Abstract
Cancer vaccines based on virus-like particles (VLPs) vectors may offer many advantages over other antigen-delivery systems and represent an alternative to the ex vivo cell therapy approach. In this study, we describe the use of penton-dodecahedron (Pt-Dd) VLPs from human adenovirus type 3 (Ad3) as cancer vaccine vehicle for specific antigens, based on its unique cellular internalization properties. WW domains from the ubiquitin ligase Nedd4 serve as an adapter to bind the antigen to Pt-Dd. By engineering fusion partners of WW with the model antigen ovalbumin (OVA), Pt-Dd can efficiently deliver WW-OVA in vitro and the Pt-Dd/WW complex can be readily internalized by dendritic cells (DCs). Immunization with WW-OVA/Pt-Dd results in 90% protection against B16-OVA melanoma implantation in syngeneic mice. This high level of protection correlates with the development of OVA-specific CD8+ T cells. Moreover, vaccination with WW-OVA Pt-Dd induces robust humoral responses in mice as shown by the high levels of anti-OVA antibodies (Abs) detected in serum. Importantly, treatment of mice bearing B16-OVA tumors with WW-OVA/Pt-Dd results in complete tumor regression in 100% of cases. Thus, our data supports a dual role of Pt-Dd as antigen-delivery vector and natural adjuvant, able to generate integrated cellular and humoral responses of broad immunogenic complexity to elicit specific antitumor immunity. Antigen delivery by Pt-Dd vector is a promising novel strategy for development of cancer vaccines with important clinical applications.
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells that orchestrate innate and adaptive immune responses, making them ideal candidates for cancer immunotherapy. Attempts to harness their potential to induce tumor-specific cytotoxic T lymphocyte (CTL) responses have mainly focused on ex vivo DC therapies. However, DCs ex vivo handling can be impractical.1 In recent years, antigen delivery to DCs in vivo2,3,4 is being proposed as alternative to the ex vivo cell therapy.
A prerequisite for stimulation of antigen-specific CD8+ T cells is the effective delivery of antigens to DCs for processing and cross-presentation by major histocompatibility complex (MHC) class I molecules. Although efforts in cancer vaccine development have mostly focused on eliciting a CTL response, recent studies support that an effective cancer vaccine should stimulate a robust, sustained and integrated cellular and humoral immune responses.5,6 Thus, the production of antibody (Ab) against the tumor antigen could offer additional protection against tumor development by Ab-dependent cellular cytotoxicity and/or inhibition of antigen function.7
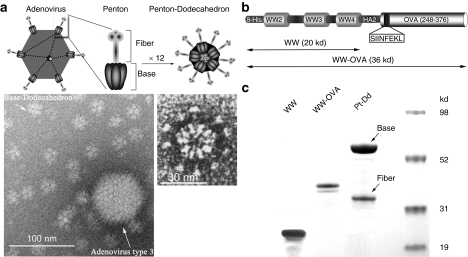
Adenoviruses (Ads), in particular their capsid components, have the natural ability to induce strong inflammatory and immune responses.8,9 Clinical studies using recombinant Ad have shown their capacity to trigger an intense and long-lasting cellular Th1 and humoral responses against the transgene product.10 Moreover, the potential of Ad capsomers as vaccination adjuvants has been reported.11 The major Ad capsomers are the trimeric hexons making the triangular facets of the icosahedral capsid (240 per virion). At each of the 12 vertices of the virion is located a noncovalent complex (penton, Pt) consisting of the Pt base and the protruding fibers (Figure 1a, upper part). Interestingly, Pt, which is involved in the virion attachment and cell entry, keeps its internalization properties when used as single-purified capsomer12,13 and has thus been used as a new vector in gene and protein delivery.12,14,15,16
Figure 1.
Description of Pt-Dd VLPs as protein delivery system for vaccine development. (a) Structure of human Ad3 and its Pt-Dd particle. Schematic representation of the Ad morphology, including an icosahedral capsid with Pt structures at the vertices (upper left diagram). Pt (zoomed diagram) comprises a noncovalent complex of trimeric fiber protein attached to a pentameric penton base. Self-association of the Pt results in the formation of the DNA devoid Pt-Dd particle (upper right diagram). Electron micrographs (obtained as described in ref. 18) illustrate the size comparison between Ad3 capsid and Dd particle (left) and the Pt-Dd detailed structure (right). (b) Schematic representation of the recombinant fusion protein WW-OVA, which comprises (i) WW2-3-4 domains from Nedd4; (ii) the NH2-terminal peptide of influenza virus HA2; (iii) a 129 amino acid C-terminal fragment of OVA containing the MHC class I immunodominant peptide SIINFEKL. (c) SDS-PAGE analysis of purified proteins. WW and WW-OVA proteins were expressed in BL21 cells and purified by affinity column. Pt-Dd VLPs were expressed in baculovirus and purified in a sucrose density gradient. Ad3, adenovirus type 3; MHC, major histocompatibility complex; OVA, ovalbumin; Pt-Dd, penton-dodecahedron; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; VLP, virus-like particle.
Among these systems, our interest has focused on the Ad type 3 (Ad3) virus-like particle (VLP) known as Pt-dodecahedron (Pt-Dd). When the Pt base and fiber proteins are expressed in a baculovirus system, they self-assemble into this dodecahedron Pt-Dd particle (Figure 1a, upper part), which can be efficiently internalized inside the cell through interaction with integrins and heparan-sulfate proteoglycans on the cell membrane.12,17,18 We have previously reported the use of Pt-Dd for protein delivery and designed an original system for protein transduction into cells.15,16 The base protein contains two strictly conserved N-terminus PPxY motifs, which are involved in the interaction with WW domains from Nedd4-like E3 ubiquitin ligases.19
We have investigated the transduction of antigen using Pt-Dd to generate integrated cellular and humoral responses of broad immunogenic complexity. To achieve this goal, we exploited the binding of Pt-Dd to WW structural domains from Nedd4 to deliver antigens into DCs. Our in vivo results using ovalbumin (OVA) as tumor model antigen fused to WW demonstrate the efficacy of a Pt-Dd vaccine in preventing tumor development and more importantly, in suppressing the growth of established tumors. The high protection achieved in the vaccinated mice correlates with the development of OVA-specific CTLs and the production of Abs against OVA, indicating that both cellular and humoral responses are taking place. Thus, these data demonstrate the potential of Pt-Dd VLPs as novel and efficient vaccine for cancer immunotherapy.
