Abstract
Duchenne muscular dystrophy is characterized by muscular atrophy, fibrosis, and fat accumulation. Several groups have demonstrated that in the mdx mouse, the exon-skipping strategy can restore a quasi-dystrophin in almost 100% of the muscle fibers. On the other hand, inhibition of the myostatin pathway in adult mice has been described to enhance muscle growth and improve muscle force. Our aim was to combine these two strategies to evaluate a possible additive effect. We have chosen to inhibit the myostatin pathway using the technique of RNA interference directed against the myostatin receptor AcvRIIb mRNA (sh-AcvRIIb). The restoration of a quasi-dystrophin was mediated by the vectorized U7 exon-skipping technique (U7-DYS). Adeno-associated vectors carrying either the sh-AcvrIIb construct alone, the U7-DYS construct alone, or a combination of both constructs were injected in the tibialis anterior (TA) muscle of dystrophic mdx mice. We show that even if each separate approach has some effects on muscle physiology, the combination of the dystrophin rescue and the downregulation of the myostatin receptor is required to massively improve both the tetanic force and the specific force. This study provides a novel pharmacogenetic strategy for treatment of certain neuromuscular diseases associated with muscle wasting.
Introduction
Duchenne muscular dystrophy is a severe muscle disorder caused by mutations in the dystrophin gene. Lack of dystrophin leads to muscle wasting and progressive weakness. Therapeutic strategies aim to restore dystrophin expression as well as muscle mass. It has been shown that quasi-dystrophin rescue can be achieved by exon-skipping approach, either using synthetic antisense oligonucleotides1,2,3,4,5,6,7,8 or engineered small nuclear RNAs (snRNAs).9,10 On the other hand, improving muscle mass has been assessed by several strategies including the modulation of IGF-1 expression (for review, see ref. 11) or the injection of myogenic cells.12,13 Among all these strategies, we focused on the regulation of the myostatin pathway. Indeed, myostatin is a key regulator of muscle mass. Previous studies have shown that natural lack of myostatin in cattle,14,15,16 sheep,17 or in dogs18 leads to spectacular increases in muscle mass resulting from both hypertrophy and hyperplasia. Moreover, a mutation in the myostatin gene has been described in a healthy child with muscle hypertrophy and important strength,19 confirming that a negative regulation of the myostatin pathway is possible in humans.
Myostatin, like other members of the TGFβ family, is produced as an immature protein, which is activated by proteolytic cleavage in the systemic compartment (for review, see ref. 20). The active fragment then forms a latent complex with follistatin or its own pro-domain. This complex remains inactive until it reaches the target tissue, where its dissociation occurs after interaction with its receptor, the activin receptor IIb (AcvRIIb).21 In order to increase muscle mass in vertebrates, different strategies of myostatin inhibition have been developed: immunotherapy by administration of neutralizing antimyostatin antibodies,22,23,24,25 in situ production of myostatin chaperone proteins (follistatin, propeptide, and soluble receptor),21,26,27,28,29,30,31,32,33,34,35 arrest of the synthesis of myostatin,36 or transgenic expression of a dominant negative form of the AcvRIIb receptor in muscle fibers.21 Except for mice mimicking the amyotrophic lateral sclerosis, for whom a muscular disorder is not the primary determinant of the pathology,32 inhibition of the myostatin pathway improved muscle mass mediated by hypertrophy without any significant hyperplasia. Moreover, an improvement in the maximal tetanic force was noted, whereas no benefit was shown in maximal specific force. Our aim was to evaluate the effect of the combination of a restoration by a quasi-dystrophin and myostatin inhibition in the dystrophic mdx mouse model.
We have chosen to restore a quasi-dystrophin using the vectorized U7 exon-skipping technique (U7-DYS). The inhibition of the myostatin pathway was mediated using the technique of RNA interference directed against the myostatin receptor AcvRIIb mRNA. RNA interference consists of introducing small synthetic double-stranded RNAs of 19–21 bases into cells37 that will induce the destruction of target mRNAs, resulting in downregulation of the expression of specific genes (for review, see ref. 38). These small synthetic RNAs can be introduced into cells by transfection, or alternatively they can be stably produced by cells when small hairpin RNAs (shRNA) are encoded by the transferred genes.39
Here, we report that in adult mdx mice, intramuscular injection of adeno-associated virus (AAV) encoding specific shRNA efficiently downregulates AcvRIIb mRNA, resulting in increased muscle mass. Moreover, we show that this increase in muscle mass is mediated preferentially by hyperplasia rather than by hypertrophy. We also show that either downmodulation of the AcvRIIb or restoration of a quasi-dystrophin by the U7-DYS AAV vectors can independently improve both specific and absolute maximal tetanic forces in mdx dystrophic mouse muscles, but more importantly, when sh-AcvRIIb is combined to U7-DYS on the same AAV vector, the absolute tetanic and specific maximal forces are significantly improved by 35 and 30%, respectively, demonstrating the additive effect of these two approaches.
