Abstract
Studies in mice indicate that gene transfer to liver with vectors based on adeno-associated viruses (AAVs) is characterized by immunological tolerance to antigenic transgene products. Mechanisms to explain host nonresponsiveness have focused on aberrant T-cell responses. We propose a distinct mechanism for conferring tolerance to AAV-transduced hepatocytes that relates to diminished sensitivity of the target organ to T cell–mediated effects. T cells to β-galactosidase (β-gal) were adoptively transferred into RAG−/− mice expressing β-gal in hepatocytes due to prior administration of either Ad or AAV vectors. Adoptive transfer was associated with extinction of LacZ expression in Ad-LacZ-transduced RAG−/− mice and had no effect on liver LacZ expression in AAV-LacZ-transduced RAG−/− mice. Systemic administration of TLR ligands lipopolysaccharide (LPS) and CpG at the time of adoptive transfer did lead to extinction of LacZ expression. Systemic TLR ligands were associated with upregulation of major histocompatibility complex (MHC) class I and the cell adhesion molecules ICAM and VCAM as was seen with Ad-LacZ alone. These data indicate that AAV transduction lacks the inflammatory signals necessary to render hepatocyte targets for cytotoxic T lymphocytes (CTLs). Underlying liver pathology may confound vector performance and should be considered in the design of clinical trials.
Introduction
The immunology of in vivo gene therapy with viral vectors has emerged as a critical path toward their successful application in the clinic. Adaptive and innate immune responses to the vector and/or vector-transduced cells are recurring problems that limit efficacy and worsen toxicity. The problem of innate and adaptive immune responses to viral vectors in vivo was initially investigated for adenovirus vectors. Systemic administration of recombinant adenoviruses was associated with relatively high levels of transduction, although expression was transient, and associated with inflammation of the target organ; attempts to readminister the vector were unsuccessful. The loss of transgene expression that occurred subsequent to vector administration was shown to be dependent on major histocompatibility complex (MHC) class I–restricted cytotoxic T lymphocytes (CTLs) directed to both viral proteins and antigenic transgene products.1 Our initial studies suggested that the strong CD8 T-cell responses were due to efficient transduction of antigen-presenting cells as well as an inherent adjuvanticity of the adenovirus that also activates the antigen-presenting cell.2
Vectors based on adeno-associated viruses (AAVs) have demonstrated interesting results following in vivo gene transfer to liver. Mice administered AAV vectors systemically failed to activate CTLs even to highly antigenic transgenes resulting in limited toxicity and prolonged transgene expression.3,4 A variety of mechanisms have been proposed to explain the apparent tolerance of mice to AAV-mediated gene transfer to liver all of which focus on dysfunctional and/or diminished activation of T cells. Our initial studies suggested that AAV very poorly transduces antigen-presenting cells leading to a state of immunologic ignorance.2 Other investigators suggested that antigen-specific T cells were indeed formed, although, in the absence of inflammation or co-stimulation, they were nonfunctional or anergic.5 Most recently, Herzog and colleagues provided data to suggest the presence of Tregs following liver-directed gene transfer leading to suppression of CTL responses.6,7 We recently extended this work showing that liver macrophages and Tregs work in concert to induce a state of tolerance.8
We initiated studies of AAV-mediated gene transfer to liver in larger animals such as rhesus and cynomolgus macaques as the next step toward clinical translation. The high efficiency of hepatocyte transduction achieved in mice with the novel AAV serotypes was indeed confirmed in nonhuman primates.9 An important difference, however, was that macaques elicit vibrant CTL responses to foreign transgenes when expressed from nontissue-specific, constitutive promoters. Most recently, we have shown that liver-specific promoters diminish the activation of CTLs to antigenic transgenes and prolong transgene expression in nonhuman primates.10
We propose in this article the existence of a mechanism of tolerance to AAV-encoded antigens, independent of dysfunctional/diminished T-cell activation, which is based on the lack of inflammatory signals necessary to render the transduced cells/target organ sensitive to the effector activities of CTLs. Our previous work with adenovirus vector–mediated gene transfer to liver supported this hypothesis. We showed delayed clearance of adenovirus-transduced hepatocytes in immunocompetent mice depleted of IFN-γ. These animals did indeed mount a robust CTL response, although, in the absence of IFN-γ, there was no upregulation of MHC class I in target hepatocytes.11 A more recent study by the Zinkernagel group confirmed the importance of hepatic innate immunity in the context of an effective CTL response to lymphocytic choriomeningitis virus–mediated hepatitis.12 In the absence of systemic activation of innate immunity, CTLs against lymphocytic choriomeningitis virus antigens expressed in hepatocytes failed to traffic to liver and exert their effector activity.
