% sodium dodecyl sulfate). qPCR was performed in Mastercycler Realplex2 (Eppendorf, Hamburg, Germany) using 2× SYBR Green PCR Master mix (Applied Biosystems, Foster City, CA). To quantify LentiVIP integrations, we used primers comprising vector (cytomegalovirus promoter) and VIP sequences: forward: 5′-GAG CTC GTT TAG TGA ACC GTC AGA-3′ and reverse: 5′-AAG GAG CTG GGC CTT ATT TCT GGT-3′. For LentiGFP detection, we used the same forward primer and the reverse primer was in the GFP sequence: 5′-TGC TTG TCG GCC ATG ATA TAG A-3′.
Genomic DNA from mouse tissues was isolated using the QIAamp DNA mini kit from Qiagen (Valencia, CA) following the manufacture's instructions.
Determination of VIP levels and cytokines by enzyme-linked immunosorbent assay. On day 7, 1 × 106 transduced DCs were plated in 12-well plates in 1 ml of complete medium and supernatants were harvested 24 hours later. VIP levels were measured using an enzyme immunoassay kit from Peninsula Laboratories (Torrance, CA). Cytokine production was determined in culture supernatants by specific sandwich enzyme-linked immunosorbent assay using capture/biotinylated detection antibodies from BD Biosciences Pharmingen according to the manufacture's recommendations.
Endocytosis assay. Mannose receptor–mediated endocytosis was measured as the cellular uptake of PE-dextran (Sigma, St Louis, MO) and was quantified by flow cytometry. Briefly, DCs (5 × 105 cells) were incubated in medium containing PE-dextran (1 mg/ml; molecular mass 40 kd) for 2 hours at 37 °C. Control cells were incubated at 4 °C. Cells were washed with cold PBS–azide (0.1%) for 3× and analyzed by flow cytometry.
Flow cytometry. For surface markers, 1 × 106 DCs were collected and washed with cold PBS–0.1% sodium azide, stained with antibodies for CD11c, CD40, CD80, CD86, MHC-II, and CD45RB and analyzed using a FACSCalibur flow cytometer (BD Biosciences).
For the staining of CCR5 and 7, we used a triple-indirect immunostaining to enhance the detection after blocking with cold PBS containing 0.1% sodium azide and 2% fetal bovine serum for 45 minutes at 4 °C. A volume of 1 × 106 cells were incubated for 1 hour at 4 °C with 1 µg of anti-CCR5 and anti-CCR7 followed by 1 µg of secondary-biotinylated antibody for 1 hour. After washing, the cells were incubated with 0.5 µg of APC-conjugated streptavidin for 45 minutes at 10 °C.
VIPase antibody treatment. The VIPase antibody (clone C23.5 IgG) was a gift from Dr Paul (University of Texas Medical School, Houston, TX). The control antibody was UPC10 (IgG2aκ) (Sigma). After LentiVIP transduction, 1 × 106 DCs were plated into a 12-well plate and daily doses of VIPase were added to the cultures (final concentration 180 µg/ml).
Mixed lymphocyte reaction assay. Different numbers of SJL/J BMDC (from 1.25 × 104 to 1 × 105 cells) were cocultured in quadruplicate with C57BL/6 splenic CD4+ T cells (5 × 104). Splenic CD4+ T cells were purified by positive selection using the autoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). 4 days later, cell proliferation was measured by [3H] thymidine uptake and was expressed as counts per minute.
Cell migration assay. The 24-well transwell plates were coated with 50 µl of fibronectin (10 µg/ml in PBS pH 8.8) O/N at 4 °C followed by blocking 30 minutes at 37 °C with 200 µl of RPMI-1640 containing 0.2% bovine serum albumin. DCs were collected, washed in PBS, and resuspended in RPMI-1640. A volume of 2.5 × 105 DCs were placed on top of transwell inserts (6.5 mm diameter and 5 µm pore size) (Corning, Lowell, MA). The chemokines (100 ng/ml) used in each case were added to the lower compartment of the transwell. The number of cells migrated to the lower chamber after 4 hours was determined by fluorescence-activated cell-sorting (120-second counts).
