Abstract
A number of oncolytic virus (OV) candidates currently in clinical trials are human viruses that have been engineered to be safer for patient administration by limiting normal cell targeting and replication. The newest OVs include viruses that cause no disease in humans, yet still have natural tumor tropism. Raccoonpox virus (RCNV) is a member of the Orthopoxvirus genus of Poxviridae and closely related to vaccinia virus, yet has no known pathogenicity in any mammalian species. A screen of cells from the NCI-60 cancer cell panel using growth curves demonstrated greater than a log increase in replication of RCNV in nearly 74% of the cell lines tested, similar to other tested OV poxviruses. In normal cell lines, pretreatment with interferon (IFN)-α/β resulted in significant inhibition of RCNV replication. In both xenograft and syngeneic models of solid tumors, injection of RCNV resulted in significantly slower tumor progression and increased survival of mice. RCNV treatment also prolonged survival in treatment-resistant models of brain tumors and decreased tumor burden by systemic administration in models of lung metastasis.
Introduction
Oncolytic viruses (OVs) are an exciting emerging targeted cancer therapy. Many tumors often sacrifice components of the inflammatory and antiviral defenses in favor of unchecked cellular growth, constituting the “Achilles heel” that OVs exploit for their own replication.
A large number of viruses have been explored for their ability to preferentially replicate in and kill tumor cells.1 Many of these viruses are responsible for naturally occurring, sometimes benign human infections, and have been genetically modified to increase selectivity to neoplastic tissue.2 Despite very good safety profiles, many of the OVs tested in the clinic do not demonstrate the robust efficacy seen in preclinical models, illustrating that new selective OV candidates need to be identified.
Poxviruses have an extensive history of use in humans either as vaccines for infectious diseases like smallpox or as vaccine vectors for the prevention and treatment of cancer.3 Vaccinia virus is a promising new OV candidate and has been engineered to be tumor selective by a variety of strategies including, but not restricted to, removal of the viral thymidine kinase (TK)4 or by deletion of the vaccinia growth factor (VGF) gene.5 A clinical candidate virus, JX-594, also expresses human GM-CSF to increase its immune-stimulating properties.6 This virus has shown encouraging safety and efficacy in early human clinical studies.7 Indeed, the safety and efficacy experience in the clinic to date with vaccinia supports the idea that the poxvirus platform is a good starting point for the identification of additional OVs.
Recently, myxoma virus, a leporipoxvirus, and Yaba-like disease virus, a yatapoxvirus, have also shown OV potential.3,8,9 Myxoma virus cannot naturally infect humans, yet it has the ability to infect many human cancer cell lines10 and shows efficacy in both immunodeficent brain models and immunocompetent models of other types of tumors.11,12 The capacity of myxoma virus to replicate productively in human tumor cells is intrinsically linked to the Akt status of the cells,13 whereas its exclusion from normal human tissue is controlled by interferons (IFNs) and TNF-α.14
The finding that myxoma virus has significant efficacy as an OV has led to examination of other nonhuman poxviruses that are more closely related to vaccinia virus. These viruses represent an exciting avenue as they likely require little genetic manipulation to be deemed safe for use in humans. Raccoonpox virus (RCNV) is a member of the Orthopoxvirus genus and closely related to vaccinia virus. RCNV was first discovered in a screen of outwardly healthy raccoons in Maryland in 1961.15 It has no known pathology in any species (including raccoons), which leads many to believe that its “natural” host may still be undiscovered. This virus has been used as a wild mammal vaccine and has been shown to successfully induce immunity, without any concurrent pathology in a number of species, including mice, prairie dogs, cats, and raccoons. In this study, we tested a novel RCNV strain that was isolated from the forepaw of a cat in Canada (this was the first report of RCNV infecting felines).16 Like vaccinia, RCNV has a large genome and the potential to harbor and express several transgenes. For the studies presented here, the enhanced green fluorescent protein was inserted into the viral TK locus for visualization purposes. We have assessed virus replication in cells from the NCI-60 cell panel, and demonstrate that RCNV is able to replicate several logs in the majority of human tumor cells tested. In normal cells, the replication of RCNV is significantly dampened by pretreatment with IFN. We also demonstrate significant efficacy of RCNV in treatment of both xenograft and syngeneic tumors in animal models, both by direct and systemic delivery of the virus. We believe that RCNV represents an alternative new oncolytic candidate that likely has an excellent safety profile and significant efficacy despite little genetic interference. Its lack of pathology may represent a unique opportunity to treat patient populations that may be contraindicated for the use of other poxviruses.