Results
Our experimental strategy was to separate the steps involved in T-cell activation from those that may impact on the receptivity of the AAV-transduced liver to T-cell effector functions. This was accomplished in a model wherein transgene-specific CD8 T cells from donor C57BL/6 mice immunized with Ad-LacZ were adoptively transferred to recipient immunocompromised RAG−/− mice previously transduced in the liver with Ad-LacZ or AAV-LacZ. The use of RAG−/− mice is well suited to dissect the role of donor CTLs versus recipient sensitivity to CTLs without interference from T and B cells of the recipient.
β-Gal-specific CTLs eliminate Ad-LacZ-transduced hepatocytes
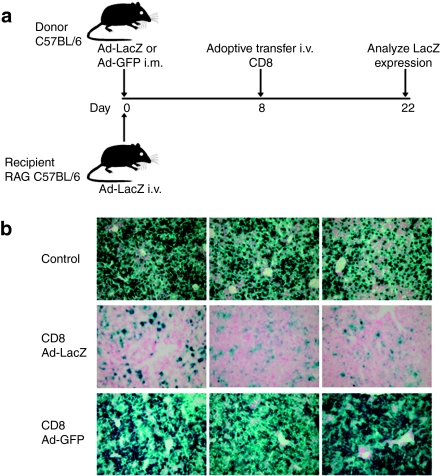
We first examined the effect of CTLs on Ad-transduced hepatocytes. To this end, we adoptively transferred β-gal-specific CD8 T cells from donor C57BL/6 mice into recipient RAG−/− mice to study the robustness of CTLs in eliminating transgene expression (Figure 1a). CD8 T cells for adoptive transfer were isolated from spleen 8 days following intramuscular (i.m.) injection of 5 × 1010 vector genomes (VG) of Ad-LacZ for 8 days that represents the peak of the β-gal-specific T-cell response. Donor T cells (2–4 × 106) were then adoptively transferred to recipient RAG−/− mice that had previously received 5 × 1010 VG of Ad-LacZ by an intravenous (i.v.) injection. As expected, in RAG−/− mice that did not receive CTLs and those that received CTLs from naive donors (Figure 1b), there was persistent β-gal expression in the liver due to the lack of a CTL response. However, mice that received CTLs from Ad-LacZ-immunized donors had nearly complete loss of transgene expression (Figure 1b). Adoptive transfer of CTLs from donor mice immunized with an Ad vector expressing an irrelevant transgene, Ad-GFP, also failed to eliminate β-gal expression suggesting that any CTLs generated to Ad viral proteins in the donor are not sufficient to target recipient hepatocytes because of vector-encoded viral gene expression (Figure 1b).
Figure 1.
β-gal-specific CTLs are sufficient to eliminate Ad-transduced hepatocytes. (a) CD8+ T cells were purified from splenocytes of C57BL/6 donor mice immunized i.m. with Ad-LacZ or Ad-GFP 8 days earlier. Purified CD8+ T cells (2–4 × 106) were then adoptively transferred into RAG−/− recipient mice administered i.v. with Ad-LacZ 8 days earlier. Control mice received CD8+ T cells purified from naive mice. (b) At 14 days after adoptive transfer, mice were euthanized, and the liver tissues were evaluated for β-gal expression by X-gal histochemistry. Columns show representative images from three individual mice. CTL, cytotoxic T lymphocyte; GFP, green fluorescent protein; i.m., intramuscular.