Results
Production of Pt-Dd VLPs and recombinant protein antigens
We use recombinant Pt-Dd VLPs from Ad3 (Figure 1a, upper part) as a vehicle to deliver protein antigens to DCs for effective immunotherapy. Pt-Dd VLPs are particles devoid of DNA of smaller size than the Ad3 capsomere (Figure 1a, electron micrograph, left, obtained as described in ref. 18) formed by the self-assembly of 12 Pt (Figure 1a, electron micrograph, right). The Pt-Dd antigen-carrier particle was constructed by engineering fusion partners to WW domains from Nedd4. OVA is a well-characterized antigen2,3,20,21 and generates the immunodominant MHC class I epitope OVA257–264 SIINFEKL.22 Thus, it represents an ideal antigen model to study the immunization potential of novel candidate vaccines. The C-terminal region of OVA (residues 248–376, comprising the SIINFEKL peptide) was cloned in frame with WW domains and expressed as recombinant fusion protein (Figure 1b). WW was also expressed individually as control protein. Both WW and WW-OVA were purified from the soluble fraction of cell lysates by affinity column, resulting in predominantly single species of the correct molecular size, as observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (Figure 1c). The Pt base and fiber proteins were expressed in a baculovirus system, where they self-assemble to form the Pt-Dd particle [Figure 1a (ref. 12)]. Pt-Dd was purified from a density sucrose gradient and the integrity and purity of both base and fiber proteins confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Figure 1c).
Pt-Dd serves as vehicle to deliver protein antigens into cells
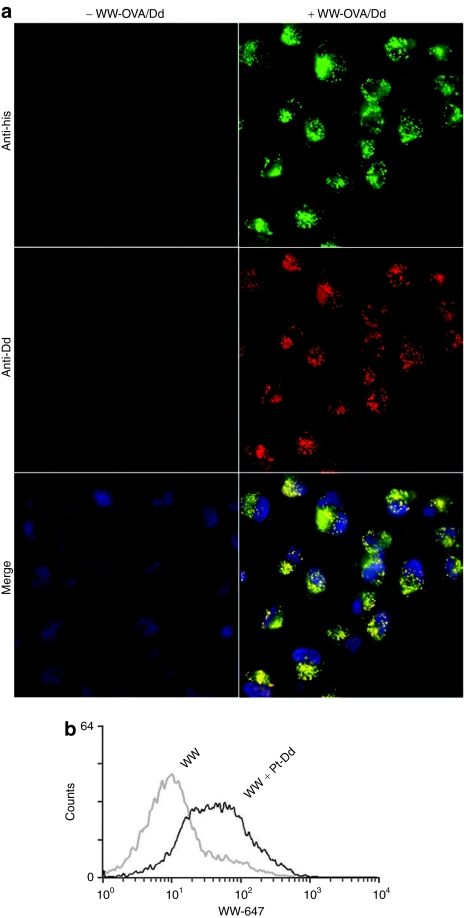
Previous studies demonstrate the ability of Pt-Dd to internalize macromolecules into cells, including WW-fusion proteins.16 To investigate whether Pt-Dd is also able to deliver protein antigens, purified WW-OVA fusion protein was incubated with Pt-Dd and HeLa cells were treated with these protein complexes. Immunofluorescence analysis of the transduced cells revealed that Pt-Dd is internalized as observed by the red punctuated signal (Figure 2a, middle right panel) and is able to deliver WW-OVA protein into cells (Figure 2a, top right panel). Control experiments using nontreated cells demonstrate that the internalization observed is not due to nonspecific signal (Figure 2a, left panels). A high degree of both Pt-Dd and WW-OVA uptake is appreciated in all cells, which colocalizes into endocytic vesicles as denoted by the yellow punctuated signal in the merge panel (Figure 2a, bottom right panel). To exclude possible artifacts arising from permeabilization and fixation of cells, live imaging confocal microscopy experiments on HeLa cells demonstrate that Pt-Dd labeled with Cy5 is correctly internalized and colocalized with endosomes marker Rab5a-RFP (see Supplementary Figure S1).
Figure 2.
Cellular uptake of proteins mediated by Pt-Dd. (a) HeLa cells were either untreated (left panel) or incubated for 1 hour with WW-OVA/Pt-Dd protein complexes (right panel). Protein internalization was detected by immunocytochemistry on fixed cells, colocalization of Pt-Dd, and WW-OVA is shown in the merge panel, with nuclei counter stained with Hoechst dye. (b) Mouse bone marrow-derived DCs were incubated for 3 hours with either Alexa 647-labeled WW protein only (gray histogram) or in complex with Pt-Dd (black histogram). Cells were trypsinized to remove surface bound proteins and protein internalization analyzed by flow cytometry. DC, dendritic cell; OVA, ovalbumin; Pt-Dd, penton-dodecahedron.
We then wanted to confirm that this efficient internalization observed also occurs in primary nonfixed cells, more specifically in DCs. To address this point, we incubated bone marrow-derived DCs with Alexa 647 WW protein in complex with Pt-Dd and analyze internalization by flow cytometry. As shown in Figure 2b, 100% of the DCs incubated with WW/Pt-Dd protein complexes show a clear shift of fluorescence (black histogram), indicating an efficient internalization of the labeled WW protein. By contrast, comparison to DCs treated with the labeled WW protein only (gray histogram) indicates that WW protein is by itself poorly internalized and evidences that protein transduction is mediated by Pt-Dd particles (Figure 2b).