Results
RCNV replicates and kills human tumor cells in vitro
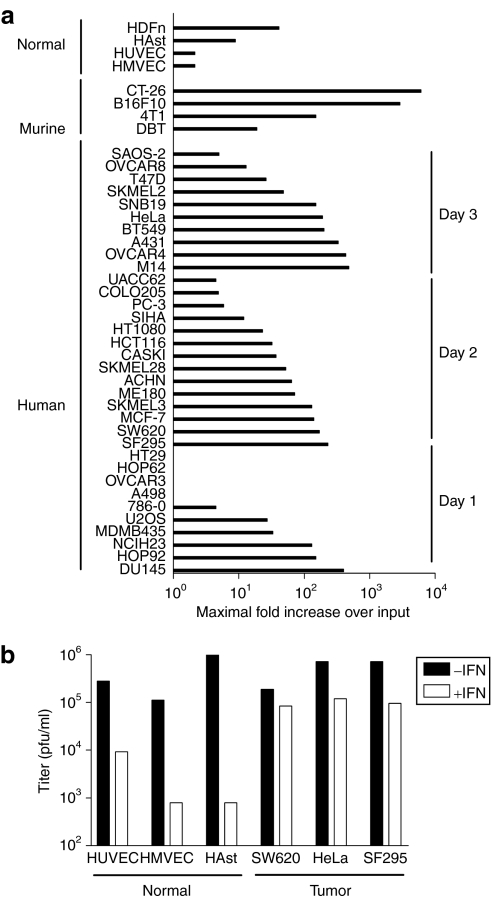
To determine whether RCNV has the ability to infect and kill human tumor cells, a screen of established cancer cell lines from the NCI-60 cell panel was undertaken. Single-step growth curves [multiplicity of infection (MOI) 3] were generated to determine permissiveness, which was defined as greater than one-log increase in virus titers over input, which is within the sensitivity of the plaque assay used to determine titer (Figure 1). Based on this criterion, 74% of cell lines tested were permissive (25 of 34 human tumor cell lines tested). Most (30 of 34 cell lines, 88%) resulted in RCNV titers that were above input virus (1.0, Figure 1a). All murine cancer cells tested showed significant RCNV replication. In addition, RCNV at low MOI (0.1) demonstrated replication in three normal human cell types: umbilical cord vein endothelial cells (HUVECs), dermal microvascular endothelial cells (HMVECs), and astrocytes (HAst) in vitro (Figure 1b). However, pretreatment of these cell lines with type I IFN resulted in an up to four-log decrease in RCNV replication. In contrast, pretreatment human cancer cells resulted in negligible (<1 log) decreases in RCNV titer (Figure 1b).
Figure 1.
Analysis of RCNV replication in normal and tumor cell lines. (a) Human tumor cell lines from the NCI-60 cell panel were screened for replication of RCNV using a single-step growth curve, as were normal cell lines and murine tumor cell lines (as indicated). The lines were infected with RCN-gfp at a multiplicity of infection (MOI) of 3, and the cellular lysates were collected every day for 4 days. The RCNV titer was then determined by titration on BGMK cells. A summary of all of the data is presented, indicating the highest fold increase in titer obtained over the input virus (as determined by collection of cellular lysate at time 0 after infection) on days 1, 2, or 3. (b) Comparison of type I interferon pretreatment on RCNV replication in normal and cancerous human cells. Normal human umbilical cord vein endothelial cells (HUVEC), dermal endothelial cells (HMVEC), astrocytes (HAst), SW620, HeLa, and SF295 were pretreated with Intron A (recombinant human IFN-β for HAst) as described in Materials and Methods, and infected with RCN-gfp at an MOI of 0.1. Cellular lysates were collected at 72 hours after infection and titer determined on BGMK cells. pfu, plaque-forming unit; RCNV, Raccoonpox virus.
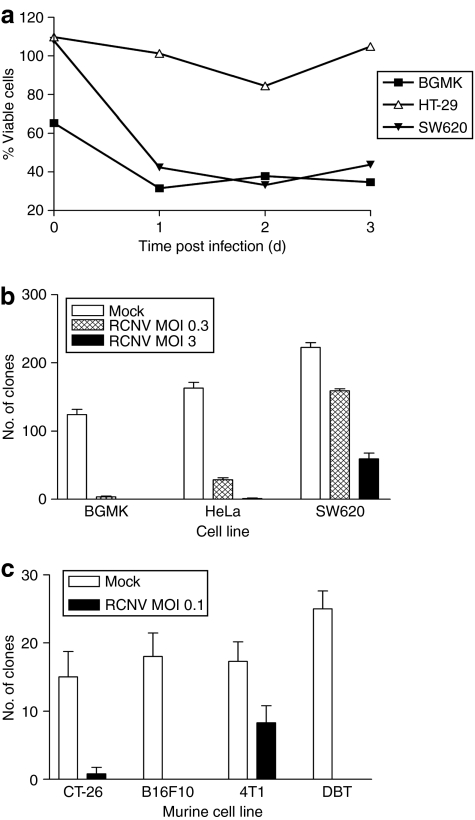
Infection with RCNV correlated with tumor cell death (Figure 2, Supplementary Figure S1). In culture, RCNV infection killed the majority of cells rapidly that can be visualized by microscopy (Supplementary Figure S1) or demonstrated by Trypan blue exclusion (Figure 2a). Both human and murine tumor cell lines have also been examined for their ability to grow after RCNV infection (Figure 2b,c). Cells were infected at the indicated MOI and 24 hours after infection, cells collected and plated in fresh wells. The ability to grow and form clones was then assessed. In human and primate cell lines, reduction in the ability to form clones correlated with viral dose (Figure 2b). The murine lines CT-26, B16F10, and delayed brain tumor (DBT) were greatly reduced in their ability to form clones after RCNV infection at an MOI of 0.1 (Figure 2c). The 4T1 cells were not as dramatically affected by virus treatment. Thus, most cells infected with RCNV are no longer able to grow in vitro, indicating viral destruction of infected cells.
Figure 2.
Tumor cell killing by RCNV. (a) Cell killing in response to virus infection. The indicated cell lines were seeded in 6-well plates and RCNV added at an MOI of 3. At each of the indicated days after infection, the cells were collected and viability determined by Trypan blue exclusion using a Vi-CELL machine. This viability was then normalized to uninfected control cells. (b) Clonogenic assays to determine cell death induced by RCNV. The indicated cell lines were infected at an MOI of 0.3 or 3, and 24 hours after infection, trypsinized and counted. 103 viable cells were added in quadruplicate to 12-well plates and allowed to grow into visible colonies, which were visualized using 0.1% crystal violet. (c) Murine cells were infected with RCN-gfp at an MOI of 0.1 and clonogenic assay performed as above 3 days after infection. MOI, multiplicity of infection; RCNV, Raccoonpox virus.