Inflammatory responses are crucial for CTL-mediated elimination of AAV-transduced hepatocytes
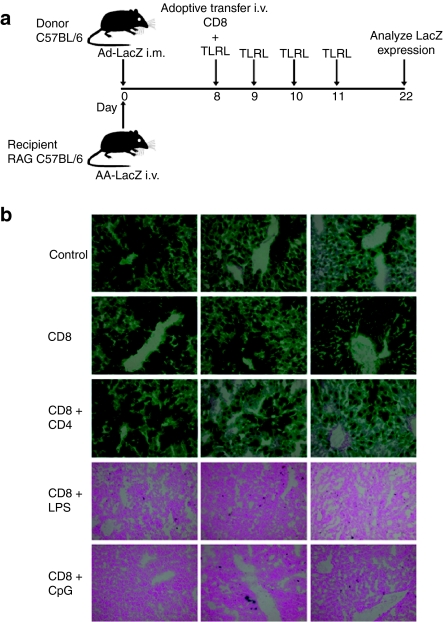
In order to study the effect of CTLs on AAV-transduced hepatocytes, we adoptively transferred Ad-LacZ-specific CTLs, isolated from donor C57BL/6 mice, to RAG−/− mice expressing AAV-LacZ (Figure 2a). There was no effect on β-gal expression in control mice that did not receive CTLs or in mice adoptively transferred with CTLs from naive mice (Figure 2b). Surprisingly, CD8 T cells isolated from Ad-LacZ-immunized mice also failed to eliminate β-gal-expressing hepatocytes in RAG−/− mice (Figure 2b). It has been observed that adoptive transfer of CD4 T cells is essential for prolonged expansion and survival of CD8 T cells.13 However, a co-transfer of CD4 and CD8 T cells from immunized mice did not result in loss of LacZ expression (Figure 2b). Because an earlier study reported the requirement for inflammation via ligation of TLR3 in the elimination of target cells in the liver,12 we assessed the role of inflammation in modulating the adaptive immune response by co-administering CD8 T cells plus daily injection of TLR ligands lipopolysaccharide (LPS) or CpG oligonucleotides (CpG) for 4 days. Interestingly, mice that received LPS or CpG plus LacZ-specific CTLs showed a complete loss of LacZ expression in hepatocytes (Figure 2b).
Figure 2.
Inflammatory signals induce CTL-mediated elimination of AAV-transduced hepatocytes. (a) RAG−/− recipient mice administered i.v. with AAV-LacZ 8 days earlier were adoptively transferred with β-gal-specific CD8+ T cells alone (CD8), with CD8+ T cells plus CD4+ T cells (CD8 + CD4), with CD8+ T cells plus daily LPS injections (CD8 + LPS) or with CD8+ T cells plus daily CpG injections (CD8 + CpG). Controls were transferred with naive CD8+ T cells. (b) At 14 days after adoptive transfer, mice were euthanized, and the liver tissues were evaluated for β-gal expression by X-gal histochemistry. Columns show representative images from three individual mice. CTL, cytotoxic T lymphocyte; i.m., intramuscular; LPS, lipopolysaccharide.
Although co-administration of LPS or CpG along with CTLs resulted in elimination of AAV-transduced cells, it was unclear whether inflammation by itself was sufficient to eliminate transgene expression. We therefore studied the role of inflammation by administering these TLR ligands along with CD8 T cells that had been activated to the irrelevant transgene, GFP. As shown in Figure 3a, CD8 T cells were isolated from donor C57BL/6 mice injected i.m. with 5 × 1010 VG of Ad-GFP and adoptively transferred to AAV-LacZ-expressing RAG−/− mice that received concurrent daily injection of LPS or CpG for 4 days. The use of donor T cells from Ad-GFP immunized mice allowed us to examine the effect of inflammation in the presence of nontransgene-specific CD8 T cells. Adoptive transfer of naive CTLs or GFP-specific CTLs caused no diminution in β-gal expression (Figure 3b). More importantly, LacZ expression also remained stable upon co-administration of either LPS or CpG along with the transferred CTLs (Figure 3b). These results indicate that only a combination of LacZ-specific CTLs and inflammatory responses, induced by LPS or CpG, can abrogate AAV-mediated β-gal expression in hepatocytes.
Figure 3.
