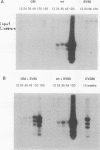
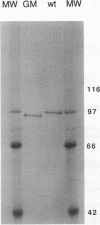
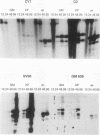
Abstract
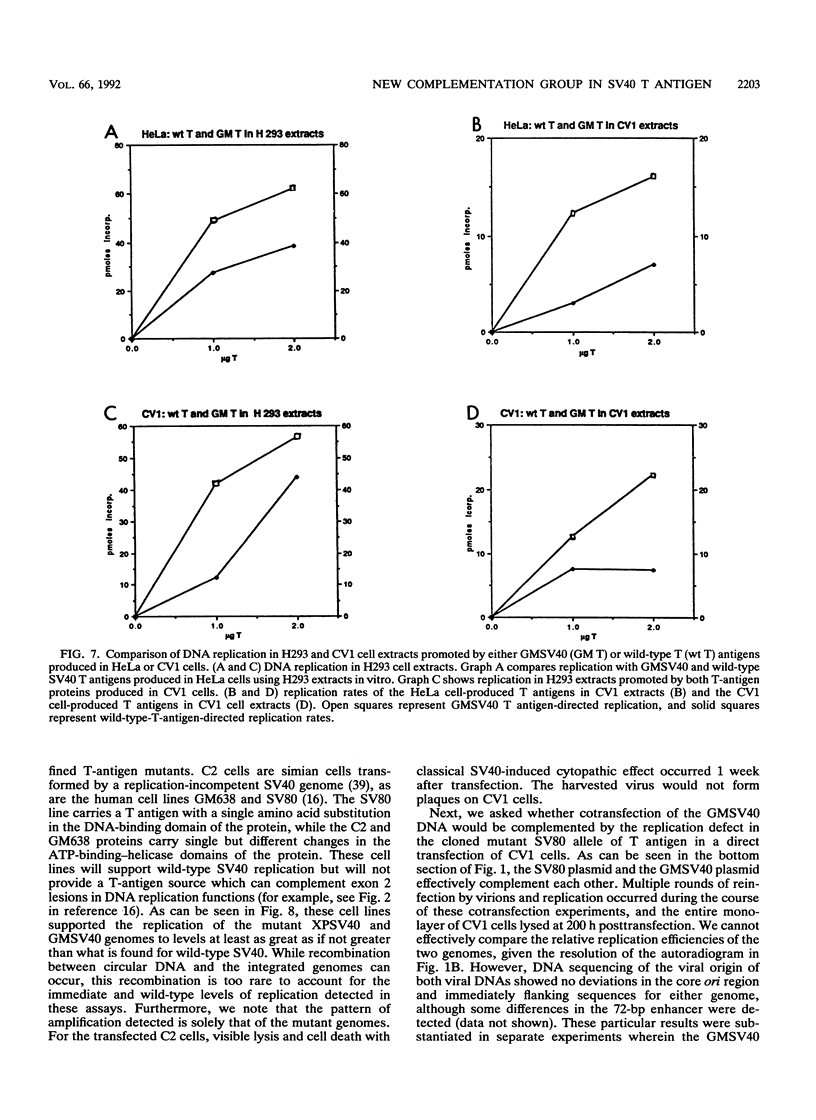
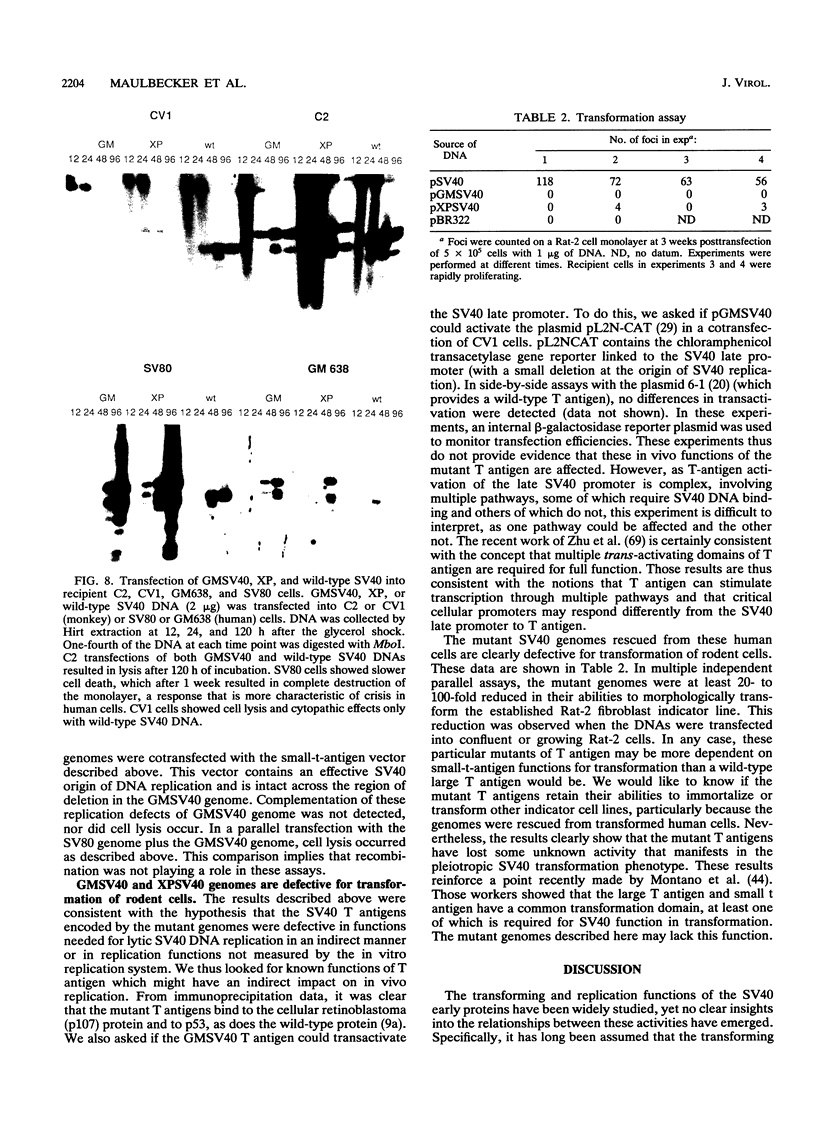
We describe a new complementation function within the simian virus 40 (SV40) A gene. This function is required for viral DNA replication and virus production in vivo but, surprisingly, does not affect any of the intrinsic enzymatic functions of T antigen directly required for in vitro DNA replication. Other well-characterized SV40 T-antigen mutants, whether expressed stably from integrated genomes or in cotransfection experiments, complement these mutants for in vivo DNA replication and plaque formation. These new SV40 mutants were isolated and cloned from human cells which stably carry the viral DNA. The alteration in the large-T-antigen gene was shown by marker rescue and nucleotide sequence analysis to be a deletion of 322 bp spanning the splice-donor site of the first exon, creating a 14-amino-acid deletion in the large T antigen. The mutant gene was expressed in H293 human cells from an adenovirus vector, and the protein was purified by immunoaffinity chromatography. The mutant protein directs greater levels of DNA replication in vitro than does the wild-type protein. Moreover, the mutant protein reduces the lag time for in vitro DNA synthesis and can be diluted to lower levels than wild-type T antigen and still promote good replication, which is in clear contrast to the in vivo situation. These biochemical features of the protein are independent of the source of the cellular replication factors (i.e., HeLa, H293, COS 7, or CV1 cells) and the cells from which the T antigens were purified. The mutant T antigen does not transform Rat-2 cells. Several different models which might reconcile the differences observed in vivo and in vitro are outlined. We propose that the function of T antigen affected prepares cells for SV40 replication by activation of a limiting cellular replication factor. Furthermore, a link between the induction of a cellular replication factor and transformation by SV40 is discussed.