Results
Choice of the best shRNA
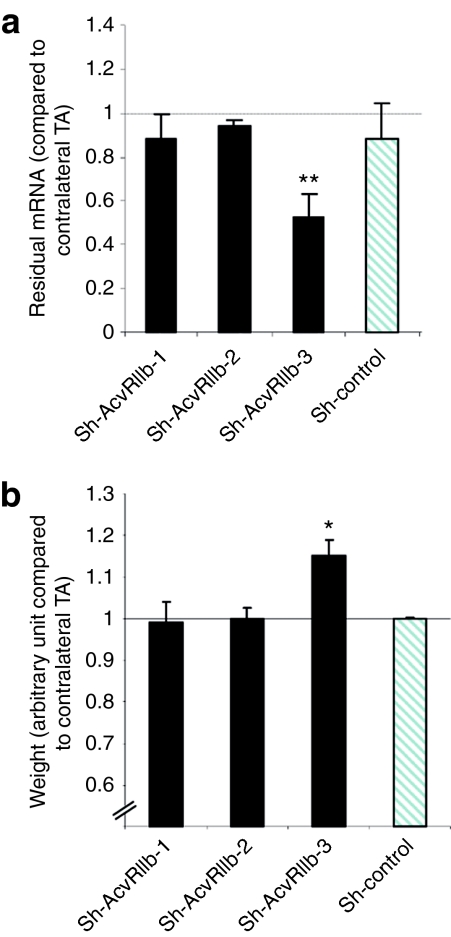
In order to efficiently downregulate the AcvRIIb mRNA, we designed three shRNAs. These shRNAs were produced from an AAV-2/1, which is known to maintain robust and long-term transduction of muscle fibers when delivered intramuscularly or intravenously. Subsequent vectors were tested by intramuscular injections of 2 × 1011 viral genomes into the tibialis anterior (TA) muscles of young adult (6–8 weeks old) mdx mice (n = 5 per construct). After 1 month, treated muscles were carefully isolated and processed for molecular and histological analyses. Figure 1 shows the residual amount of AcvRIIb-targeted mRNA (Figure 1a) and the muscle weights (Figure 1b) in treated muscles compared to contralateral muscles injected with only phosphate-buffered saline (PBS). As expected, a shRNA harboring a random shRNA (sh-control) did not affect AcvRIIb mRNA expression levels. Quantitative RT-PCR showed a highly significant downregulation of AcvRIIb mRNA only in muscles treated with sh-AcvRIIb-3 AAV (53 ± 10% of residual mRNA, P = 0.01) (Figure 1a). As expected, a statistically significant increase in weight was observed only for this shRNA (+15 ± 3.8%; P = 0.03) (Figure 1b). Sh-control did not affect the muscle mass. Incidentally, qPCR directed against the viral genome revealed that the absence of effect of the other constructs was not due to inefficient vector entry (data not shown).
Figure 1.
Effect of activin receptor IIb-shRNA. mdx mice were intramuscularly injected with an AAV-1 vector carrying sh-RNA directed against activin receptor IIb (sh-AcvRIIb). One month after injection, mice were killed, and (a) the tibialis anterior muscles were weighed and (b) the expression level of the targeted mRNA was measured by quantitative RT-PCR. The results obtained with the phosphate-buffered saline-injected contralateral TA (for either the weight or the residual mRNA) were used as references. *P < 0.05; **P < 0.01. Graphs below show result from n = 5 mice for each point. TA, tibialis anterior.