Inflammatory signals in the absence of transgene-specific CTLs fail to eliminate transgene expression. (a) RAG−/− recipient mice administered i.v. with AAV-LacZ 8 days earlier were adoptively transferred with GFP-specific CD8+ T cells alone (CD8), with CD8+ T cells plus daily LPS injections (CD8 + LPS), or with CD8+ T cells plus daily CpG injections (CD8 + CpG). Controls were transferred with naive CD8+ T cells. (b) At 14 days after adoptive transfer, mice were euthanized, and the liver tissues were evaluated for β-gal expression by X-gal histochemistry. Columns show representative images from three individual mice. CTL, cytotoxic T lymphocyte; i.m., intramuscular; LPS, lipopolysaccharide.
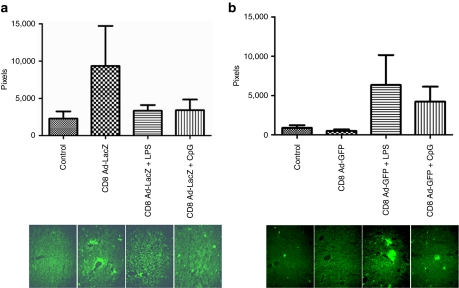
To investigate whether there was evidence of CTL killing of hepatocytes, we quantified AAV-LacZ vector genomes in livers 14 days after adoptive transfer from mice described in Figures 2 and 3. AAV-LacZ genomes were persistent in control mice that did not receive CD8 T cells, as well as in those mice that received Ad-LacZ-specific CD8 T cells (Figure 4a). However, adoptive transfer of CD8 T cells plus LPS or CpG dramatically reduced AAV vector genomes, when compared to control animals (Figure 4a). As expected, there was no loss of vector genomes in mice adoptively transferred with CD8 T cells from Ad-GFP immunized mice even in the presence of inflammation (Figure 4b). These results support the hypothesis that loss of β-gal expression was due to CTL-mediated elimination of AAV-LacZ vector genomes, although the mechanism by which this occurred, e.g., actual destruction of the target cells or inflammation-induced destabilization of the vector genome, was not investigated.
Figure 4.
Elimination of AAV-transduced hepatocytes correlates with the loss of genome copies. (a) CD8+ T cells were purified from splenocytes of C57BL/6 donor mice immunized i.m. with Ad-LacZ 8 days earlier. Then, RAG−/− recipient mice (n = 3 per group) administered i.v. with AAV-LacZ 8 days earlier were adoptively transferred with CD8+ T cells alone (CD8 Ad-LacZ), with CD8+ T cells plus daily LPS injections (CD8 Ad-LacZ + LPS), or with CD8+ T cells plus daily CpG injections (CD8 Ad-LacZ + CpG). Controls were transferred with naive CD8+ T cells. (b) CD8+ T cells were purified from splenocytes of C57BL/6 donor mice immunized i.m. with Ad-GFP 8 days earlier. Then, RAG−/− recipient mice (n = 3 per group) administered i.v. with AAV-LacZ 8 days earlier were adoptively transferred with CD8+ T cells alone (CD8 Ad-GFP), with CD8+ T cells plus daily LPS injections (CD8 Ad-GFP + LPS), or with CD8+ T cells plus daily CpG injections (CD8 Ad-GFP + CpG). Controls were transferred with naive CD8+ T cells. (a,b) At 14 days after adoptive transfer, mice were euthanized, total cellular DNA was extracted from the liver, and AAV2/8 vector genomes were quantified by real-time PCR. AAV, adeno-associated virus; GC, genome copies; GFP, green fluorescent protein; i.m., intramuscular; LPS, lipopolysaccharide.