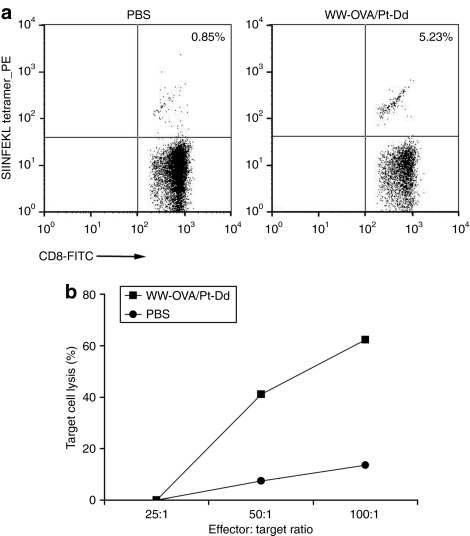
Vaccination with WW-OVA/Pt-Dd induces OVA-specific CTL responses
We investigated the ability of WW-OVA/Pt-Dd to stimulate naive CTL precursors in vivo. To this end, we immunized naive mice subcutaneously (s.c.) with WW-OVA/Pt-Dd protein complexes and harvested splenocytes at day 14. First, we used the MHC class I H-2Kb-OVA(257–264) (SIINFEKL) tetramer to identify the OVA-specific CD8+ T cells responding to WW-OVA/Pt-Dd vaccination. Staining with anti-CD8+ Ab and SIINFEKL tetramer demonstrated that the frequency of OVA-specific CD8+ cells increased from 0.85% in control mice receiving phosphate-buffered saline (PBS) to 5.23% in WW-OVA/Pt-Dd vaccinated mice (Figure 3a). We then evaluated the functionality of OVA-specific CD8+ cells from WW-OVA/Pt-Dd vaccinated mice by cytotoxicity assay. Vaccination with WW-OVA/Pt-Dd induced effective priming of OVA-specific CTL responses with robust cytotoxic activity toward the melanoma B16-OVA target cell line (Figure 3b, black squares). The cytotoxicity observed is effector to target ratio dependent, with target cell lysis of 37.2 and 60% at effector to target ratios of 50:1 and 100:1, respectively. Mice that received PBS only did not elicit CTL-specific responses (Figure 3b, black circles) with only a response slightly above background <14% at the highest effector to target ratio (100:1).
Figure 3.
Vaccination with WW-OVA/Pt-Dd generates OVA-specific CD8+ T-cell responses. C57BL/6 naive mice (N = 3) were immunized twice with WW-OVA/Pt-Dd protein complexes or PBS. CD8+ T-cell responses were measured 14 days postinjection on splenocytes. (a) H-2Kb-OVA (SIINFEKL) tetramer staining on CD8+ cells from mice injected with PBS (left panel) or Pt-Dd/WW-OVA (right panel). (b) Specific killing of B16-OVA target cells by FACS-based cytotoxicity assay. A quantitative analysis of viable target cells was performed using FITC-labeled calibration beads and the percentage of specific lysis was calculated with the formula [1 − (R1:1/R1:A)] × 100, as described in Supplementary Materials and Methods. FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; OVA, ovalbumin; PBS, phosphate-buffered saline; Pt-Dd, penton-dodecahedron.
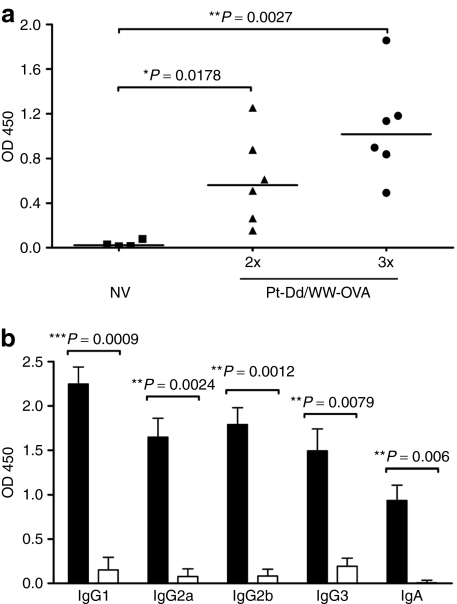
Immunization with WW-OVA/Pt-Dd induces a robust humoral response specific to OVA
To evaluate the humoral immune response elicited by WW-OVA/Pt-Dd vaccination, serum OVA-specific Abs were measured by enzyme-linked immunosorbent assay using OVA as capture antigen. As shown in Figure 4a, mice that received two vaccinations of Pt-Dd/WW-OVA (black triangles) elicited serum anti-OVA immunoglobin G (IgG) responses as compared to nonvaccinated naive mice (black squares). Moreover, the IgG production can be boosted with a third vaccination (black circles), resulting in twofold overall increase. To further characterize the humoral response upon WW-OVA/Pt-Dd vaccination, we determined the titers of serum IgG isotypes IgG1, IgG2a, IgG2b and IgG3, and IgA in vaccinated mice. All mice that received WW-OVA/Pt-Dd showed significantly increased titers of all IgG isotypes and IgA (Figure 4b, black bars) compared to nonvaccinated naive mice (Figure 4b, white bars). Although there was no dominant serum IgG isotype subclass in the vaccinated group, the high levels of IgG2a and IgG1 indicates that a roughly balanced Th1 and Th2 immune response is induced with the WW-OVA/Pt-Dd vaccine.
Figure 4.
Humoral response elicited by vaccination with WW-OVA/Pt-Dd. (a) C57BL/6 naive mice (N = 6) were immunized twice (triangles) or thrice (circles) with Pt-Dd/WW-OVA protein complexes, with 1-week intervals between each boost. A control group included nonvaccinated (NV, squares) naive mice (N = 4). Serum antibodies specific for OVA were determined by ELISA at week 11 after the first immunization, using OVA as antigen. Each point represents the mean of duplicate OD values from each mouse serum at dilution 1/50. (b) Geometric mean and SEM of serum-specific IgG isotypes and IgA from WW-OVA/Pt-Dd vaccinated mice (black bars, N = 5) were determined by ELISA and compared to nonvaccinated mice (white bars, N = 3).The unpaired t-test with Welch's correction was used to compare Ab levels between vaccination groups and a value of P < 0.05 was considered statistically significant. Ab, antibody; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobin G; NV, nonvaccinated; OVA, ovalbumin; Pt-Dd, penton-dodecahedron.