RNA extraction, reverse transcription reaction, and real-time PCR. To prepare total RNA from cells we used Ultraspec (Biotecx, Houston, TX) and followed the manufacture's instructions. To extract total RNA from spinal cord we used Trizol (Invitrogen) according to the manufacturer's recommendations. We used 2 µg of total RNA for the reverse transcription reaction, which was performed with 300 U of Moloney murine leukemia virus reverse transcriptase, 40 U of RNasin, Moloney murine leukemia virus buffer, 500 ng of random primers, 1% BSA and 500 µmol/l of dNTP mix, in a total volume of 30 µl. All reagents were supplied from Promega (Madison, WI). Before adding the mix to the RNA, the samples together with random primers were heated at 70 °C for 5 minutes and chilled immediately in ice. The reverse transcription reaction was performed at 37 °C for 90 minutes.
The primers used to amplify are in Supplementary Table S1.
EAE induction. Female SJL/J and C57BL/6 mice (6 weeks old, 17–20 g) were obtained from Jackson Laboratories and Taconic, respectively. PLP139–151 (HCLGKWLGHPDKF) and MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptides were synthesized by Peptides International (Louisville, KY) and Anaspec (San Jose, CA) respectively.
To induce relapsing–remitting-EAE, SJL/J mice were immunized subcuataneously with 200 µg of PLP139–151 emulsified in complete Freund's adjuvant containing 5 mg/ml of Mycobacterium tuberculosis H37 RA (Difco, Detroit, MI) on day 0. Mice received intraperitoneally 200 ng of pertussis toxin (Sigma) on days 0 and 2. For chronic EAE, C57BL/6 mice were immunized subcuataneously with 200 µg of MOG35–55 on days 0 and 7 and received 200 ng of pertussis toxin intraperitoneally on days 0 and 2.
Mice were scored daily for clinical signs of EAE according to the following clinical scoring system: 0, no clinical signs; 0.5, partial loss of tail tonicity; 1, complete loss of tail tonicity; 2, flaccid tail and abnormal gait; 3, hind legs paralysis; 4, hind legs paralysis with hind body paresis; 5, hind and fore leg paralysis; and 6, death. If necessary, food and hydrogel were provided on the cage floor.
CLP. BALB/c mice (6–8 weeks old) were anesthetized with 30 µl of ketamine (80 mg/kg) and xylazine (10 mg/kg) given intraperitoneally Under aseptic conditions, a 2-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture at its base, below the ileo-cecal valve, and was perforated once with a 19½-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation sites, returned to the peritoneal cavity, and the laparotomy was closed with 4.0 silk sutures. The animals were returned to their cages with free access to food and water.
Statistical analysis. The results were expressed as mean ± SD of at least three independent experiments. The Student t-test was used to compare control and experimental groups. Statistical significance was based on P values <0.05.
SUPPLEMENTARY MATERIALFigure S1. BMDC are efficiently transduced with lentiviral vector LentiGFP. (a) Effect of protamine sulfate. BMDC were transduced as described in Methods with LentiGFP at MOI 10 in the presence of different concentrations of PS. Transduction efficiency is determined by percentage of eGFP+ cells. (b) Density plots of LentiGFP-DC by FACS. (c). Light and fluorescent microscopy: Upper panels: Untransduced DC. Lower panels: LentiGFP-DC.Figure S2. CCR5 and CCR7 patterns in LentiVIP-DC. CCR5 and CCR7 mRNA expression in untransduced-, LentiGFP- and LentiVIP-DC in the presence and absence of LPS (0.1 μg/ml). Semiquantitative PCR were performed using GAPDH as a housekeeping gene.Table S1. Primers for qPCR.