Safety and viral biodistribution of RCNV
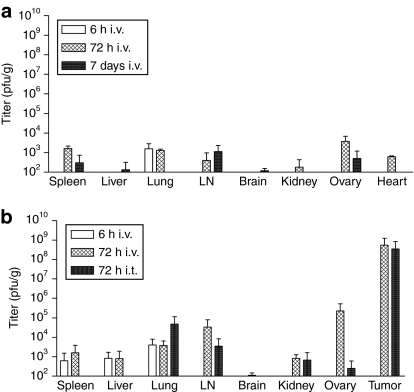
RCNV has been used as a vaccine vector in mice, eliciting excellent immunity to Yersinia pestis and rabies virus without any adverse effects of the viral vector.17,18,19,20,21,22,23,24,25,26,27 Prior to our therapy experiments, we confirmed the safety of our strain in adult, immunocompetent mice. In toxicity experiments, a single intraperitoneal (i.p.) injection of up to 5 × 107 plaque-forming units (pfu) or intravenous (i.v.) dose of up to 108 pfu of virus did not yield measurable signs of illness in naive immunocompetent (Balb/c) mice. A biodistribution experiment in CD-1 nude mice after i.v. dose of 107 pfu of RCNV indicated little virus in any of the organs tested at 6 hours, 72 hours, or 7 days after virus injection (Figure 3a), thereby validating the lack of illness in our safety experiments. A subsequent biodistribution assay was performed in tumor-bearing immunocompetent animals (Figure 3b). A dose of 108 pfu RCN-gfp was injected into CT-26 tumor-bearing Balb/c mice (two per group) by the i.v. or intratumoral (i.t.) route, and at both early (6 hours) and later (72 hours) time points, the organs were collected and RCNV titer determined. At 6 hours after i.v. injection, RCNV could be detected in the spleen, liver, and lung, at relatively low titer (white bars). By 72 hours after injection, RCNV could be detected at low levels in all organs tested, with at least four logs more RCNV detected within the tumor, underlying its proclivity for tumors (hatched and black bars). The titers in all compared organs were very similar regardless of the route of injection, indicating that i.v. delivered RCNV can enter and replicate in tumors. Of note, the highest titers of RCNV in normal tissues were detected in the ovaries of i.v. injected mice. This propensity of orthopoxviruses to enter the ovaries has also been demonstrated using vaccinia virus.5 In nontumor-bearing animals, RCNV could only be detected in the lungs after 3 days (~2 × 104 pfu/g, data not shown). As RCNV was originally discovered in the lungs of raccoons, this tissue tropism is not unexpected.
Figure 3.
Organ distribution of RCNV in mice. (a) Biodistribution experiment was performed in CD-1 nude, naive mice (two per group) and they were injected i.v. with 107 pfu RCN-gfp. At either 6 hours, 72 hours, or 7 days, the mice were euthanized and the indicated organs were collected and homogenized, and the viral titer contained in the lysate was measured using BGMK cells. (b) Biodistribution of RCNV in immunocompetent, tumor-bearing mice. Mice bearing s.c. CT-26 tumors (10 days after implantation) (two mice per group) were injected either i.v. or i.t. with 108 pfu of RCNV. At either 6 or 72 hours after injection, the mice were euthanized and the indicated organs were collected and homogenized, and the viral titer from each individual organ was measured using BGMK cells. The data represented is the average titer from these organs. LN, lymph node; pfu, plaque-forming unit; RCNV, Raccoonpox virus.
RCNV is effective in the treatment of human tumors in a xenograft model
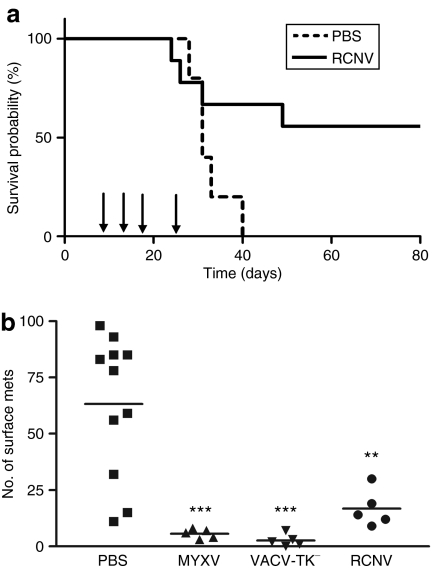
Human colon cancer cell line SW620 was chosen from the screen to examine the efficacy of RCNV treatment of established tumors in nude mice (Figure 4). Tumors were established under the skin and at ~9–12 days postimplantation, we began i.t. injections of RCNV. Treated mice (four mice) received a total of four i.t. injections (time points indicated by arrows) of RCNV (107 pfu/dose), whereas the tumors of control mice (five mice) were injected with phosphate-buffered saline (PBS). Mice that received RCNV exhibited no ill effects from virus treatment. Mice that had PBS-treated tumors rapidly grew to end point and one RCNV-treated tumor demonstrated a similar progression to control tumors. Another treated tumor had significant delay in progression and two of four RCNV-treated mice were cured of their tumors (P = 0.047).
Figure 4.