Full text
PDF


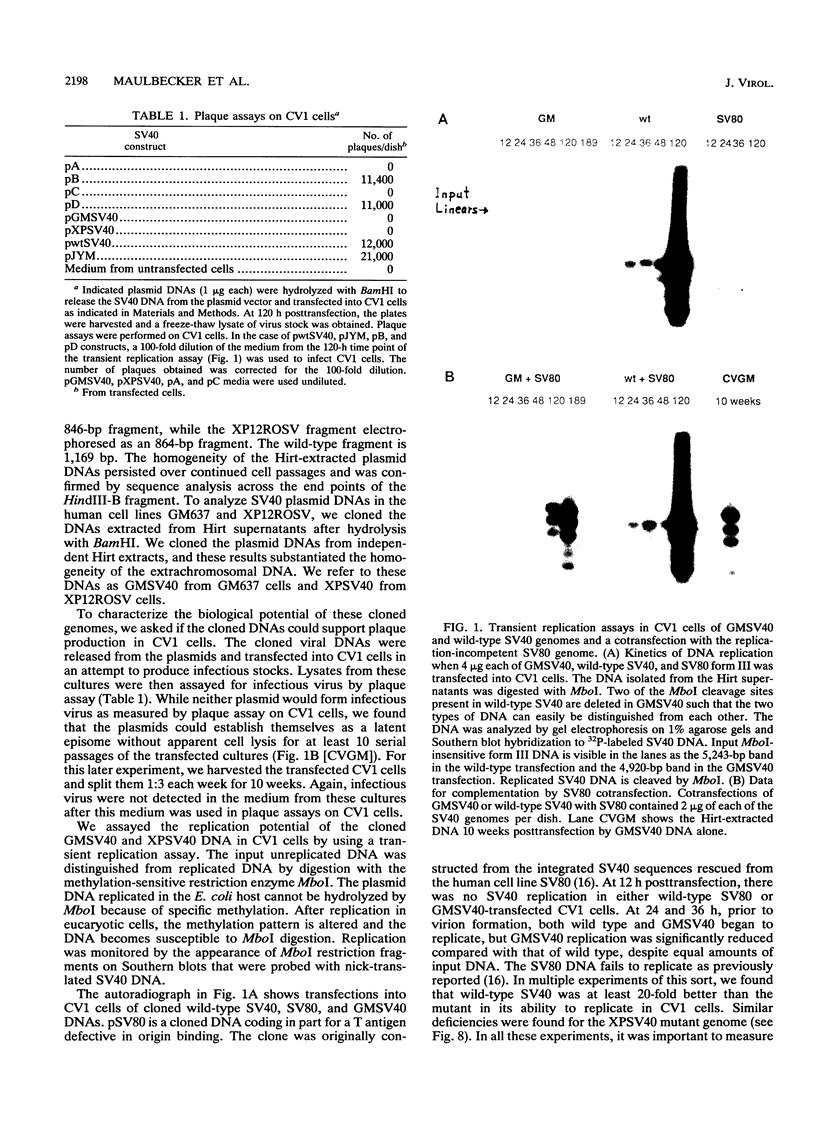



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Fromm M., Berg P. Complex regulation of simian virus 40 early-region transcription from different overlapping promoters. Mol Cell Biol. 1984 Sep;4(9):1900–1914. doi: 10.1128/mcb.4.9.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Cherington V., Brown M., Paucha E., St Louis J., Spiegelman B. M., Roberts T. M. Separation of simian virus 40 large-T-antigen-transforming and origin-binding functions from the ability to block differentiation. Mol Cell Biol. 1988 Mar;8(3):1380–1384. doi: 10.1128/mcb.8.3.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Yamaguchi M., Possenti R., DePamphilis M. L. Initiation of simian virus 40 DNA replication in vitro: aphidicolin causes accumulation of early-replicating intermediates and allows determination of the initial direction of DNA synthesis. Mol Cell Biol. 1986 Nov;6(11):3815–3825. doi: 10.1128/mcb.6.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peden K. W., Nathans D. trans-dominant defective mutants of simian virus 40 T antigen. J Virol. 1987 Feb;61(2):436–445. doi: 10.1128/jvi.61.2.436-445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. J., Gilinger G., Alwine J. C. Simian virus 40 T antigen alters the binding characteristics of specific simian DNA-binding factors. Mol Cell Biol. 1988 Apr;8(4):1648–1656. doi: 10.1128/mcb.8.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Fareed G. C. Transformation of cultured human vascular endothelium by SV40 DNA. Cell. 1976 Dec;9(4 Pt 2):685–693. doi: 10.1016/0092-8674(76)90132-x. [DOI] [PubMed] [Google Scholar]
- Gish W. R., Botchan M. R. Simian virus 40-transformed human cells that express large T antigens defective for viral DNA replication. J Virol. 1987 Sep;61(9):2864–2876. doi: 10.1128/jvi.61.9.2864-2876.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Ahrens B. SV40 early mutants that are defective for viral DNA synthesis but competent for transformation of cultured rat and simian cells. Virology. 1982 Nov;123(1):78–92. doi: 10.1016/0042-6822(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIUNG G. D., GAYLORD W. H., Jr The vacuolating virus of monkeys. I. Isolation, growth characteristics, and inclusion body formation. J Exp Med. 1961 Dec 1;114:975–986. doi: 10.1084/jem.114.6.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Analysis of an activatable promoter: sequences in the simian virus 40 late promoter required for T-antigen-mediated trans activation. Mol Cell Biol. 1985 Aug;5(8):1859–1869. doi: 10.1128/mcb.5.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Khalili K., Brady J., Khoury G. Translational regulation of SV40 early mRNA defines a new viral protein. Cell. 1987 Feb 27;48(4):639–645. doi: 10.1016/0092-8674(87)90242-x. [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Mochly-Rosen D., Koshland D. E., Jr, Linn S. Exposure of HeLa DNA polymerase alpha to protein kinase C affects its catalytic properties. J Biol Chem. 1987 Mar 15;262(8):3432–3435. [PubMed] [Google Scholar]
- Kucherlapati R., Hwang S. P., Shimizu N., McDougall J. K., Botchan M. R. Another chromosomal assignment for a simian virus 40 integration site in human cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4460–4464. doi: 10.1073/pnas.75.9.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Y., Simmons D. T. Stable T-p53 complexes are not required for replication of simian virus 40 in culture or for enhanced phosphorylation of T antigen and p53. J Virol. 1991 Apr;65(4):2066–2072. doi: 10.1128/jvi.65.4.2066-2072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Fairman M. P., Stillman B., Gluzman Y. Large T-antigen mutants define multiple steps in the initiation of simian virus 40 DNA replication. J Virol. 