Supplementary Material
BMDC are efficiently transduced with lentiviral vector LentiGFP. (a) Effect of protamine sulfate. BMDC were transduced as described in Methods with LentiGFP at MOI 10 in the presence of different concentrations of PS. Transduction efficiency is determined by percentage of eGFP+ cells. (b) Density plots of LentiGFP-DC by FACS. (c). Light and fluorescent microscopy: Upper panels: Untransduced DC. Lower panels: LentiGFP-DC.
CCR5 and CCR7 patterns in LentiVIP-DC. CCR5 and CCR7 mRNA expression in untransduced-, LentiGFP- and LentiVIP-DC in the presence and absence of LPS (0.1 μg/ml). Semiquantitative PCR were performed using GAPDH as a housekeeping gene.
Primers for qPCR.
Acknowledgments
This work was supported by NIH/NIAID R01AI052306 and Alfonso Martin Escudero Foundation. We thank Alexis Agelan (Temple University), for his support with animal work. We are also grateful to Didier Trono (Geneva, Switzerland) for supplying us with HIV-packaging pCMVΔR8.91 and envelope pMD.G plasmids and Sudhir Paul (University of Texas Medical School, Houston, TX) who provided us with the VIPase antibody. We thank Varshana Gurusamy for her technical support.
REFERENCES
- Wolfraim LA. Treating autoimmune diseases through restoration of antigen-specific immune tolerance. Arch Immunol Ther Exp (Warsz) 2006;54:1–13. doi: 10.1007/s00005-006-0001-7. [DOI] [PubMed] [Google Scholar]
- Roth JC, Curiel DT., and , Pereboeva L. Cell vehicle targeting strategies. Gene Ther. 2008;15:716–729. doi: 10.1038/gt.2008.38. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Banchereau J., and , Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steptoe RJ., and , Thomson AW. Dendritic cells and tolerance induction. Clin Exp Immunol. 1996;105:397–402. doi: 10.1046/j.1365-2249.1996.d01-779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F., and , Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Schmitt E, Bonifaz L, Enk AH., and , Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D., and , Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H., and , Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998;66:1567–1574. doi: 10.1097/00007890-199812270-00001. [DOI] [PubMed] [Google Scholar]
- Lee WC, Zhong C, Qian S, Wan Y, Gauldie J, Mi Z, et al. Phenotype, function, and in vivo migration and survival of allogeneic dendritic cell progenitors genetically engineered to express TGF-β. Transplantation. 1998;66:1810–1817. doi: 10.1097/00007890-199812270-00040. [DOI] [PubMed] [Google Scholar]
- Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, et al. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001;107:1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Pozo D., and , Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J., and , Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Keino H, Kezuka T, Takeuchi M, Yamakawa N, Hattori T., and , Usui M. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol. 2004;122:1179–1184. doi: 10.1001/archopht.122.8.1179. [DOI] [PubMed] [Google Scholar]
- Herrera JL, Fernández-Montesinos R, González-Rey E, Delgado M., and , Pozo D. Protective role for plasmid DNA-mediated VIP gene transfer in non-obese diabetic mice. Ann N Y Acad Sci. 2006;1070:337–341. doi: 10.1196/annals.1317.041. [DOI] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D., and , Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102:13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E., and , Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- Pozo D, Anderson P., and , Gonzalez-Rey E. Induction of alloantigen-specific human T regulatory cells by vasoactive intestinal peptide. J Immunol. 2009;183:4346–4359. doi: 10.4049/jimmunol.0900400. [DOI] [PubMed] [Google Scholar]