The additional requirement for inflammation to eliminate AAV-LacZ but not Ad-LacZ-transduced hepatocytes indicates that LacZ-specific CD8 T cells may not effectively traffic to the liver. AAV-LacZ transduced mice adoptively transferred with splenocytes from Ad-LacZ or Ad-GFP immunized donors were evaluated for infiltration of CD8 T cells at the time of necropsy. Figure 5 shows representative immunohistochemical micrographs and morphometric quantifications of CD8 T cells in histological sections from animals that received cells from Ad-LacZ (Figure 5a) or Ad-GFP (Figure 5b) immunized mice. Significantly, more CD8 T cells were detected in livers of animals who received cells from Ad-LacZ immunized animals than those from either naive animals (labeled control) suggesting substantial migration of transgene-specific CTLs to the liver. Inflammation induced by systemic TLR ligands in the absence of antigen-specific T cells did increase the presence of CD8 T cells in liver tissue (Figure 5b). However, the number of CD8 T cells in liver were diminished following adoptive transfer of transgene-specific T cells in the presence of systemic TLR ligands when evaluated 14 days later (Figure 5a), which was the only condition that lead to apparent in vivo CTL activity. We believe the apparent reduction of CD8 T cells is due to activation-induced T-cell death that occurs in the context of CTL effector activity. Loss of CTLs following strong activation may also be exaggerated due to the absence of IL-2-secreting CD4 T cells in the immunocompromised recipient mice.
Figure 5.
Role of inflammation in CTL targeting to the liver. RAG−/− mice that received AAV-LacZ were adoptively transferred with (a) Ad-LacZ or (b) Ad-GFP CTLs, and killed 7 days later. Where indicated, LPS or CpG was coadministered for 4 days starting on the day of adoptive transfer. Control represents AAV-LacZ transduced mice adoptively transferred with splenocytes from naive donors. Livers were sectioned and stained for the presence of CD8 T cells by indirect immunofluorescence. Histograms show the average number of CD8 staining pixels from three mice per group; corresponding microscopy images from one animal in each group are shown below. CTL, cytotoxic T lymphocyte; GFP, green fluorescent protein.
We also evaluated the possibility that the TLR-induced inflammation enhanced apparent CTL killing by promoting the expansion of adoptively transferred CTLs. This was evaluated by labeling the donor cells with CFSE prior to transplantation and subsequent recovery of splenocytes and analysis by flow cytometry. There was no difference in the abundance or fluorescence profile of the recovered CFSE positive cells in animals that did or did not receive TLR ligands (data not shown).
Upregulation of class I MHC molecules and cell adhesion molecules
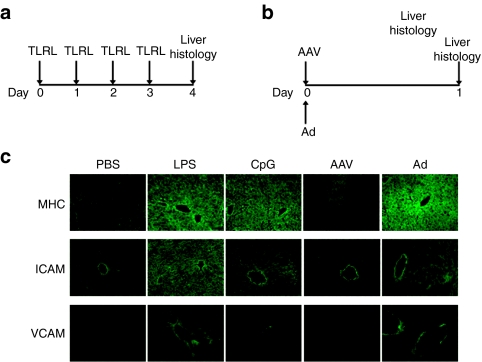
To test the role of inflammation in enhancing the CTL response, we analyzed the expression of molecules involved in peptide “display” (MHC class I) along with those involved in CTL homing to the liver (VCAM-1 and ICAM-1). C57BL/6 mice received daily injection of LPS or CpG for 4 days, and on day 5, livers were isolated and analyzed for upregulation of molecules (Figure 6a). A separate group of mice received either AAV-LacZ or Ad-LacZ, and livers were isolated 1 day later for analysis (Figure 6b). LPS or CpG treatment resulted in a strong upregulation of MHC class I, VCAM-1 and ICAM-1 at the 24-hour time point, in comparison to control mice injected with phosphate-buffered saline (Figure 6c). A similar increase in the expression of all three molecules was also observed 24 hours following administration of Ad-LacZ (Figure 6c). Notably, no upregulation was observed in any of the molecules following administration of AAV-LacZ (Figure 6c). These results indicate that CTL homing to the liver, and increased antigen display to CTLs, may be the mechanisms mediating the elimination of AAV-LacZ expression.
Figure 6.
Inflammatory signals enhance the expression of MHC class I and cell adhesion molecules. C57BL/6 mice (n = 3 per group) received (a) daily LPS or CpG injections for 4 days, (b) AAV-LacZ or Ad-LacZ injection. (c) Mice were euthanized 1 day (Ad and AAV) or 5 days (LPS and CpG) later, and liver tissue harvested and stained for MHC class I, ICAM-1 and VCAM-I expression. Control mice were injected with PBS. MHC, major histocompatibility complex; PBS, phosphate-buffered saline.
