Abstract
A free-standing, robust cell sheet comprising aligned human mesenchymal stem cells (hMSCs) offers many interesting opportunities for tissue reconstruction. As a first step toward this goal, a confluent, uniform hMSC layer with a high degree of alignment and stemness maintenance needs to be created. Hypothesizing that topographical cue and a physiologically relevant low-oxygen condition could promote the formation of such an hMSC layer, we studied the culture of hMSCs on synthetic nanogratings (350 nm width and 700 nm pitch) and either under 2 or 20% O2. Culturing hMSCs on the nanogratings highly aligned the cells, but it tended to create patchy layers and accentuate the hMSC differentiation. The 2% O2 improved the alignment and uniformity of hMSCs, and reduced their differentiation. Over a 14-day culture period, hMSCs in 2% O2 showed uniform connexon distribution, secreted abundant extracellular matrix (ECM) proteins, and displayed a high progenicity. After 21-day culture on nanogratings, hMSCs exposed to 2% O2 maintained a higher viability and differentiation capacity. This study established that a 2% O2 culture condition could restrict the differentiation of hMSCs cultured on nanopatterns, thereby setting the foundation to fabricate a uniformly aligned hMSC sheet for different regenerative medicine applications.
Introduction
Human mesenchymal stem cells (hMSCs) can differentiate into multiple cell lineages, serving as an excellent cell source for regenerative medicine.1,2,3,4,5 Among different forms of applying hMSCs to engineer tissues, a scaffold-free approach is particularly attractive. It avoids any foreign-body response to the scaffold and other complications arising from the by-products of scaffold biodegradation.6,7,8 A micromass pellet culture of hMSCs to generate cartilaginous tissue exemplifies the appeal of this approach.9 A free-standing MSC sheet comprising only cells and their deposited extracellular matrix (ECM) is another prominent example for the regeneration of scarred myocardium10 and bone tissues.11 Although cell sheets alone are restricted in clinical application by their insufficient mechanical strength, three-dimensional tissue structure may be created by utilizing laminar cellular assemblies.12 In addition, fragments of MSC sheet can serve as cell delivery vehicle by providing a favorable ECM environment to retain the transplanted cells and improve the efficacy of therapeutic cell transplantation via direct intramyocardial13 or intramuscular14 injection. Although the multilineage differentiation capability allows hMSC sheets to reconstruct complex tissues, even more attractive would be a uniform cell sheet with aligned hMSCs in a relatively undifferentiated state.
Cellular organization, in many cases alignment, provides functional competence to many tissue types. We have previously fabricated an hMSC sheet from aligned, electrospun thermosensitive chitosan fibers.15 We have also studied the alignment of hMSC on nanogratings fabricated by soft lithography and nanoimprinting, and established that nanopatterns exert a more pronounced effect than micropatterns in aligning cells.16,17 To form an aligned hMSC sheet, the first crucial step would be to grow hMSCs into confluency with a high degree of alignment. We frequently observe hMSCs forming clusters when cultured on a flat surface, consistent with reports in the literature.18 On nanogratings, the hMSCs have an even greater tendency to grow into an uneven patchy layer. A desirable cell sheet should comprise cells forming tight junctions with each other and secrete plenty of ECM proteins to hold the cell sheet together.6,19,20 A nonuniform or patchy structure could make the cell sheet vulnerable to tearing during handling, in addition to compromising the quality of the engineered tissue. Another complication of culturing hMSCs on nanopatterns is the differentiation driven by nanotopographical cues. Nanostructures stimulate hMSCs to differentiate along the neuronal, myogenic, and osteogenic lineages in a proliferative, nondifferentiation medium, while decrease their proliferation.15,17,21