Treatment of human xenograft tumors in nude mice with RCNV. Human SW620 cells (106 cells) were injected s.c. into the right flank of naive CD-1 nude mice. At 10 days after implantation, RCN-gfp (107 pfu) was injected into the tumor. RCNV was also injected at days 14, 17, and 24 after tumor implantation. Mice were followed until end point that was based on high tumor volume (P = 0.047). PBS, phosphate-buffered saline; pfu, plaque-forming unit; RCNV, Raccoonpox virus.
RCNV is effective in the treatment of syngeneic mouse tumors in immunocompetent animal models
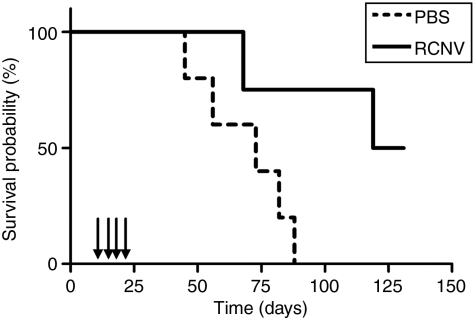
We conducted a similar experiment to those done in the nude mice (Figure 5a) in immunocompetent Balb/c mice bearing syngeneic CT-26 tumors. Repeated intratumoral injections of RCNV into CT-26 tumors improved the survival of mice compared to control animals. In examining individual treated animals, 5 of 11 of the mice were cured of their tumors (Figure 5a, P = 0.0511). As in the xenograft model, there were no visible signs of illness in RCNV-treated mice, yet several mice failed to respond to treatment. Multiple i.v. injections of RCNV did not result in an increased survival in this model, although at early time points following treatment with RCNV delivered i.v. and i.t., we observed significant (P = 0.005) retardation of tumor growth as compared to control tumors (Supplementary Figure S2a). This suggests that early doses of virus reach the tumors and retard their progression; however, insufficient virus from subsequent doses is delivered to establish a curative infection in these subcutaneous tumor models.
Figure 5.
Treatment of syngeneic tumors in immunocompetent mice with RCNV. (a) Treatment of syngeneic solid tumors. CT-26 cells (3 × 105 cells) were injected s.c. into the right flank of Balb/c mice. At 10 days after injection, RCNV (107 pfu) was injected directly into the tumor. Virus was also injected at days 14, 17, and 24 days after implantation. Tumor volume for each mouse was followed in both the PBS-treated (dashed line) and RCNV-treated groups (solid line) until end point (based on high tumor volume) (P = 0.0511). (b) Treatment of metastatic CT-26 tumors with oncolytic poxviruses. CT-26-LacZ cells (105 cells) were injected i.v. into the tail vein of Balb/c mice. RCNV, MYXV, or VACV (107 pfu) was injected i.v. into the mice via the tail vein on days 1, 3, and 8 after cell injection. At day 10, mice were killed, their lungs excised, fixed and stained with an X-gal-containing solution. The lung lobes were then separated and the surface lung metastases counted under a dissecting microscope (**P < 0.01, ***P < 0.001 by analysis of variance). PBS, phosphate-buffered saline; pfu, plaque-forming unit; RCNV, Raccoonpox virus.
In contrast, intravenous administration of RCNV to a lung metastasis model with CT-26 did have a significant impact on tumor burden (Figure 5b). In addition, the poxvirus OVs MYXV and VACV were also administered, and all viruses demonstrated significant efficacy in this model. In a separate experiment, RCNV was injected i.v. to CT-26 lung tumor–bearing animals, and at 24 hours, we collected the lungs. Both microscopy and titration indicated that RCNV is delivered to the lung and it colocalizes with the tumor tissue (Supplementary Figure S2b). This does not rule out a role of immune-mediated destruction of the tumor, yet shows that detectable virus can be found in tumor-bearing lungs.
RCNV can extend survival in treatment-resistant brain tumors in immunocompetent mice
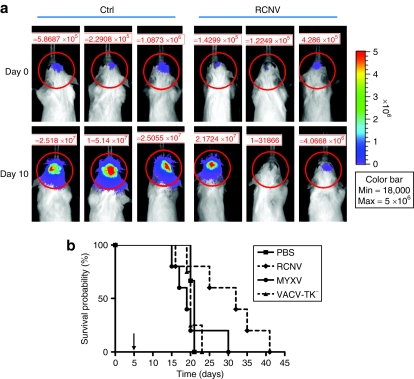
In addition to subcutaneous tumor models, we wanted to assess virus efficacy on malignant glioma, which is both difficult to treat and located in a sensitive organ.28 A single injection of 107 pfu RCNV, MYXV, or VACV was given into the brains of animals harboring intracranial DBTFluc tumors (Figure 6). By IVIS, we saw that several tumors injected with RCNV responded to treatment, with the tumor produced IVIS signal either decreasing or remaining steady 10 days after treatment (Figure 6a) compared to the rapid increase in signal in the control-treated animals. Overall, treatment with RCNV stabilized disease for a short period of time and showed a trend toward prolonged survival in these mice (P = 0.0941) (Figure 6b). In comparison, MYXV and VACV, which has provided significant survival advantage in other brain tumor models,29,30 demonstrated no survival advantage in this particular model (Figure 6b). Importantly, RCNV injections were well tolerated with no acute toxicity or neurological symptoms observed during at least 10 days after infection (when mice started dying of tumor burden).
Figure 6.
Treatment of syngeneic orthotopic glioma model with RCNV. Intracranial DBTFluc tumors were established in Balb/c mice as described in Materials and Methods. After 5 days, 107 pfu RCNV, MYXV, or VACV was injected into the same stereotactic coordinates. (a) IVIS imaging of mice at day 0 (at the time of virus injection) and day 10 after injection. (b) Kaplan–Meier survival curve shows a trend toward increased survival upon RCNV treatment compared to controls (P = 0.0941). PBS, phosphate-buffered saline; pfu, plaque-forming unit; RCNV, Raccoonpox virus.