Discussion
Many studies have reported stable expression of nonself-transgenes following AAV gene transfer to the mouse liver (reviewed in ref. 14). Such persistent gene expression was mediated by splenic,6,15,16 as well as by combined action of liver-resident Tregs and Kupffer cells8 that actively suppress the CTL responses to AAV-encoded transgenes. Additionally, such active suppression continued to persist even when AAV-administered mice were challenged with an Ad vector that is known to induce a robust CTL response (E. Breous, S. Somanathan, P. Bell, and J.M. Wilson, unpublished results).6 The ability of AAV to circumvent activation of effector T cells is a potential safety advantage in several clinical applications of gene therapy such as gene replacement in recessive genetic diseases, delivery of therapeutic proteins containing potential T-cell epitopes, and the use of nonmammalian transcription factors to pharmacologically regulate transgene expression.
The ability of AAV to efficiently transduce hepatocytes in vivo without activating effector T-cell responses is not a universal finding. Activation of T cells following gene transfer to liver has been demonstrated in mice and is a function of dose, strain of mice, promoter, and capsid structure.16,17 Importantly, larger animals that presumably would include humans are less tolerant to AAV-encoded antigens as it relates to both T- and B-cell responses.9,18 Finally, one can envision situations in which Treg-mediated tolerance is broken subsequent to the initial gene therapy. In evaluating the consequences of these scenarios, we asked the question: will AAV-transduced hepatocytes be effectively targeted by CTLs if the gene therapy recipient did generate transgene-specific T cells? The answer is yes when there are inflammatory signals present to promote migration of T cells and enhance MHC class I presentation.
This finding has important implications in the preclinical and clinical development of gene therapy. One aspect of preclinical studies of gene therapy that has been difficult to reconcile is the fairly significant differences in host-vector responses observed in different species such as enhanced innate immunity to adenovirus vectors observed in primates as compared to mice19,20 and the appearance of destructive T-cell responses to AAV-encoded antigens in primates not routinely observed in mice.9 It is possible that some of this species-specific variation is caused by genetic or environmental differences that modulate the sensitivity of the target organ to effector T-cell responses. Such differences are evident in studies that noted increased inflammation following portal vein delivery of AAV vectors to hepatitis C virus–positive chimpanzees when compared to hepatitis C virus–negative animals.21 Similarly, gender-specific differences in the expression from AAV vectors in mice have been observed,22 which may then influence the ensuing innate and adaptive immune response. In this regard, one may better simulate the biology of the human host in mouse models by exposing the mice to toxic, infectious, or allergic stimuli that induce chronic or episodic activation of innate immunity in humans.
The present study was limited to analyzing the impact of inflammation on the T-cell response to AAV-encoded transgenes. It is important to note that inflammation may also have an effect on the T-cell response to the AAV capsid antigens. Indeed, the influence of inflammation on AAV capsid-specific T cells may be pronounced in gene therapy recipients who are likely to mount an anamnestic T-cell response.
The dramatic impact that inflammation has on the outcome of AAV gene therapy, as shown in our study, should be considered in the design of clinical trials. A recent study showed that adaptive immune responses to AAV-encoded transgenes were diminished in TLR9−/− mice.23 Although the serotype of AAV used (AAV2) as well as route of administration (i.m.) were different from those used in our study, this report suggests a role for inflammatory mediators in enhancing transgene-specific adaptive immune responses. Conditions leading to liver inflammation at the time of gene transfer such as underlying liver pathology due to the disease being treated or concomitant disease may have a further bearing on the adaptive immune response and should be avoided. It is also possible that the development of liver inflammation subsequent to gene transfer due to an unrelated event may provoke adaptive immune responses to the transduced cells by promoting T-cell migration and/or T cell–mediated killing. This scenario presumes either the existence of vector/transgene-specific T cells at the time of initial gene transfer or the reactivation of suppressed T cells subsequent to gene transfer.
Materials and Methods
Vectors. The E1/E3-deleted adenovirus type 5 vector, expressing a nuclear targeted form of β-galactosidase under control of a chicken β-actin promoter (Ad.CB.nLacZ), and the AAV8-pseudotyped vector, expressing a similar transgene cassette, were obtained from Penn Vector Core at the University of Pennsylvania. The adenovirus vectors used in the study were correct by restriction analysis, and both adenovirus and AAV vectors had endotoxin levels below the level of detection (<20 EU/ml). Physical titer of the adenovirus vector was determined by optical density and of the AAV vector by PCR.