Morphometric analyses after intramuscular injection of AAV downregulating the AcvRIIb mRNA
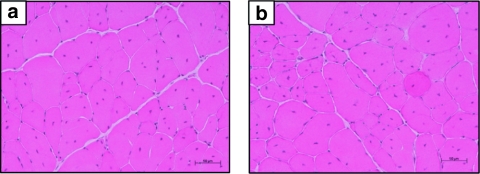
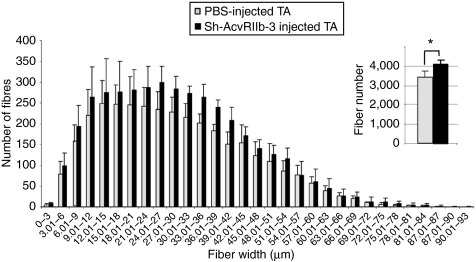
We next examined the physiological aspects of TA transduced with the sh-AcvRIIb-3 AAV vector. Standard histological staining with hematoxylin and eosin did not reveal any major histological changes between AAV-treated muscles and PBS-injected muscles (Figure 2): centrally localized nuclei, pockets of myonecrosis and inflammation were present in both muscles. Morphometric analyses were carried out to determine whether the gain in muscle mass was due to hypertrophy and/or hyperplasia. Laminin staining in muscles treated with sh-control did not show any change either in total fiber number or size distribution (data not shown). In the sh-AcvRIIb-3-treated muscles, morphometric analysis showed a significant increase of fiber number (Figure 3). The mean fiber number in the control TA was 3,380 ± 235, whereas it was 4,085 ± 148 in the sh-AcvRIIb-3-injected TAs, which corresponds to a 20% increase in the number of fibers in the treated muscles. Interestingly, morphometric analysis did not show fiber hypertrophy: the mean smallest fiber diameters were the same in injected and control TA (30.2 ± 13.3 µm versus 30.2 ± 12.8 µm, respectively), and the medians were very similar (medians: 28.2 µm in the injected TA versus 28.4 µm in the noninjected TA). Moreover, the single fiber width distribution (Figure 3) for muscles treated with AAV carrying the sh-AcvRIIb-3 was not statistically different from PBS-injected muscles (n = 5, χ2 = 3.6, P = 1).
Figure 2.
Hematoxylin and eosin staining of muscle cross sections. The tibialis anterior muscles of mdx dystrophic mice were injected with (a) phosphate-buffered saline or (b) an AAV-1 carrying the sh-AcvRIIb-3. One month after injection, mice were killed, and the muscles were mounted in OCT and then frozen. Transverse 8 µm frozen sections were performed using a cryostat, and sections were stained with hematoxylin and eosin.
Figure 3.
Distribution of fiber diameter. The tibialis anterior muscles of mdx mice were injected with 2 × 1011 viral genome of sh-AcvRIIb-3 AAV-1 or with PBS (50 µl). One month after injection, mice (n = 5) were killed, and muscles were sectioned and labeled with laminin. The fiber number and their size were measured using the Ellix software. Gray bars represent muscles injected with PBS. Black bars represent muscles injected with AAV coding for sh-AcvRIIb-3. *P < 0.05. PBS, phosphate-buffered saline.
Combination of myostatin pathway interference and dystrophin rescue in mdx adult mice
For this study, we designed new AAV constructs (U7-DYS/sh-AcvRIIb) capable of both downregulating AcvRIIb mRNA and rescuing the dystrophin expression by using the exon-skipping strategy (modified U7-DYS snRNA targeting exon 23). mdx mice were injected with an AAV vector carrying either the U7-DYS/sh-AcvRIIb construct, the U7-DYS construct alone, or the sh-AcvRIIb construct alone. Resulting AAV vectors were tested via intramuscular injections (TA) in mdx dystrophic mice. Contralateral muscles received either PBS or a vector harboring the control sh-RNA and were used as internal references.
Three months after injection, treated TA muscles were harvested and weighed, and dystrophin expression was analyzed. The weight of both treated TA and contralateral TA muscles remained unchanged (Figure 4a). This is in contrast to the increase in weight that we had observed at 1 month using the sh-AcvRIIb construct alone. As expected, immunolabeling of dystrophin showed efficient dystrophin rescue in the muscles injected with either U7-DYS or U7-DYS/sh-AcvRIIb vectors (Figure 4b). It should be noted that the dystrophin-positive clusters observed in the control mdx mice correspond to revertant fibers (Figure 4b, B4). Similar clusters were also observed in the TA muscles injected with the sh-AcvRIIb vector (Figure 4b, B3). We next counted the number of centrally located nuclei in the different groups. We observed a significant reduction in the percentage of fibers with centrally located nuclei in the treated U7-DYS/shAcvRIIb group (57 ± 7%) compared to the contralateral TA (72 ± 5%) (P < 0.05) (Figure 4c). No difference was observed with the sh-AcvRIIb vector injected, whereas a significant reduction was also observed between the U7-DYS-treated group and the contralateral TA (53 ± 8% versus 69 ± 9%, respectively, P < 0.01), thus showing that the dystrophin rescue is the main parameter mediating the repositioning of the nuclei at the fiber periphery.