Discussion
RCNV represents a unique OV candidate. It is related to other OV candidates such as vaccinia virus and myxoma virus, and has no known significant pathology in any mammalian species. It was first described in a screen of an outwardly healthy raccoon population, and was not associated with any known illness.31 RCNV has demonstrated utility as a vaccine vector in wild animal vaccines for rabies virus and sylvatic plague, suggesting its safety in multiple animal species.17,19,23,24,25,26,27,32,33,34 RCNV has also been inadvertently introduced into humans by way of a laboratory accident, and even with direct injection by needle-stick, there were a few symptoms beyond an injection site reaction and a small blister, suggesting further safety for eventual use in humans.35 Our biodistribution experiments also suggest little viral replication in mice after i.v. administration in both naive and tumor-bearing mice (Figure 3). It appears therefore that RCNV is a naturally attenuated poxvirus, and we show here that it has inherent oncolytic activity.
In this report, we directly correlate the OV capacity of RCNV to the virus' ability to replicate in vitro, in a manner similar to what has been done to describe the OV capacity of viruses, such as HSV and adenovirus.36,37 In many other reports, the cellular death induced by the virus is sufficient to argue significant replication. However, poxviruses are known to induce death in vitro independent of viral replication (particularly at very high MOI) due to their ubiquitous ability to enter cells and initiate early gene transcription and stimulate death programs.38 Thus, we have chosen significant viral replication as our indication of oncolytic capacity and then correlated this to specific cellular killing in vitro.
RCNV replicates in most tumor cell lines tested, similar to that seen with other OV poxviruses.10 Comparison of the tumor tropism of RCNV to that of myxoma virus, where a screen of human tumor cell lines has been completed, revealed that cell lines, such as HCT116 (colon cancer), are susceptible to both poxviruses; however, RCNV is able to infect and kill human cancer cell lines such as M14 (melanoma), ACHN (renal), and MCF-7 (breast) that restrict myxoma virus replication. Conversely, cell lines such as PC3 (prostate) are restrictive to RCNV but permissive to myxoma virus.10 A more systemic comparison of these viruses is required, but the observed differences in the level of susceptibility indicate that these viruses have unique mechanisms of action. In addition, the finding that pretreatment of normal cells with type I IFN largely inhibits RCNV replication is also interesting as it has been shown that myxoma requires the action of both IFN and TNF-α to preclude its replication in normal cells.14 However, other OV candidates such as VSV and NDV have also demonstrated this differential sensitivity to IFN.39,40 We believe that the sequencing of RCNV and study of its unique immunomodulatory genes may give additional clues into its tumor tropism.
RCNV treatment significantly delayed the progression of solid tumors in both xenograft and syngeneic tumor models (Figures 3–6). However, some tumors did not respond to RCNV despite significant viral titers within the tumor, perhaps due to physical barriers within the tumor as has been shown with other viruses. For instance, extracellular matrix that walls off tumor nests can impede the spread of OVs within tumors11,41 arguing that strategies that provide widespread infection of tumors is key for effective therapy.
How is RCNV a distinctive oncolytic poxvirus candidate? It appears to have a natural ability to kill certain tumors that are known to be refractory to other poxvirus candidates suggesting that it targets unique tumor-specific signaling pathways. Its apparent safety in a number of mammalian species argues that it could be added to the arsenal of poxviruses under development for treatment of human tumors. An encouraging observation was that RCNV seemed to provide therapeutic activity in a syngeneic brain tumor model (Figure 6). This may represent a unique niche for RCNV as it showed no obvious effects on normal brain tissue, yet was able to impact on a highly treatment-resistant brain tumor model. Brain tumors in general are an unmet clinical need that would welcome a new OV strategy.
Materials and Methods
Cell lines and viruses
Cell lines: Human tumor cell lines tested were from the NCI-60 reference collection and maintained in media supplemented with a 3:1 mix of fetal calf serum (PAA Laboratories, Etobicoke, Ontario, Canada) and fetal bovine serum (Invitrogen, Burlington, Ontario, Canada), and grown at 37 °C, 5% CO2. The human tumor cell lines used include the following: DU145, HOP 92, HOP62, NCIH23, 786-0, A498, HT29, SW620, MCF-7, SKMEL3, ME180, ACHN, SKMEL28, CASKI, HCT116, HT1080, SIHA, PC-3, COLO205, OVCAR3, OVCAR 4, A431, BT549, SNB19, T47D, MDMB435, OVCAR8, SAOS-2, M14, SF295, UACC 62, HeLa, and U2OS. The murine tumor cell lines used include 4T1, B16F10, CT-26, and DBT. B16F10 expressing LacZ (B16F10-LacZ) was obtained from Ann Chambers (London Regional Cancer Program, London, Ontario, Canada).11 CT-26-LacZ cells have been described.42 DBT cells28 were kindly provided by Robert C Rostomily, University of Washington, School of Medicine, Seattle, WA. DBT cells expressing firefly luciferase (DBTFluc) were generated by plasmid transfection of DBT cells at no more than five passages, with subsequent maintenance in medium supplemented with 1 mg/ml G418 (Sigma, Oakville, Ontario, Canada). Baby green monkey kidney (BGMK) cells were obtained from Grant McFadden (University of Florida, Gainesville, FL). All remaining cell lines were obtained from ATCC, Manassas, VA. Most cell lines, with the exception of the murine melanoma B16F10/B16F10LacZ cells which are grown in α-MEM (HyClone, Logan, UT), were maintained in Dulbecco's modified Eagle's medium (HyClone). HMVEC-dBlAd (adult dermal blood microvascular endothelial) and HUVEC (umbilical vein endothelial) cells (Lonza, Basel, Switzerland) were maintained in EGM-2 MV or EGM-2 medium, respectively, with appropriate supplements (Lonza) for no more than nine passages.