Vaccination with WW-OVA/Pt-Dd protects against B16-OVA tumor challenge
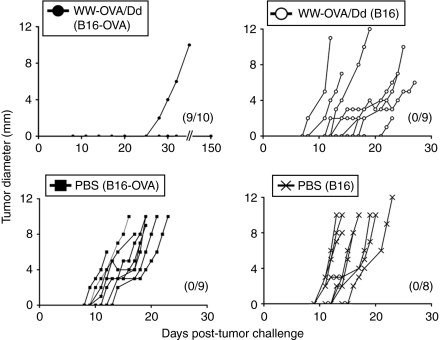
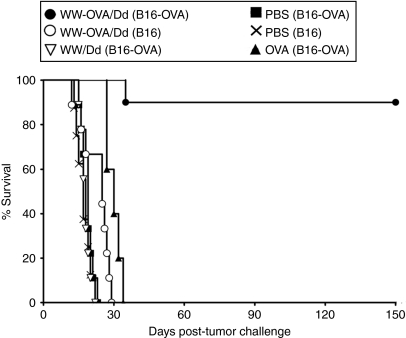
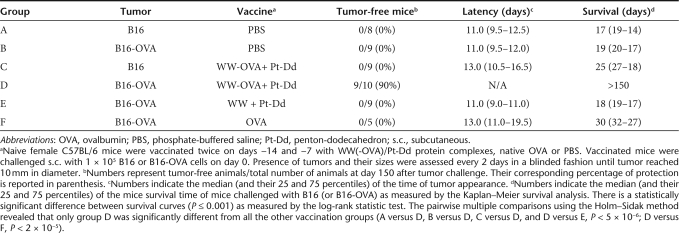
To evaluate whether the OVA-specific CTL responses and humoral immunity observed upon vaccination with WW-OVA/Pt-Dd could lead to protection against tumor growth, we performed an in vivo tumor assay. Mice received a two-vaccination boost schedule followed by s.c. challenge with B16-OVA or B16 melanoma cells and were monitored for tumor growth and survival (Figures 5 and 6, respectively and Table 1). Vaccination with WW-OVA/Pt-Dd induced a robust and long-lasting protective effect, resulting in suppression of B16-OVA tumor development (Figures 5 and 6, black circles). There was a complete tumor rejection in 9 out of 10 vaccinated mice (90%) over 5 months post-tumor challenge and a delay in tumor growth in one mouse (10%), whereas the B16-OVA tumor kinetics in nonvaccinated mice is highly aggressive (Figures 5 and 6, black squares), with a median of tumor appearance and survival time of 11 and 19 days, respectively (Table 1, group B). To prove the specificity of the vaccine toward the OVA antigen, WW-OVA/Pt-Dd vaccinated mice were challenged with B16 cells (Figures 5 and 6, open circles). Although the tumor growth in this group is more heterogeneous, no overall significant effects were observed compared to mice challenged with B16 that received PBS (Figures 5 and 6, black crosses), with only a modest delay in tumor appearance and increased survival (Table 1, groups A and C). Animals vaccinated with native OVA (Figure 6 and Supplementary Figure S2, black triangles) developed B16-OVA tumors similarly to mice receiving either PBS (Figure 5, black squares) or Pt-Dd/WW without OVA (Figure 6 and Supplementary Figure S2, open inverted triangles), although with slightly slower tumor growth, resulting in a very modest delay in tumor appearance and increased median survival from 19 to 30 days (Table 1, groups B, E, and F).
Figure 5.
Vaccination with WW-OVA/Pt-Dd prevents tumor engraftment. C57BL/6 naive mice (N = 5–10) were immunized on days –14 and –7 and challenged s.c. in the opposite flank (day 0) with 1 × 105 B16 or B16-OVA melanoma cells. Tumor sizes were monitored every 2 days for 150 days or until tumors reached 10 mm in diameter. Data show tumor growth curves for individual mice, with number of tumor-free mice indicated in parenthesis. OVA, ovalbumin; PBS, phosphate-buffered saline; Pt-Dd, penton-dodecahedron; s.c., subcutaneous.
Figure 6.
A Pt-Dd-based vaccine is a potent immunogen in a prophylactic tumor model and elicit tumor immunity. C57BL/6 naive mice (N = 5–10) were immunized on days –14 and –7 and challenged s.c. in the opposite flank (day 0) with 1 × 105 B16 or B16-OVA melanoma cells. Data are representative of two independent experiments. Results of the statistical analysis are shown in the legend to Table 1. OVA, ovalbumin; PBS, phosphate-buffered saline; Pt-Dd, penton-dodecahedron; s.c., subcutaneous.
Table 1.
Vaccination with WW-OVA/Pt-Dd protein complexes efficiently protects against B16-OVA melanoma
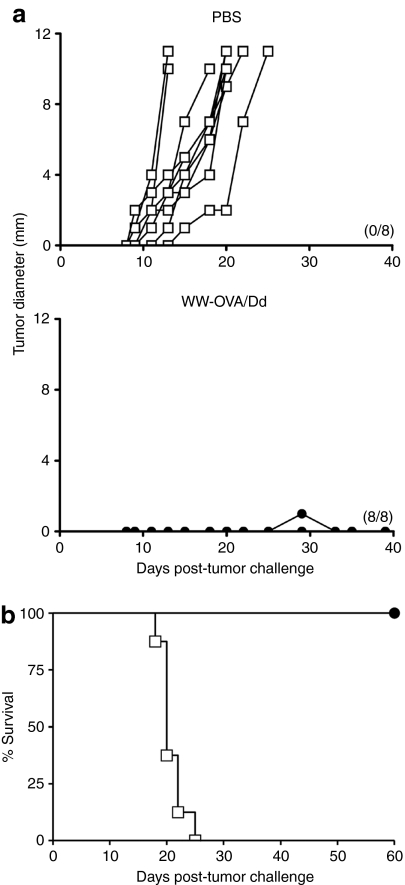
Treatment of tumor-bearing animals with WW-OVA/Pt-Dd
Prophylactic vaccination in mice is a well-established and powerful tool to validate a vaccine candidate and assesses its efficacy to induce robust tumor immunity. However, this approach has a limited clinical relevance in humans and therefore it has to be tested in therapeutic assays using tumor-bearing mice. Based on the highly aggressive growth of B16 tumors, therapeutic assays usually start after 24 hours s.c. implantation.20 Therapeutic vaccination with WW-OVA/Pt-Dd on tumor-bearing mice resulted in permanent tumor rejection in 100% of the mice (Figure 7b, black circles), whereas all mice in the control group developed tumors (Figure 7a,b, white squares).