Figure 4.
Long-term effect of intramuscularly injected AAV-1 vectors carrying either sh-AcvRIIb, U7-DYS, or U7-DYS/sh-AcvRIIb construct. Mice were killed 3 months after injection. (a) Relative weight expressed as arbitrary units. Each TA is represented by a black diamond, and the mean is represented by a red square. (b) Dystrophin immunostaining of whole transverse sections from the tibialis anterior injected with U7-DYS AAV (B1), U7-DYS/sh-AcvRIIb AAV (B2), sh-AcvRIIb AAV (B3), and untreated mdx mice (B4). (c) Percentage of central nucleated fibers. The number of central nucleated fibers was counted in five random fields (containing between 120 and 350 fibers) per TA (n = 5 TA/group). (d) AcvRIIb mRNA expression levels were compared to sh-control-injected contralateral TA that corresponds to 1. (e) Relative tetanic force expressed as arbitrary units. A Student's t-test (represented with #) was first used to compare treated muscles versus the contralateral-injected legs (PBS injected for the U7-DYS construct or sh-control injected for the sh-AcvRIIb or U7-DYS/sh-AcvRIIb) in each condition. ##P < 0.01, ###P < 0.001. An ANOVA PLSD Fisher test (represented with *) was then used to compare each treatment: mdx versus sh-AcvRIIb: P > 0.05; mdx versus U7-DYS: P < 0.01; mdx versus U7-DYS/sh-AcvRIIb: P < 0.0001; mdx versus C57Bl6: P < 0.0001; sh-AcvRIIb versus U7-DYS: P > 0.05; sh-AcvRIIb versus U7-DYS/sh-AcvRIIb: P < 0.001; sh-AcvRIIb versus C57Bl6: P < 0.05; U7-DYS versus U7-DYS/sh-AcvRIIb: P < 0.01; U7-DYS versus C57Bl6: P > 0.05; U7-DYS/sh-AcvRIIb versus C57Bl6: P > 0.05. (f) Relative specific force. The results obtained with the mdx mouse (for either the weight or the force) are used as references. The ANOVA PLSD Fisher test was used to analyze the statistics: mdx versus sh-AcvRIIb: P > 0.05; mdx versus U7-DYS: P < 0.05; mdx versus U7-DYS/sh-AcvRIIb: P < 0.001; mdx versus C57Bl6: P < 0.001; sh-AcvRIIb versus U7-DYS: P > 0.05; sh-AcvRIIb versus U7-DYS/sh-AcvRII: P < 0.05; sh-AcvRIIb versus C57Bl6: P < 0.0001; U7-DYS versus U7-DYS/sh-AcvRIIb: P < 0.05; U7-DYS versus C57Bl6: P < 0.0001; U7-shAcvRIIb versus C57Bl6: P < 0.0001. *P < 0.05; **P < 0.01; ***P < 0.001; NS, nonsignificant P > 0.05. ANOVA, analysis of variance; PBS, phosphate-buffered saline; PLSD, protected least-squares difference; TA, tibialis anterior.
We next assessed the level of residual mRNA in the TA muscles (Figure 4d). The restoration of the dystrophin did not induce any modulation in the expression of the AcvRIIb mRNA expression because the level of residual AcvRIIb mRNA was the same in both the treated TA compared to the contralateral TA. However, we did observe that the amount of AcvRIIb mRNA was dramatically reduced in the TA muscles where the sh-AcvRIIb was expressed (36 ± 5% of residual mRNA, P < 0.001 for the U7-DYS/sh-AcvRIIb and 60 ± 6% of residual mRNA, P < 0.001 for sh-AcvIIb-3) confirming that there is a long-term expression of the interfering RNAs, and a subsequent decrease in muscle degeneration and regeneration in these muscles. It can also be noted that the downregulation of AcvRIIb mRNA was more efficient when the dystrophin was also restored probably due to the decreased cellular turnover in these muscles.
Finally, we examined whether the treated mdx muscles would display an increase in muscle strength. In situ contractile properties were analyzed (Figure 4e). We observed that the downregulation of AcvRIIb alone was not sufficient to modify the strength of the treated TA, but if dystrophin was expressed alone, we could demonstrate an increase of 16% (P < 0.01) in the absolute maximal force. More importantly, a combination of the downregulation of AcvRIIb and the dystrophin rescue enhanced muscle maximal strength by 35% (P < 0.001), suggesting there is an additive effect of the two cassettes (Figure 4e). The absolute maximal force of mdx muscles coexpressing the U7-DYS and the sh-AcvRIIb was comparable to the absolute maximal force developed by C57Bl6 mice (P > 0.05) and was greater than the force developed when U7-DYS or the sh-AcvRIIb were expressed alone (P < 0.01).