1989 Oct;63(10):4181–4188. doi: 10.1128/jvi.63.10.4181-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Gluzman Y., Fairman M. P., Strauss M., McVey D., Stillman B., Gerard R. D. Production of simian virus 40 large tumor antigen in bacteria: altered DNA-binding specificity and dna-replication activity of underphosphorylated large tumor antigen. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6479–6483. doi: 10.1073/pnas.86.17.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano X., Millikan R., Milhaven J. M., Newsom D. A., Ludlow J. W., Arthur A. K., Fanning E., Bikel I., Livingston D. M. Simian virus 40 small tumor antigen and an amino-terminal domain of large tumor antigen share a common transforming function. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7448–7452. doi: 10.1073/pnas.87.19.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld D. S., Ripley S., Henderson A., Ozer H. L. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987 Aug;7(8):2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Carroll D. Role of defective simian virus 40 genomes in establishment and maintenance of persistently infected primate cell lines. Intervirology. 1983;19(4):181–194. [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., D'Urso G. An origin unwinding activity regulates initiation of DNA replication during mammalian cell cycle. Science. 1988 Sep 16;241(4872):1486–1489. doi: 10.1126/science.2843984. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Peterson W. D., Jr, Haseltine W. A. Biological and biochemical characterization of an SV40-transformed xeroderma pigmentosum cell line. Exp Cell Res. 1984 Apr;151(2):408–420. doi: 10.1016/0014-4827(84)90391-4. [DOI] [PubMed] [Google Scholar]
- Rutila J. E., Christensen J. B., Imperiale M. J. Viral growth, origin binding, and p53 binding properties of simian virus 40 large T antigen transformation and replication mutants. Oncogene Res. 1989;4(4):303–310. [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Peden K. W., Pipas J. M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989 Dec;63(12):5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Maimets T., Addison C., Rudge K., Jenkins J. R. Mouse p53 blocks SV40 DNA replication in vitro and downregulates T antigen DNA helicase activity. Oncogene. 1988 Oct;3(4):405–413. [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kelly T. J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989 May;86(10):3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Green M. R. Retinoblastoma. A transcriptional tryst. Nature. 1991 Jul 18;352(6332):189–190. doi: 10.1038/352189a0. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Friedman P. N., Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Simian virus 40 enhancer increases RNA polymerase density within the linked gene. Nature. 1985 May 2;315(6014):75–77. doi: 10.1038/315075a0. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F. B., Murakami Y., Weissbach L., Hurwitz J. Simian virus 40 DNA replication in vitro: study of events preceding elongation of chains. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4612–4616. doi: 10.1073/pnas.83.13.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Y., Rice P. W., Chamberlain M., Cole C. N. Mapping the transcriptional transactivation function of simian virus 40 large T antigen. J Virol. 1991 Jun;65(6):2778–2790. doi: 10.1128/jvi.65.6.2778-2790.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Jha K. K., Mulder C., Basilico C., Ozer H. L. Human fibroblasts transformed by the early region of SV40 DNA: analysis of "free" viral DNA sequences. Virology. 1980 Jul 30;104(2):439–453. doi: 10.1016/0042-6822(80)90346-3. [DOI] [PubMed] [Google Scholar]