Figure 7.
Treatment of tumor-bearing mice with WW-OVA/Pt-Dd. C57BL/6 naive mice (N = 8) were challenged s.c. with 1 × 105 B16-OVA melanoma cells on day 0 and immunized with WW-OVA/Pt-Dd protein complexes or PBS (control group) on days 1 and 8. (a) Tumor growth curves for individual mice. Tumor sizes were monitored every 2 days for 60 days or until tumors reached 10 mm in diameter. The number of tumor-free mice are shown in parenthesis. (b) Kaplan–Meier survival analysis showing the percentage of surviving mice. OVA, ovalbumin; PBS, phosphate-buffered saline; Pt-Dd, penton-dodecahedron; s.c., subcutaneous.
Discussion
In this study, we have explored the Pt-Dd VLP from human Ad3 as carrier macromolecule for novel vaccine development. The versatility of the Pt-Dd VLPs allows a protein of choice, in this case the model antigen OVA, to be delivered into cells when fused to WW binding domains (Figure 2). Vaccination with other VLPs results in trafficking into lymph nodes23 and free drainage to these organs is possible for particles with the size of Pt-Dd,24 allowing the targeting of resident cells to elicit antigen-specific cellular responses. Importantly, in vivo delivery of OVA by WW-OVA/Pt-Dd immunization elicits robust and sustained immune responses capable of rejecting the highly aggressive and poorly immunogenic B16-OVA melanoma (Figures 5 and 6).
Most efforts in vaccine development focus in the generation of CD8+ T-cell responses by improving antigen delivery and presentation on DC cell surface for initial T-cell encounter.4,21 Various strategies have been used to actively direct the endocytosed antigens to the MHC class I cross-presentation pathway.1 In contrast to protein and peptide antigens, most viral targeting vectors have an inherent capacity to escape from the endosome, and drive expression of antigens directly into the cytosol, resulting in effective MHC class I loading. Vaccination with WW-OVA/Pt-Dd protein complexes leads to the efficient generation of CD8+ T cells specific for the OVA immunodominant MHC class I epitope SIINFEKL (>5%, Figure 4a). This result indicates that the Pt-Dd VLP particle can deliver antigens to DCs, allowing proteasome processing and cross-presentation onto MHC class I molecules to generate specific CD8+ T cells. Although not formally tested, Pt-Dd by itself could favor the endosomal scape, because Dd Pt base is thought to be involved in the release of cargo from endosomes by pH-induced conformational changes leading to membrane disruption.25,26
Our vaccination strategy with WW-OVA/Pt-Dd is highly effective in protecting animals to specifically reject B16-OVA tumor implantation (Figures 5 and 6) and correlates with the induction of functional CTL responses capable of B16-OVA killing in vitro (Figure 3b). More importantly, mice that received the WW-OVA/Pt-Dd vaccine in curative assays display a total tumor regression after 2-month postvaccination (Figure 7). We should take into account, however, that coupling tumor-associated antigens to WW instead of the exogenous OVA is unpredictable and would need to be assessed experimentally. It is noteworthy that the frequency of antigen-specific CD8+ T cells does not consistently correlate with the control of viral infection or tumor growth, which depends on qualitative rather than quantitative parameters.27 High-avidity CD8+ T cells recognize epitopes at low densities on the cell surface and preferentially undergo rapid expansion in vivo.28
The efficacy of a vaccine is greatly dependent upon its ability to induce DC activation and maturation, which is necessary for effective CTL responses. Targeted antigen vaccination without additional maturation stimuli results in the induction of tolerance rather than immunity.1 Stimulation of the innate immune system via toll-like receptors plays an important role in the generation and maintenance of acquired immune responses.29 toll-like receptors recognize conserved molecular patterns present on pathogens, including bacteria and viruses, and these ligands can serve as adjuvants in vaccine formulation for effective CD8+ T-cell priming and maturation of DCs. VLPs-based vaccines can acts as a natural adjuvant and in particular, the presence of the fiber protein in Pt-Dd would further potentiate this effect.30 Interestingly, Dd have been recently found to be a potent activator of human myeloid DCs that induce the release of proinflammatory cytokines, which strengthen its adjuvant effect in vaccination.31
VLPs can activate both the endogenous and exogenous antigen pathways, leading to viral peptide presentation by MHC class I and II molecules.32 Additionally, VLPs could also bind to Abs in circulation and be taken up by phagocytic cells via Fc receptors, leading to MHC class II presentation.33 Vaccination with WW-OVA/Pt-Dd promotes the production of all IgG subtypes and IgA (Figure 5b), resulting in a strong OVA-specific humoral immune response. Such a broad immune response, including Th1 and Th2 immune responses, highlights the potent adjuvant effect by Pt-Dd itself. Thus, this effect could be contributing to the protection against tumor implantation elicited by T-cell responses (Figure 3) and would offer a robust long-term protection in the event of subsequent exposure to the antigen. It should be noted, however, that pre-existing immunity is often a drawback in gene delivery using adenovirus vectors. Nevertheless, it has been demonstrated that this effect is less critical and can even be favorable for vaccination purposes.9,34 Although it is generally assumed that Abs against a vaccine carrier impair protective immune responses, the presence of high carrier-specific VLP Ab titers had a marginal influence on the induction of T cell responses.35 Therefore, the generation of Pt-Dd Abs upon vaccination would not constitute an impediment toward antitumor immunity.