The specific maximal force was also analyzed by normalizing the maximal power to the muscle mass. Because at 3 months the weights of the treated muscle were not modified by the different injections, it was not surprising to observe comparable results for both absolute and specific maximal forces: the downregulation of the AcvRIIb alone did not modify the specific force (Figure 4f), whereas the expression of the dystrophin alone did (+14%, ±4.1%; P < 0.01). Similarly the coexpression of the sh-AcvRIIb and the U7-DYS enhanced the specific maximal force of the injected muscle by >31% (P < 0.001). However, it should be noted that whereas the absolute maximal force of the muscles that had received the double constructs was similar to that observed in normal C57Bl6 mice, the specific maximal force of the treated mdx muscles was below that measured in C57Bl6 mice but in the same range as previous studies.2,10,40,41
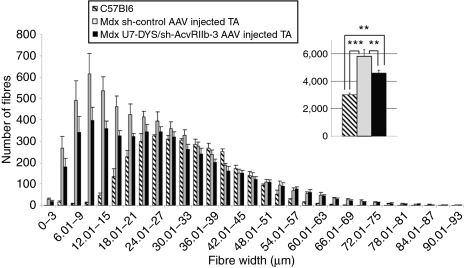
Finally, in order to determine whether the expression of the U7-DYS/sh-AcvRIIb transgene causes hypertrophy and/or hyperplasia, morphometric analyses were performed on muscles treated by this vector (Figure 5). Interestingly, we observed that the number of fibers was decreased in U7-DYS/sh-AcvRIIb-treated muscles compared to sh-control-injected muscles (4,581 ± 212 versus 5,809 ± 474, P = 0.01) but did not reach the number of fibers observed in C57Bl6 mice. As stated previously, this could be a direct consequence of the dystrophin rescue that would stabilize the muscle integrity and stop the repeated cycles of necrosis and regeneration, a phenomenon that persisted in control samples leading to a classical and well-known pseudohypertrophy in dystrophic individuals.
Figure 5.
Fiber diameter and fiber type distributions in U7-DYS/ sh-AcvRIIb-3 AAV-1-injected TA muscles. The tibialis anterior muscles of mdx mice were injected with 2 × 1011 viral genome of either U7-DYS/sh-AcvRIIb-3 AAV or sh-control AAV. Muscles injected with AAV coding for sh-control are in gray and U7-DYS/sh-AcvRIIb-3-injected TA are in black. Three months after injection, mice (n = 9) were killed, and muscles were sectioned and labeled with laminin. The number and size of each fiber was measured. As an indicator, the data obtained with age-matched C57/Bl6 were added (hatched bars). **P < 0.01; ***P < 0.001. PBS, phosphate-buffered saline; TA, tibialis anterior.
Discussion
In this study, we have developed a double strategy combining the restoration of a quasi-dystrophin and the inhibition of myostatin pathway in the mdx mouse model of Duchenne muscular dystrophy. We show that the improvement in both absolute and specific maximal forces is much greater when the two therapeutic strategies are combined. Interestingly, the downregulation of AcvRIIb mRNA was even more efficient when the dystrophin was also restored. This may be explained by the fact that when the fiber integrity is restored, this will protect the fiber from damage and consequently reduce myonuclei renewal in the fibers and allow maintenance of the viral genome. This may explain why in the muscles injected with both sh-AcvRIIb and U7-DYS, the distribution of fiber diameter reveals a clear decrease in the number of small diameter fibers. The rescue of the fiber integrity may also maintain the expression of the sh-AcvRIIb and therefore reduce the AcvRIIb mRNA expression by >60%. It is interesting to note that heterozygote dogs for myostatin mutation are more muscled than normal and are among the fastest dogs in competitive racing events,18 thus suggesting the partial maintenance of the pathway is a benefit.
A second important result that emerged from our study was that the sh-AcvRIIb vector when administered alone induced hyperplasia. Myostatin is expressed during early stages of embryogenesis and its expression remains active although at a lower level in adult animals.42 The developmental stage at which the myostatin pathway is inhibited during development directly influences if the increase in skeletal muscle mass results from hyperplasia and/or hypertrophy. When the myostatin pathway is modified in utero, muscle fibers are always bigger and most of the time more numerous as observed in different myostatin-deficient animals.21,28,29,42,43 On the other hand, some studies do not report any hyperplasia but only hypertrophy.44 When the myostatin pathway is modulated after birth, only hypertrophy is observed in both wild-type23,30,45 and mdx mice.22,27,34
In our experiments, the downregulation of AcvRIIb mRNA resulted in a 20% increase in fiber number 1 month after treatment. This increase could be due either to branching or to hyperplasia. We have favored the hyperplasia hypothesis (although we cannot exclude a role of branching) because the increase in the fiber number was accompanied by a similar increase in the number of nuclei (data not shown).