Viruses. RCNV used in this study was isolated from a cat in Southern Ontario, Canada.16 This viral isolate was used to create RCN-gfp, a recombinant with the enhanced green fluorescent protein inserted in the TK open-reading frame, using the homologous sequence for vaccinia virus TK. The viruses myxoma virus (vMyxgfp) and TK– vaccinia virus (JX-594) were as previously described.43,44
Animals. Female 6- to 8-week-old Balb/c, C57BL6, or CD-1 nude mice were supplied by Charles River Canada (St Constant, Quebec, Canada). Mice were housed in groups of up to six mice in microisolator cages within a level 2 biocontainment unit of the Animal Care and Veterinary Services facility (University of Ottawa, Ottawa, Ontario, Canada) in a scheduled 12-hour light/dark environment. All animal protocols were carried out according to standard operating procedures of the Animal Care and Veterinary Services.
Viral growth curves and viability assay. Single-step growth curves were conducted to assess RCNV replication in human tumor cells. Tumor cell monolayers in 6-well plates at 80–95% confluence were infected with RCN-gfp at an MOI of 3, in a volume of 0.5 ml, for 1 hour at 37 °C, 5% CO2. The virus inoculum was then removed and replaced with growth media. At the indicated time points (0, 24, 48, 72, and 96 hours after infection), adherent cells were dislodged using a cell scraper, and both the cells and corresponding supernatants were collected and frozen at −80 °C. Lysates were then subjected to three freeze–thaw cycles to release the virus from the cells. The RCNV contained in the cellular lysates were determined by titration on BGMK cells. Serially diluted samples from lysates were applied to 95% confluent monolayers of BGMK. Following 1-hour adsorption, the virus inoculum was removed, and replaced with Dulbecco's modified Eagle's medium supplemented with 10% serum and 1.5% carboxymethylcellulose (Sigma). The cells were incubated with virus for 4–5 days. The monolayers were then stained with 1% crystal violet in methanol to allow for visualization of plaques and determination of viral titer.
Replication in the following cell lines was also tested in the presence of Intron A (IFN-α): HUVEC, HMVEC, SW620, SF295, and HeLa. Cell monolayers in 12-well plates were treated with Intron A at a concentration of 200 IU/ml overnight prior to infection. Normal human embryonal astrocytes (ScienceCell, Carlsbad, CA) maintained in 10% fetal bovine serum–Dulbecco's modified Eagle's medium were seeded in 12-well plates at 150,000 cells per well (1 ml) in either the absence or presence of 200 IU/ml recombinant human IFN-β (Betaseron). The cells were inoculated with RCN-gfp at an MOI of 0.1 with 200 IU/ml of Intron A in a volume of 100 µl for 2 hours. The inoculum was then removed and replaced with growth media supplemented with Intron A (200 IU/ml). Seventy-two hours after infection, cell lysates were collected and the virus samples titered (as described above).
Viability/clonogenic assays were performed using the same method as the viral growth curves. The indicated cells were prepared and infected with RCN-gfp at the indicated MOI (or mock-infected) as above. At the indicated time points, cells were collected from the wells as above and the cell viability assessed using Trypan blue exclusion via the Vi-CELL instrument. Cells were then replated at 103 viable cells/well in quadruplicate wells of a 12-well plate. Single resuspended cells form clones in 7–14 days in cell culture medium. The monolayers were then stained with 1% crystal violet in methanol to allow for visualization and quantification of clones.
Maximum tolerated dose/biodistribution experiment. To determine the safety of administration of RCNV in mice, increasing doses of RCN-gfp were administered to Balb/c mice by both the intravenous and intraperitoneal routes. Mice (five per group) were injected i.p. with virus with titers ranging from 5 × 104 to 5 × 107 pfu/dose. In a separate experiment, mice (five per group) were injected i.v. with either 106, 107, or 108 pfu of RCN-gfp. The animals were followed for signs of illness for 14 days, examining physical changes such as weight loss, general appearance, lesion formation, and respiratory distress.
The biodistribution of RCNV was determined in naive CD-1 nude mice, tumor-bearing, or naive Balb/c mice. Nude mice (two per group) received a dose of 107 pfu of RCN-gfp, and the indicated organs were collected and titered at 6 hours, 72 hours, or 7 days after injection. Mice with CT-26 tumors (10 days postimplantation) (two mice per group) were injected, either i.v. or i.t., with 108 pfu of RCNV. Naive mice were injected i.v. as a control. At either 6 or 72 hours after injection, the mice were euthanized and the indicated organs were collected, homogenized, and the viral titer contained in the lysate from each individual organ determined using BGMK cells, as above.
In vivo tumor models
Primary tumor models: A single injection of 106 SW620 cells was given subcutaneously to the right flank of nude mice or 3 × 105 CT-26 cells was given subcutaneously to the right flank of Balb/c mice. The mice received four intratumoral injections of RCN-gfp (107 pfu in 100 µl PBS) or 100 µl of PBS on days 11, 14, 17, and 24. Tumors were measured (length and width) using calipers beginning on day 11, until end point (when tumors reached a maximum diameter of 15 mm). At end point, the CT-26 tumors were resected, homogenized and the RCNV viral titer determined on BGMK cells.