There is a growing interest for vaccine development using VLPs, based on their great potential to induce cellular and humoral immune responses, in particular potent CTL responses compared with other vectors.36,37,38 VLPs are also cost-effective and highly scalable to produce, safe for human use as they cannot replicate and cause infection, induce T cells with higher avidity39 and have adjuvant effects.40 The Pt-Dd vaccination approach offers the unique advantage of easy full-length antigens incorporation by fusion to WW domains without compromising the integrity of the Pt-Dd because they are produced as separate entities. Incorporation of the protein antigen instead of the CD4 and CD8 cognate epitopes allows the generation of a T-cell response of a broader repertoire and its therapeutic value is less likely to be restricted to patients of specific human histocompatibility leukocyte antigen haplotype. Our Pt-Dd vaccination system could also be envisaged as a versatile approach suitable for simultaneous multiantigen delivery to alleviate tumor evasion due to immunoediting41 or human histocompatibility leukocyte antigen class I loss or antigen heterogeneity in tumor cells.42 In summary, our data demonstrates the suitability of a Pt-Dd vaccine to deliver antigens in an in vivo setting to target DCs and induce integrated humoral and antigen-specific T-cell responses required for effective prophylactic and therapeutic protection toward tumor development.
Materials and Methods
Generation of expression constructs. The complementary DNA of WW domains from human ubiquitin ligase Nedd4 (WW2-3-4) was amplified by standard PCR techniques and cloned into pET15bΔt (modified pET15b by deletion of thrombin cleavage site downstream the histidine tag, amino acids SSGLVPRGS) to generate the pET15bΔt− WW2-3-4 expression vector. The WW-OVA construct was generated by cloning in frame the N-terminal domain of HA2 (ref. 33) and the OVA complementary DNA sequence coding for residues 248–376 of OVA into pET15bΔt-WW2-3-4 (Figure 1b).
Protein expression and purification. WW and WW-OVA proteins were expressed in Escherichia coli strain BL21 (DE3) (Novagen, Madison, WI). Protein expression was induced with 0.2 mmol/l isopropyl β-D-thiogalactopyranoside (see Supplementary Materials and Methods for detailed list of reagents and Abs) and bacterial cultures grown overnight at 20 °C. WW and WW-OVA were purified by affinity chromatography methods as detailed in Supplementary Materials and Methods. Pt-Dd was expressed in baculovirus and purified on a density sucrose gradient, as previously described.12,43 Protein concentration and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with PageBlue (Fermentas France, St Rémy Les Chevreuse, France).
Cells and culture conditions. HeLa cells, B16, and B16-OVA (B16 cell line transfected with OVA) murine melanoma cell lines were grown in Dulbecco's modified Eagle's medium with GlutaMAX, supplemented with 10% fetal calf serum and 1% antibiotics. Maintenance of B16-OVA cells was ensured by addition of 400 µg/ml G418 to the growth medium.
Protein internalization experiments. Pt-Dd delivery of WW-OVA in HeLa cells was assessed by immunofluorescence as detailed in Supplementary Materials and Methods. Cells were grown to confluency on eight-well Lab-Tek chamber slides (Thermo Fisher Scientific, Langenselbold, Germany). 0.35 µg WW-OVA was incubated with 0.25 µg Pt-Dd for 30 minutes to allow protein complexes formation. Samples were added to 100 µl of supplemented Dulbecco's modified Eagle's medium and incubated with HeLa cells for 1 hour. Control experiments included incubation of cells with medium only. Internalized proteins were visualized using a Nikon Eclipse TE 2000 inverted fluorescence microscopy (Nikon, Champigny Sur Marne, France). The internalization of WW by Pt-Dd in DCs was analyzed by flow cytometry. To allow protein complex formation, 1 µg of Alexa 647 fluorescently labeled WW was incubated with 1 µg Pt-Dd for 30 minutes and added to mouse bone marrow-derived DCs for 3 hours in a 12-well culture plate. Control experiment included incubation of DCs with Alexa 647-WW only. After treatment, cells were washed twice with PBS, trypsinized for 10 minutes at 37 °C to remove proteins bound to the cell surface and trypsin inactivated with RPMI medium supplemented with 10% serum. Cells were washed with PBS and resuspended in supplemented RPMI medium. Internalized proteins were monitored by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD, Biosciences, Pont de Claix, France).
Immunization schedules. C57BL/6 mice were purchased from Janvier Breeding Laboratories (Le Genest St Isle, France) and treated in accordance with European Community guidelines. All in vivo experimentation was approved by the Animal Experimentation Ethical Committee and conducted in compliance with the Université Joseph Fourier and CIEMAT guidelines. For all the vaccination experiments, 8-week-old female C57BL/6 mice were vaccinated s.c. with WW-OVA (12 µg)/Pt-Dd (20 µg) or WW (8 µg)/Pt-Dd (15 µg) protein complexes, 8 µg OVA or PBS.
Tetramer staining. Mice were vaccinated twice every 7 days with WW-OVA/Pt-Dd complexes or PBS (three animals per group). On day 14, spleens were harvested and splenocytes resuspended in PBS with 1% bovine serum albumin and 2 mmol/l EDTA. 1 × 106 splenocytes were stained with fluorescein isothiocyanate anti-CD8 Ab and PE-H-2Kb-OVA(257–264) (SIINFEKL) tetramer, according to the manufacturer's instructions. Double positive CD8 tetramer+ cells were gated excluding 7-amino-actinomycin+ cells by fluorescence-activated cell sorting analysis.
Cytotoxicity assay. Mice received two s.c. vaccinations of WW-OVA/Pt-Dd protein complexes or PBS every 7 days. On day 14, their spleens were harvested and single-cell suspensions of splenocytes resuspended in RPMI with GlutaMAX (supplemented with 20% fetal calf serum, 50 µmol/l 2-ME, 10 mmol/l HEPES, 1 mmol/l sodium pyruvate, and 1% antibiotics) at a concentration of 20 × 106/ml. CTL activity was measured as detailed in Supplementary Materials and Methods.