Muscle hyperplasia is sometimes perceived as a double-edged sword because it can possibly deplete the muscle repair in the long term. Nevertheless, this hyperplasia is only observed when the downregulation of the AcvRIIb is induced without dystrophin rescue. When both strategies are combined, there is a decrease in the number of small diameter fibers.
It cannot be excluded that this hyperplasia could be due to another ligand in addition to myostatin being affected by the downregulation of the AcvRIIb mRNA in adult mdx mice. Indeed, if the consequence of the downregulation of the AcvRIIb mRNA was only to inhibit the binding of the myostatin to the AcvRIIb, the phenotype we observed would have been the same as that observed when the propeptide is overexpressed, i.e., hypertrophy. It is thus reasonable to think that an uncharacterized ligand might play a role in this hyperplasia. This hypothesis is supported by the fact that interactions between the activin receptors and their ligands are complex. First of all, myostatin is not the only protein that can inhibit muscle growth via AcvRIIb. Indeed, in the myostatin−/− mice, the injection of AcvRIIb soluble receptor increases the muscle weight by at least 14% (ref. 45), suggesting that at least one protein in addition to myostatin interacts with AcvRIIb to inhibit muscle growth. This hypothesis is comforted by the fact that BMP-11 and activins A, B, and AB bind the activin receptor IIA and IIB, and can also be blocked from inhibiting the myogenic differentiation with soluble AcvRIIb.46 Second, the AcvRIIb ligands are able to bind receptors other than AcvRIIb. For instance, myostatin has been shown to bind AcvRIIA and AcvRIIb.21 These AcvRIIb ligands can also inhibit muscle growth via an unknown receptor, as suggested by the AcvR2 knockout mouse, where the injection of soluble AcvRIIb enhances the increase in muscle weight observed in the AcvR2−/− mice.45
Another important point to consider for the understanding of the mechanism inducing this hyperplasia is the role of the satellite cells. It was recently shown that muscle hypertrophy driven by myostatin blockade does not require stem/precursor cell activation.47 However, because the strategy used in our study is different from that previously described, a participation of the satellite cells cannot be excluded.
In conclusion, the double approach we have designed and tested has provided us with a new strategy that could improve muscle quality in older Duchenne patients where muscle atrophy and fibrosis are already present. It now deserves to be tested in larger animals, such as in the dystrophic GRMD dog, that has a very similar disease evolution.
Materials and Methods
shRNA constructs. The mouse AcvRIIb gene sequence (Ensembl accession number ENSMUSG00000061393) was analyzed for siRNA target using the siSearch software on Erik Sonnhammer's Web site (http://sonnhammer.sbc.su.se/index.html). Three siRNAs were selected (Table 1) and synthesized to be directly cloned in pSUPER under the control of the H1 promoter. shRNA consisted of a 19-nt sense sequence followed by a 9-nt loop (TTCAAGAGA), a 19-nt reverse sequence, and a RNA pol III terminator (TTTTT). The H1 cassette was then introduced into an AAV-2-based vector between the two ITRs using the blunted SpeI and SalI sites on pSUPER-shRNA plasmid and XbaI site on the pAAV plasmid (Généthon, Evry, France). For the double constructs (U7-DYS/shRNA constructs), the U7-DYS plasmid was digested using XbaI restriction enzyme to allow the cloning of the BamH1/HindIII fragment from the pAAV-shRNA plasmid.
Table 1.
Sequences of hairpin siRNA inserts used in this study
Virus production and titration. All the vectors were produced in human embryonic kidney 293 cells by triple-transfection method using the calcium phosphate precipitation technique with the pAAV-shRNA plasmid, the pXX6 plasmid (Généthon) coding for the adenoviral sequences essential for AAV production, and the pRepCAp plasmid (Généthon) coding for AAV-1 capsid. The virus is then purified by two cycles of cesium chloride gradient centrifugations and concentrated by dialysis. The final viral preparations were kept in PBS solution at −80 °C. The particle titer (number of viral genomes) was determined by quantitative PCR.