- Fraccaroli L, Alfieri J, Larocca L, Calafat M, Roca V, Lombardi E, et al. VIP modulates the pro-inflammatory maternal response, inducing tolerance to trophoblast cells. Br J Pharmacol. 2009;156:116–126. doi: 10.1111/j.1476-5381.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL., and , McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- Penna G., and , Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Paul S, Sun M, Mody R, Tewary HK, Stemmer P, Massey RJ, et al. Peptidolytic monoclonal antibody elicited by a neuropeptide. J Biol Chem. 1992;267:13142–13145. [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland HF., and , Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu X, Hsu HC, Tousson A, Yang PA, Wu Q, et al. CII-DC-AdTRAIL cell gene therapy inhibits infiltration of CII-reactive T cells and CII-induced arthritis. J Clin Invest. 2003;112:1332–1341. doi: 10.1172/JCI19209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Tesmer LA, Hindnavis V, Endres JL., and , Fox DA. Interleukin-17 as a molecular target in immune-mediated arthritis: immunoregulatory properties of genetically modified murine dendritic cells that secrete interleukin-4. Arthritis Rheum. 2007;56:89–100. doi: 10.1002/art.22311. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Yu Q, Piraino ST, Pennington SE, Shankara S, Woodworth LA, et al. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J Immunol. 1999;163:699–707. [PubMed] [Google Scholar]
- Roe T, Reynolds TC, Yu G., and , Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J, Latouche JB, Schnell S., and , Sadelain M. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97:114–121. doi: 10.1182/blood.v97.1.114. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Keating K., and , Thorpe R. Comparison of toxicogenomic profiles of two murine strains treated with HIV-1-based vectors for gene therapy. Toxicol Appl Pharmacol. 2007;225:189–197. doi: 10.1016/j.taap.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Dullaers M, Bonehill A, van Meirvenne S, Heirman C, de Greef C, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–667. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang J, Mi Z, Robbins P., and , Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- Henry E, Desmet CJ, Garzé V, Fiévez L, Bedoret D, Heirman C, et al. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J Immunol. 2008;181:7230–7242. doi: 10.4049/jimmunol.181.10.7230. [DOI] [PubMed] [Google Scholar]
- Delgado M, Toscano MG, Benabdellah K, Cobo M, O'Valle F, Gonzalez-Rey E, et al. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum. 2008;58:1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- Noser JA, Towers GJ, Sakuma R, Dumont JM, Collins MK., and , Ikeda Y. Cyclosporine increases human immunodeficiency virus type 1 vector transduction of primary mouse cells. J Virol. 2006;80:7769–7774. doi: 10.1128/JVI.02427-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE., and , Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Chan J, Ban EJ, Chun KH, Wang S, Bäckström BT, Bernard CC, et al. Transplantation of bone marrow transduced to express self-antigen establishes deletional tolerance and permanently remits autoimmune disease. J Immunol. 2008;181:7571–7580. doi: 10.4049/jimmunol.181.11.7571. [DOI] [PubMed] [Google Scholar]
- Makar TK, Trisler D, Bever CT, Goolsby JE, Sura KT, Balasubramanian S, et al. Stem cell based delivery of IFN-β reduces relapses in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;196:67–81. doi: 10.1016/j.jneuroim.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MG, Frecha C, Ortega C, Santamaría M, Martín F., and , Molina IJ. Efficient lentiviral transduction of Herpesvirus saimiri immortalized T cells as a model for gene therapy in primary immunodeficiencies. Gene Ther. 2004;11:956–961. doi: 10.1038/sj.gt.3302259. [DOI] [PubMed] [Google Scholar]
- Jing H, Yen JH., and , Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem. 2004;279:55176–55186. doi: 10.1074/jbc.M409816200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BMDC are efficiently transduced with lentiviral vector LentiGFP. (a) Effect of protamine sulfate. BMDC were transduced as described in Methods with LentiGFP at MOI 10 in the presence of different concentrations of PS. Transduction efficiency is determined by percentage of eGFP+ cells. (b) Density plots of LentiGFP-DC by FACS. (c). Light and fluorescent microscopy: Upper panels: Untransduced DC. Lower panels: LentiGFP-DC.
CCR5 and CCR7 patterns in LentiVIP-DC. CCR5 and CCR7 mRNA expression in untransduced-, LentiGFP- and LentiVIP-DC in the presence and absence of LPS (0.1 μg/ml). Semiquantitative PCR were performed using GAPDH as a housekeeping gene.
Primers for qPCR.