Serological assays. Blood from mice vaccinated with WW-OVA/Pt-Dd or PBS was collected from tail vein and allowed to clot at room temperature. Sera were recovered by centrifugation and stored at −80 °C. The production of anti-OVA specific total IgG, IgG isotypes, and IgA in immunized mice was assessed by enzyme-linked immunosorbent assay as detailed in Supplementary Materials and Methods.
In vivo tumor assays. For tumor protection experiments, mice (5–10 animals per group) were vaccinated on days −14 and −7 as stated as stated above. On day 0, mice were challenged s.c. in the opposite flank with 1 × 105 B16 or B16-OVA cells. Tumor progression was monitored every 2 days in a blinded fashion for 150 days. Mice were sacrificed when tumors ulcerated or reached 1 cm in diameter. For curative assays, mice were challenged s.c. with 1 × 105 B16-OVA melanoma cells on day 0 and subsequently immunized with WW-OVA/Pt-Dd protein complexes or PBS on days 1 and 8. The survival curves and statistical analysis from each animal group were plotted using the Sigma Stat software, version 3.5 (Erkrath, Germany).
SUPPLEMENTARY MATERIALFigure S1. Colocalization of Bs-Dd and endosomes in HeLa cells. (a) Bs-Dd labelled with Cy5 (red signal) is incubated for 30 minutes at 37°C on living HeLa cells expressing Rab5a/RFP in their endosomes (orange). Z-series are performed with 0,2 mm sections. (b) Colocalisation analysis showing in green all pixels labelled both in red and orange.Figure S2. Vaccination with control group protein antigens has no effect on tumor growth. C57BL/6 naive mice were immunized on days -14 and -7 with OVA or WW/Pt-Dd and challenged s.c. in the opposite flank (day 0) with 1×105 B16-OVA melanoma cells. Tumor sizes were monitored every two days until tumors reached 10 mm in diameter. Data show tumor growth curves for individual mice, with number of tumor-free mice indicated in parenthesis.Supplementary Materials and Methods.
Supplementary Material
Colocalization of Bs-Dd and endosomes in HeLa cells. (a) Bs-Dd labelled with Cy5 (red signal) is incubated for 30 minutes at 37°C on living HeLa cells expressing Rab5a/RFP in their endosomes (orange). Z-series are performed with 0,2 mm sections. (b) Colocalisation analysis showing in green all pixels labelled both in red and orange.
Vaccination with control group protein antigens has no effect on tumor growth. C57BL/6 naive mice were immunized on days -14 and -7 with OVA or WW/Pt-Dd and challenged s.c. in the opposite flank (day 0) with 1×105 B16-OVA melanoma cells. Tumor sizes were monitored every two days until tumors reached 10 mm in diameter. Data show tumor growth curves for individual mice, with number of tumor-free mice indicated in parenthesis.
Acknowledgments
We thank L. Cuppini and M. Derouazi (TIMC-ThereX, UMR 5525 CNRS-UJF), E. Grueso, P. del Busto, J. Martínez, and E. de Almeida (CIEMAT) for their technical assistance and support; B. Toussaint and O. Epaulard (TIMC-ThereX, UMR 5525 CNRS-UJF and CHU-Grenoble) for the B16 and B16-OVA cell lines; Alexei Grichine for access to the confocal platform, Stephanie Corjon for her help in confocal image acquisition and P. Marche (Institute Albert Bonniot, Grenoble) for providing bone marrow-derived DCs. This work was supported by a Marie Curie Excellence Grant #014320, by the Spanish Grants from the Ministerio de Ciencia e Innovación SAF 2007-66227 (to M.I.G.) and Ramón y Cajal Program (to E.P.-M.), by a FEBS fellowship (to A.V.-M.) and by a scholarship of the Leonardo da Vinci project Unipharma Graduates (www.unipharmagraduates.it) coordinated by Sapienza University of Rome (to E.V.). We also thank the “Factoría Española de Cristalización,” Consolider-Ingenio 2010 project (MEC) and Region Rhône-Alpes for its financial support through the clusters.
REFERENCES
- Tacken PJ, de Vries IJ, Torensma R., and , Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- Epaulard O, Toussaint B, Quenee L, Derouazi M, Bosco N, Villiers C, et al. Anti-tumor immunotherapy via antigen delivery from a live attenuated genetically engineered Pseudomonas aeruginosa type III secretion system-based vector. Mol Ther. 2006;14:656–661. doi: 10.1016/j.ymthe.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Radford KJ, Higgins DE, Pasquini S, Cheadle EJ, Carta L, Jackson AM, et al. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 2002;9:1455–1463. doi: 10.1038/sj.gt.3301812. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi F, Venanzi FM, Concetti A, Yamauchi H, Tiwari S, Norton L, et al. Antibody and CD8+ T cell responses against HER2/neu required for tumor eradication after DNA immunization with a Flt-3 ligand fusion vaccine. Clin Cancer Res. 2007;13:6195–6203. doi: 10.1158/1078-0432.CCR-07-0258. [DOI] [PubMed] [Google Scholar]
- Julien S, Picco G, Sewell R, Vercoutter-Edouart AS, Tarp M, Miles D, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L., and , Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Gahery-Segard H, Mehtali M, Le Boulaire C, Ribault S, Boulanger P, et al. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Le Boulaire C, Le Gal FA, Gahéry-Segard H, Tursz T, Guillet JG, et al. Longitudinal follow-up of cellular and humoral immunity induced by recombinant adenovirus-mediated gene therapy in cancer patients. Hum Gene Ther. 2000;11:1911–1920. doi: 10.1089/10430340050129521. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Ségard H, et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender P, Ruigrok RW, Gout E, Buffet S., and , Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat Biotechnol. 1997;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- Hong SS, Gay B, Karayan L, Dabauvalle MC., and , Boulanger P. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology. 1999;262:163–177. doi: 10.1006/viro.1999.9864. [DOI] [PubMed] [Google Scholar]
- Smith CC, Kulka M., and , Aurelian L. Modified adenovirus penton base protein (UTARVE) as a non-replicating vector for delivery of antisense oligonucleotides with antiviral and/or antineoplastic activity. Int J Oncol. 2000;17:841–850. doi: 10.3892/ijo.17.4.841. [DOI] [PubMed] [Google Scholar]