Injections. All researches have been conducted according to the French and European regulations. Our animal facility is fully licensed by the French competent authorities and has an animal welfare insurance NIH#A5326-01. So far, the French legislation does not require any approval by a committee. However, each animal experimentation and the protocol are discussed and approved by our animal facility accredited veterinary. Animal experimentation was carried out strictly following French and European laws, directives, and regulations. All animal procedures were performed according to an institution-approved protocol and under appropriate biological containment. The injections in TA were performed on 6- to 8-week-old female mdx mice with 1011 AAV viral genomes. The contralateral TA was injected with 50 µl of PBS or with control shRNA. Mice were killed at different times following injection.
Histological and immunofluorescence analysis. Muscles were weighed, mounted in OCT and then frozen in isopentane cooled in liquid nitrogen. Transverse sections (8 µm) were performed on a cryostat. Sections for labeling were cut from the midbelly region of each muscle and then stained with hematoxylin and eosin, or laminin. Laminin labeling allows us to analyze the number and the smallest diameter of fibers per TA muscle using the Ellix software (Microvision, Evry, France). Dystrophin labeling was carried out using the dystrophin epitope–specific rabbit antibody (Interchim, Paris, France). Briefly, sections were blocked in 20% fetal calf serum (FBS) PBS. The antidystrophin antibody was diluted 1:500 in 2% FBS–PBS and incubated overnight at 4 °C. After three washes, the sections were incubated for 1 hour with a goat anti-rabbit Alexa 488 (Invitrogen, Cergy-Pontoise, France) in 2% FBS–PBS. After three washes, the samples were mounted in Fluoromount-G (CliniSciences, Montrouge, France).
Quantitative RT-PCR. Total RNA was extracted from muscle using the RNeasy Mini Kit (Invitrogen). After DNaseI digestion, the RNA was eluated in 30 µl. Reverse transcription was done on 8 µl of total RNA with random hexamer primers and 50 units of superscript II in a 20 µl final volume. After treatment, the cDNAs were digested with 2 units of RNase H (Invitrogen) for 20 minutes at 37 °C.
For the quantitative PCR, reactions were carried out in a 96-well plate with (per well) 0.8 µl of RT product, 0.17 µl of 20 µmol/l forward (5′CGTGGTGAGGGCCACAA3′) and reverse primers (5′GTGAGGTCG CTCTTCAGCAGTA3′), 0.17 µl of 10 µmol/l FAM-TAMRA probe (5′AT TGCCCACAGGGACTTCAAAAGCAA3′), and 8.5 µl of 2X Master Mix solution (ABgene, Courtaboeuf, France).
The sample transcripts of the PO gene encoding (PO) ubiquity are expressed as the endogenous RNA control.
The PO gene encoding human acidic ribosomal phosphoprotein was used as the endogenous control and each sample was normalized on the basis of its PO content (forward primer: 5′CTCCAAGCAGATGCAGCA GA3′ reverse primer: 5′ATAGCCTTGCGCATCATGGT3′ probe: 5′CC GTGGTGCTGATGGGCAAGAA3′). Each PCR was performed in duplicate on at least three serial dilutions.
Force measurements. Measurements were performed as described previously.48,49 Briefly, mice were anesthetized (pentobarbital sodium, 60 mg/kg), and the limbs were fixed with clamps. The distal tendon of the TA muscle was attached to a dual-mode lever arm system that measures muscle isometric force (300C; Aurora Scientific, Aurora, Ontario, Canada) using a silk ligature. Great care was taken to ensure that the blood and nerve supply remained intact during surgery. All data obtained from the isometric transducer were recorded and analyzed on a microcomputer, using the PowerLab system (4SP; ADInstruments, Paris, France) and software (Chart 4; ADInstruments). The sciatic nerve was crushed proximally and stimulated distally by a bipolar silver electrode using supramaximal square wave pulses of 0.1 ms duration. All isometric measurements were made at an initial muscle length of L0 (length at which maximal tension was obtained during the twitch). Force productions in response to tetanic stimulation were successively recorded (pulse frequency from 25, 50, and 100 to 143 Hz) and at least 1 minute was allowed between each contraction. The absolute maximal force was determined. The muscle mass was measured to calculate specific maximal force (specific force = absolute force/muscle weight). After contractile measurements, the animals were killed with an overdose of pentobarbital. Muscles were then dissected.
Statistical analysis. All data reported here are expressed as mean ± SE of the mean. Muscle growth, mRNA quantification, and fiber counts were analyzed with a Student's t-test. χ2 test was used to analyze fiber width distribution. An analysis of variance protected least-squares difference Fisher test was used to compare the maximum and specific forces that were analyzed 3 months after injection.