Adoptive transfer of CD4 and CD8 T cells. All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (University of Pennsylvania, Bar Harbor, ME). Six- to eight-week-old age-matched male C57BL/6 mice (Jackson Laboratory) were injected i.m. with Ad5-LacZ (5 × 1010 VG). Splenocytes were harvested 8 days later, and CD8 or CD4 T cells were isolated using MACS cell separation kits (Miltenyi Biotec, Auburn, CA). The purity of the isolated cells was >95% as determined by subsequent flow cytometry (FACS) analysis. Freshly isolated CD8 or CD4 cells (2–4 × 106/mouse) were adoptively transferred by i.v. injection (tail vein) to congenic mice homozygous for the RAG1−/− mutation (Jackson Laboratory).
Analysis of LacZ, MHC class I, VCAM-1 and ICAM-1 expression. Animals were killed 14 days after adoptive transfer of cells, and livers were sectioned and stained for β-gal expression. Additionally, MHC class I, VCAM-1 and ICAM-1 expression was detected by indirect immunofluorescent staining of livers. Control animals injected with only TLR ligands were killed 24 hours after final injection (day 5). Similarly, control animals that only received AAV-LacZ or Ad-LacZ were killed 1 day later. All images were collected on a Nikon microscope (Nikon, Melville, NY) fitted with a digital camera.
Quantitative PCR analysis. DNA was isolated from livers using the QIAamp DNA mini kit (Qiagen, Valencia, CA). For real-time TaqMan PCR, a TBG promoter assay was designed against the TBG moiety present in the cis-plasmids used to manufacture the target vectors. The assay is as follows: TBG Fwd: AAACTGCCAATTCCACTGCTG; TBG Rev: CCATAGGCAAAAGCACCAAGA; and TBG Probe: 6FAM-TTG GCC CAA TAG TGA GAA CTT TTT CCT GC-TAMRA. All PCR reactions were carried out in duplicate sets and included 100 ng of sample.
TLR ligands. TLR 4 (Ultra-Pure E. coli LPS) and TLR 9 ligand (Type C CpG oligonucleotide) were purchased from Invivogen, San Diego, CA. Mice were administered i.p. with LPS (10 ng) or CpG (50 µg) on the day of adoptive transfer. Ligands were given for 3 additional days for a total of 4 consecutive days of administration.
Acknowledgments
We thank the Animal Models Program, Cell Morphology Core and the Quality Control Core personnel (University of Pennsylvania Gene Therapy Program) for help with the rodent studies, processing of tissue samples, histological staining, and quantitative PCR. We also thank Penn Vector at the University of Pennsylvania for providing the vectors used in this research. This research was supported by a grant from NICHD P01 HD57247 and NIDDK P30 DK47757. J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. E.B. is currently at Department of Medicine II, University of Freiburg, Freiburg, Germany.
REFERENCES
- Yang Y, Jooss KU, Su Q, Ertl HC., and , Wilson JM. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- Jooss K, Yang Y, Fisher KJ., and , Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M, et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol. 2009;182:6051–6060. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L., and , Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM, et al. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS ONE. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S, Vandenberghe LH., and , Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xiang Z, Ertl HC., and , Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Williams MA., and , Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander IE, Cunningham SC, Logan GJ., and , Christodoulou J. Potential of AAV vectors in the treatment of metabolic disease. Gene Ther. 2008;15:831–839. doi: 10.1038/gt.2008.64. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O., and , Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang H, Bell P, McCarter RJ, He J, Calcedo R, et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3 5 Pt 1:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3 5 Pt 1:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Goetzmann J, Caridi J, Paolillo J, Conlon TJ, Potter M, et al. Apparently nonspecific enzyme elevations after portal vein delivery of recombinant adeno-associated virus serotype 2 vector in hepatitis C virus-infected chimpanzees. Hum Gene Ther. 2008;19:681–689. doi: 10.1089/hum.2007.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ, et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20:908–917. doi: 10.1089/hum.2009.031. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang X., and , Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]