Metastasis tumor models: 105 CT-26-LacZ cells were injected i.v. via tail vein into Balb/c mice. Mice were treated with RCN-gfp, vMyxgfp, or VACV (JX-594) (107 pfu in 100 µl PBS) i.v. on days 1, 3, and 8, and end point was 10 days (CT-26) postimplantation. Mice were anesthetized with Euthanyl, and after a terminal bleed via cardiac puncture, lungs were excised and stained with X-gal solution as previously described.11 The number of metastases on the surface of each lung lobe was determined after physical separation of the lungs. They were counted under a dissecting microscope (Leica, Richmond Hill, Ontario, Canada).
Syngeneic orthotopic glioma model: DBTFluc cells were trypsinized, washed with PBS and suspended in PBS at 1 × 106 cells/10 µl. Mice were kept under continuous 2.5% isoflurane anesthesia during intracranial surgeries and IVIS imaging. Mice were affixed to a stereotactic frame, the scalp was disinfected, and the skull exposed by midline scalpel incision. Cells were injected 1.5 mm lateral and 0.5 mm anterior to the rostral confluence of the sinus20 at a depth of 3.5 mm over 2 minutes. A fresh batch of cells was used for every five mice.
Mice were imaged 4 days after surgery and divided into groups based on positive tumor (Fluc) signal. Five days postimplantation, a group of five mice received an intracranial injection of 1 × 107 pfu RCN-gfp in 10 µl PBS (surgeries as above). Another group of five mice received 107 pfu vMyxgfp, or 107 pfu JX-594 (TK− vaccinia virus) and three mice serving as controls, received PBS. Animals were imaged at 3- to 4-day intervals over 45 days. Mice reaching end point were killed and the brains stored at −80 °C for titration.
For detection of tumor (Fluc) activity, mice were injected intraperitoneally with 3 mg beetle D-luciferin (Promega, Madison, WI) and imaged 7 minutes later in the IVIS 200 apparatus (Xenogen, Hopkinton, MA) with 1-minute exposure.
Statistical analysis. All statistical analyses were performed using the GraphPad Prism 3.0 software (GraphPad Software, La Jolla, CA). Survival curves were generated by the Kaplan–Meier method, and the log-rank test was used to compare groups.
SUPPLEMENTARY MATERIALFigure S1. Representative microscopy of cell lines infected with RCN-gfp.Figure S2. RCNV treatment in CT-26 solid tumor and lung models.
Supplementary Material
Representative microscopy of cell lines infected with RCN-gfp.
RCNV treatment in CT-26 solid tumor and lung models.
Acknowledgments
We thank Grant McFadden of the University of Florida for providing vMyxgfp, and Jan Brun at the Children's Hospital of Eastern Ontario for providing DBTFluc cells. Research support for this project was provided by the Canadian Institute for Health Research and the Ontario Institute for Cancer Research.
REFERENCES
- Vähä-Koskela MJ, Heikkilä JE., and , Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and , Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Kirn DH., and , Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- Liu TC, Hwang TH, Bell JC., and , Kirn DH. Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop. Mol Ther. 2008;16:1006–1008. doi: 10.1038/mt.2008.70. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Stanford MM., and , McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- Hu Y, Lee J, McCart JA, Xu H, Moss B, Alexander HR, et al. Yaba-like disease virus: an alternative replicating poxvirus vector for cancer gene therapy. J Virol. 2001;75:10300–10308. doi: 10.1128/JVI.75.21.10300-10308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypula, J,, Wang, F,, Ma, Y,, Bell, J., and , McFadden, G. Myxoma virus tropism in human cells. Gene Ther Mol Biol. 2004;8:102–115. [Google Scholar]
- Stanford MM, Shaban M, Barrett JW, Werden SJ, Gilbert PA, Bondy-Denomy J, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther. 2008;16:52–59. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gao X, Barrett JW, Shao Q, Bartee E, Mohamed MR, et al. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 2008;4:e1000099. doi: 10.1371/journal.ppat.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EK, Palmer EL, Obijeski JF., and , Nakano JH. Further characterization of Raccoonpox virus. Arch Virol. 1975;49:217–227. doi: 10.1007/BF01317540. [DOI] [PubMed] [Google Scholar]
- Yager JA, Hutchison L., and , Barrett JW. Raccoonpox in a Canadian cat. Vet Dermatol. 2006;17:443–448. doi: 10.1111/j.1365-3164.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- Osorio JE, Powell TD, Frank RS, Moss K, Haanes EJ, Smith SR, et al. Recombinant raccoon pox vaccine protects mice against lethal plague. Vaccine. 2003;21:1232–1238. doi: 10.1016/s0264-410x(02)00557-1. [DOI] [PubMed] [Google Scholar]
- Rocke TE, Iams KP, Dawe S, Smith SR, Williamson JL, Heisey DM, et al. Further development of raccoon poxvirus-vectored vaccines against plague (Yersinia pestis) Vaccine. 2009;28:338–344. doi: 10.1016/j.vaccine.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Mencher JS, Smith SR, Powell TD, Stinchcomb DT, Osorio JE., and , Rocke TE. Protection of black-tailed prairie dogs (Cynomys ludovicianus) against plague after voluntary consumption of baits containing recombinant raccoon poxvirus vaccine. Infect Immun. 2004;72:5502–5505. doi: 10.1128/IAI.72.9.5502-5505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio JE, Frank RS, Moss K, Taraska T, Powell T., and , Stinchcomb DT. Raccoon poxvirus as a mucosal vaccine vector for domestic cats. J Drug Target. 2003;11:463–470. doi: 10.1080/10611860410001670062. [DOI] [PubMed] [Google Scholar]