- Fender P, Schoehn G, Foucaud-Gamen J, Gout E, Garcel A, Drouet E, et al. Adenovirus dodecahedron allows large multimeric protein transduction in human cells. J Virol. 2003;77:4960–4964. doi: 10.1128/JVI.77.8.4960-4964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcel A, Gout E, Timmins J, Chroboczek J., and , Fender P. Protein transduction into human cells by adenovirus dodecahedron using WW domains as universal adaptors. J Gene Med. 2006;8:524–531. doi: 10.1002/jgm.862. [DOI] [PubMed] [Google Scholar]
- Vivès RR, Lortat-Jacob H, Chroboczek J., and , Fender P. Heparan sulfate proteoglycan mediates the selective attachment and internalization of serotype 3 human adenovirus dodecahedron. Virology. 2004;321:332–340. doi: 10.1016/j.virol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Fender P, Schoehn G, Perron-Sierra F, Tucker GC., and , Lortat-Jacob H. Adenovirus dodecahedron cell attachment and entry are mediated by heparan sulfate and integrins and vary along the cell cycle. Virology. 2008;371:155–164. doi: 10.1016/j.virol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Galinier R, Gout E, Lortat-Jacob H, Wood J., and , Chroboczek J. Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry. 2002;41:14299–14305. doi: 10.1021/bi020125b. [DOI] [PubMed] [Google Scholar]
- Bellone M, Cantarella D, Castiglioni P, Crosti MC, Ronchetti A, Moro M, et al. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J Immunol. 2000;165:2651–2656. doi: 10.4049/jimmunol.165.5.2651. [DOI] [PubMed] [Google Scholar]
- Shibagaki N., and , Udey MC. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168:2393–2401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- Rötzschke O, Falk K, Stevanovic S, Jung G, Walden P., and , Rammensee HG. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991;21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- Cubas R, Zhang S, Kwon S, Sevick-Muraca EM, Li M, Chen C, et al. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32:118–128. doi: 10.1097/CJI.0b013e31818f13c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P., and , Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Greber UF, Willetts M, Webster P., and , Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Douek DC., and , Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Dzutsev AH, Belyakov IM, Isakov DV, Margulies DH., and , Berzofsky JA. Avidity of CD8 T cells sharpens immunodominance. Int Immunol. 2007;19:497–507. doi: 10.1093/intimm/dxm016. [DOI] [PubMed] [Google Scholar]
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Prévost-Blondel A, Hong SS, Lengagne R, Boudaly S, Magnusson MK, et al. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J Biol Chem. 2003;278:37175–37182. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- Naskalska A, Szolajska E, Chaperot L, Angel J, Plumas J., and , Chroboczek J. Influenza recombinant vaccine: matrix protein M1 on the platform of the adenovirus dodecahedron. Vaccine. 2009;27:7385–7393. doi: 10.1016/j.vaccine.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Storni T, Lechner F, Bächi T., and , Bachmann MF. Cross-presentation of virus-like particles by skin-derived CD8(−) dendritic cells: a dispensable role for TAP. Eur J Immunol. 2002;32:818–825. doi: 10.1002/1521-4141(200203)32:3<818::AID-IMMU818>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- Singh R., and , Kostarelos K. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol. 2009;27:220–229. doi: 10.1016/j.tibtech.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Schwarz K, Jegerlehner A, Storni T, Manolova V., and , Bachmann MF. J Virol. 2005. pp. 717–724. [DOI] [PMC free article] [PubMed]
- Allsopp CE, Plebanski M, Gilbert S, Sinden RE, Harris S, Frankel G, et al. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur J Immunol. 1996;26:1951–1959. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- Sedlik C, Saron M, Sarraseca J, Casal I., and , Leclerc C. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc Natl Acad Sci USA. 1997;94:7503–7508. doi: 10.1073/pnas.94.14.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lu X, Kang SM, Chen C, Compans RW., and , Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313:502–513. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- Sedlik C, Dadaglio G, Saron MF, Deriaud E, Rojas M, Casal SI, et al. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J Virol. 2000;74:5769–5775. doi: 10.1128/jvi.74.13.5769-5775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgérault F, Rueda P, Sun CM, Hervas-Stubbs S, Rojas M., and , Leclerc C. Cross-priming of T cell responses by synthetic microspheres carrying a CD8+ T cell epitope requires an adjuvant signal. J Immunol. 2005;174:3432–3439. doi: 10.4049/jimmunol.174.6.3432. [DOI] [PubMed] [Google Scholar]
- Singh R., and , Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67:1887–1892. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- Elkord E, Dangoor A, Burt DJ, Southgate TD, Daayana S, Harrop R, et al. Immune evasion mechanisms in colorectal cancer liver metastasis patients vaccinated with TroVax (MVA-5T4) Cancer Immunol Immunother. 2009;58:1657–1667. doi: 10.1007/s00262-009-0674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuschiotti P, Schoehn G, Fender P, Fabry CM, Hewat EA, Chroboczek J, et al. Structure of the dodecahedral penton particle from human adenovirus type 3. J Mol Biol. 2006;356:510–520. doi: 10.1016/j.jmb.2005.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colocalization of Bs-Dd and endosomes in HeLa cells. (a) Bs-Dd labelled with Cy5 (red signal) is incubated for 30 minutes at 37°C on living HeLa cells expressing Rab5a/RFP in their endosomes (orange). Z-series are performed with 0,2 mm sections. (b) Colocalisation analysis showing in green all pixels labelled both in red and orange.
Vaccination with control group protein antigens has no effect on tumor growth. C57BL/6 naive mice were immunized on days -14 and -7 with OVA or WW/Pt-Dd and challenged s.c. in the opposite flank (day 0) with 1×105 B16-OVA melanoma cells. Tumor sizes were monitored every two days until tumors reached 10 mm in diameter. Data show tumor growth curves for individual mice, with number of tumor-free mice indicated in parenthesis.