Acknowledgments
We thank the in vivo and production departments of Généthon (Evry, France).
REFERENCES
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD., and , Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- McClorey G, Moulton HM, Iversen PL, Fletcher S., and , Wilton SD. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–1381. doi: 10.1038/sj.gt.3302800. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ.et al. (2009Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino Gene Therepub ahead of print). [DOI] [PubMed]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C, et al. Chimeric adeno-associated virus/antisense U1 small nuclear RNA effectively rescues dystrophin synthesis and muscle function by local treatment of mdx mice. Hum Gene Ther. 2006;17:565–574. doi: 10.1089/hum.2006.17.565. [DOI] [PubMed] [Google Scholar]
- Barberi L, Dobrowolny G, Pelosi L, Giacinti C., and , Musarò A. Muscle involvement and IGF-1 signaling in genetic disorders: new therapeutic approaches. Endocr Dev. 2009;14:29–37. doi: 10.1159/000207474. [DOI] [PubMed] [Google Scholar]
- Negroni E, Butler-Browne GS., and , Mouly V. Myogenic stem cells: regeneration and cell therapy in human skeletal muscle. Pathol Biol (Paris) 2006;54:100–108. doi: 10.1016/j.patbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP., and , Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- McPherron AC., and , Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobet L, Poncelet D, Royo LJ, Brouwers B, Pirottin D, Michaux C, et al. Molecular definition of an allelic series of mutations disrupting the myostatin function and causing double-muscling in cattle. Mamm Genome. 1998;9:210–213. doi: 10.1007/s003359900727. [DOI] [PubMed] [Google Scholar]
- Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibé B, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38:813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- Lee SJ., and , McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, McNally EM., and , Khurana TS. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve. 2008;37:308–316. doi: 10.1002/mus.20920. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Poupiot J, Vulin A, Fougerousse F, Arandel L, Daniele N, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther. 2007;14:733–740. doi: 10.1038/sj.gt.3302928. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA., and , Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:19546–19551. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsakas A, Foster K, Otto A, Macharia R, Elashry MI, Feist S, et al. Molecular, cellular and physiological investigation of myostatin propeptide-mediated muscle growth in adult mice. Neuromuscul Disord. 2009;19:489–499. doi: 10.1016/j.nmd.2009.06.367. [DOI] [PubMed] [Google Scholar]
- Foster K, Graham IR, Otto A, Foster H, Trollet C, Yaworsky PJ, et al. Adeno-associated virus-8-mediated intravenous transfer of myostatin propeptide leads to systemic functional improvements of slow but not fast muscle. Rejuvenation Res. 2009;12:85–94. doi: 10.1089/rej.2008.0815. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhao B, Kim YS, Hu CY., and , Yang J. Administration of a mutated myostatin propeptide to neonatal mice significantly enhances skeletal muscle growth. Mol Reprod Dev. 2010;77:76–82. doi: 10.1002/mrd.21111. [DOI] [PubMed] [Google Scholar]
- Magee TR, Artaza JN, Ferrini MG, Vernet D, Zuniga FI, Cantini L, et al. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J Gene Med. 2006;8:1171–1181. doi: 10.1002/jgm.946. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W., and , Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung RK., and , Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R., and , Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Watchko J, O'Day T, Wang B, Zhou L, Tang Y, Li J, et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- Abmayr S, Gregorevic P, Allen JM., and , Chamberlain JS. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol Ther. 2005;12:441–450. doi: 10.1016/j.ymthe.2005.04.001. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM., and , Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mendias CL, Marcin JE, Calerdon DR., and , Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hadhazy M, Wehling M, Tidball JG., and , McNally EM. Dominant negative myostatin produces hypertrophy without hyperplasia in muscle. FEBS Lett. 2000;474:71–75. doi: 10.1016/s0014-5793(00)01570-2. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22:2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA. 2009;106:7479–7484. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud A, Caruelle JP, Martelly I., and , Ferry A. Differential effects of post-natal development, animal strain and long term recovery on the restoration of neuromuscular function after neuromyotoxic injury in rat. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:1–8. doi: 10.1016/j.cbpc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Vignaud A, Noirez P, Besse S, Rieu M, Barritault D., and , Ferry A. Recovery of slow skeletal muscle after injury in the senescent rat. Exp Gerontol. 2003;38:529–537. doi: 10.1016/s0531-5565(03)00007-x. [DOI] [PubMed] [Google Scholar]