- Hu L, Ngichabe C, Trimarchi CV, Esposito JJ., and , Scott FW. Raccoon poxvirus live recombinant feline panleukopenia virus VP2 and rabies virus glycoprotein bivalent vaccine. Vaccine. 1997;15:1466–1472. doi: 10.1016/s0264-410x(97)00062-5. [DOI] [PubMed] [Google Scholar]
- Lodmell DL, Esposito JJ., and , Ewalt LC. Rabies virus antinucleoprotein antibody protects against rabies virus challenge in vivo and inhibits rabies virus replication in vitro. J Virol. 1993;67:6080–6086. doi: 10.1128/jvi.67.10.6080-6086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini JC, Bickle HM, Brodie SJ, He BX., and , Esposito JJ. Raccoon poxvirus rabies virus glycoprotein recombinant vaccine in sheep. Arch Virol. 1993;133:211–222. doi: 10.1007/BF01309757. [DOI] [PubMed] [Google Scholar]
- Hable CP, Hamir AN, Snyder DE, Joyner R, French J, Nettles V, et al. Prerequisites for oral immunization of free-ranging raccoons (Procyon lotor) with a recombinant rabies virus vaccine: study site ecology and bait system development. J Wildl Dis. 1992;28:64–79. doi: 10.7589/0090-3558-28.1.64. [DOI] [PubMed] [Google Scholar]
- Fekadu M, Shaddock JH, Sumner JW, Sanderlin DW, Knight JC, Esposito JJ, et al. Oral vaccination of skunks with raccoon poxvirus recombinants expressing the rabies glycoprotein or the nucleoprotein. J Wildl Dis. 1991;27:681–684. doi: 10.7589/0090-3558-27.4.681. [DOI] [PubMed] [Google Scholar]
- Lodmell DL, Sumner JW, Esposito JJ, Bellini WJ., and , Ewalt LC. Raccoon poxvirus recombinants expressing the rabies virus nucleoprotein protect mice against lethal rabies virus infection. J Virol. 1991;65:3400–3405. doi: 10.1128/jvi.65.6.3400-3405.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito JJ, Knight JC, Shaddock JH, Novembre FJ., and , Baer GM. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology. 1988;165:313–316. doi: 10.1016/0042-6822(88)90692-7. [DOI] [PubMed] [Google Scholar]
- Mourad PD, Farrell L, Stamps LD, Chicoine MR., and , Silbergeld DL. Why are systemic glioblastoma metastases rare? Systemic and cerebral growth of mouse glioblastoma. Surg Neurol. 2005;63:511–9; discussion 519. doi: 10.1016/j.surneu.2004.08.062. [DOI] [PubMed] [Google Scholar]
- Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barrett JW, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Adventures with poxviruses of vertebrates. FEMS Microbiol Rev. 2000;24:123–133. doi: 10.1016/S0168-6445(00)00027-9. [DOI] [PubMed] [Google Scholar]
- Rocke TE, Smith SR, Stinchcomb DT., and , Osorio JE. Immunization of black-tailed prairie dog against plague through consumption of vaccine-laden baits. J Wildl Dis. 2008;44:930–937. doi: 10.7589/0090-3558-44.4.930. [DOI] [PubMed] [Google Scholar]
- Wasmoen TL, Kadakia NP, Unfer RC, Fickbohm BL, Cook CP, Chu HJ, et al. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus. Adv Exp Med Biol. 1995;380:221–228. doi: 10.1007/978-1-4615-1899-0_36. [DOI] [PubMed] [Google Scholar]
- Spatz SJ, Rota PA., and , Maes RK. Identification of the feline herpesvirus type 1 (FHV-1) genes encoding glycoproteins G, D, I and E: expression of FHV-1 glycoprotein D in vaccinia and raccoon poxviruses. J Gen Virol. 1994;75 (Pt 6):1235–1244. doi: 10.1099/0022-1317-75-6-1235. [DOI] [PubMed] [Google Scholar]
- Rocke TE, Dein FJ, Fuchsberger M, Fox BC, Stinchcomb DT., and , Osorio JE. Limited infection upon human exposure to a recombinant raccoon pox vaccine vector. Vaccine. 2004;22:2757–2760. doi: 10.1016/j.vaccine.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Peng KW, Ranki T, Kangasniemi L, Chaudhuri TR, et al. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Ther. 2005;12:87–94. doi: 10.1038/sj.gt.3302387. [DOI] [PubMed] [Google Scholar]
- Toda M, Iizuka Y, Kawase T, Uyemura K., and , Kawakami Y. Immuno-viral therapy of brain tumors by combination of viral therapy with cancer vaccination using a replication-conditional HSV. Cancer Gene Ther. 2002;9:356–364. doi: 10.1038/sj.cgt.7700446. [DOI] [PubMed] [Google Scholar]
- Taylor JM., and , Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–150. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Takimoto T, Scroggs RA., and , Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80:5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YS, Kim H, Huang JH, Yoon AR., and , Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282–5292. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Chang W, Chung CS, Zeng W, Masters J, et al. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J Virol. 2003;77:5877–5888. doi: 10.1128/JVI.77.10.5877-5888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative microscopy of cell lines infected with RCN-gfp.
RCNV treatment in CT-26 solid tumor and lung models.