To fully exploit the cell sheet engineering concept with hMSCs, it is highly desirable to form an aligned, confluent hMSC layer while keeping the cells in a relatively undifferentiated state. We propose to achieve this by culturing hMSCs under physiologically relevant oxygen tension and on substrates with nanogratings. Low-oxygen tension is a native physiological condition of the hMSC niche.22 It maintains the undifferentiated state of hMSCs, stimulates hMSC proliferation, and upregulates the secretion of ECM proteins in both two- and three-dimensional cultures.18,22 Low-oxygen tension, when in a suitable range (1–3%), also increases cell motility in vitro.23
In this study, hMSCs were cultured on nanopatterned poly(dimethylsiloxan) (PDMS) under a physiologically relevant low-oxygen (2%) condition over a 21-day period, and compared with three control groups that cultured on flat surface-2% O2, nanograted surface-20% O2, and flat surface-20% O2. The cultures from the four conditions will be hereafter abbreviated as: NN: 20% O2, normoxic, nanopatterned surface; NF: 20% O2, normoxic, flat surface; HN: 2% O2, hypoxic, nanopatterned surface; HF: 2% O2, hypoxic, flat surface. The experimental group showed a highly confluent hMSC layer with a uniformly aligned morphology, abundant ECM protein deposition, tight gap junctions, and improved stemness as reflected in subsequent differentiation assays. This study established that a 2% O2 culture condition could curtail the differentiation tendency of hMSCs cultured on nanopatterns, thereby rendering it possible to fabricate a confluent and uniform layer of aligned hMSCs with plasticity for different regenerative medicine applications.
Results
Cell alignment and nuclear morphology
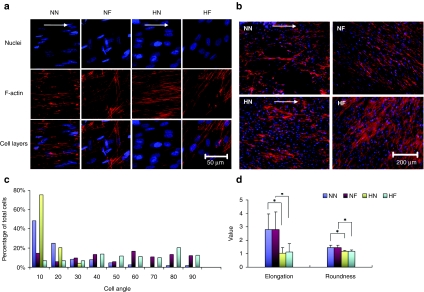
The cell layer uniformity and cell alignment on different surfaces were examined by F-actin and nuclear staining at day 14. The four groups studied are denoted by two letters: the first letter H or N represents either hypoxic or normoxic condition, and the second N or F represents nanopatterned or flat surface. The cells displayed even and fine F-actin fibers in the HN sample, while forming fiber bundles in the NF samples (Figure 1a). A lower magnification (Figure 1b) showed clearly a higher uniformity of the cell layer formed at the two 2% O2 samples (HN and HF). The cytoskeleton of hMSCs was more regularly aligned along the grating axis on the HN samples than their counterparts cultured at NN condition, which demonstrated a patchy pattern. Cell alignment was defined as the angle between the major axis of each cell nucleus and the main direction of the grating axis. The percentage of aligned cells with angles <10° was 75% in HN samples, and 58% in NN samples (Figure 1c). The distribution of alignment angles was also narrower in the HN condition than the NN condition. The alignment angles of HN samples were confined within 30° whereas only ~80% in the NN samples fell within this range and the rest distributed in higher angles up to 90°. Cells cultured on flat surfaces under both normoxic and hypoxic conditions showed no directionality as expected (NF and HF).
Figure 1.
Morphology of nuclei and F-actin, and cell nuclear parameters of human mesenchymal stem cells (hMSCs) grown on conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF) on day 14. (a) Morphology of nuclei (blue) and F-actin (red) of hMSCs. The cells exhibited slightly elongated nuclei and finer F-actin fibers in the two 2% O2 samples (HN and HF), whereas cultures from the two 20% O2 samples displayed significantly more elongated nuclei. White arrows indicate the direction of nanogratings on the surface of PDMS. (b) Morphology of F-actin of hMSC layers at day 14 at conditions of NN, NF, HN, and HF in a low magnification. Uniform cell layers were formed at the two 2% O2 samples (HN and HF), whereas the NN samples displayed a patchy structure. (c) The distribution of the cell nuclear alignment angles in the four conditions. The percentage of aligned cells with angles <10&Ring; was significantly higher in HN samples than NN samples, and the distribution of alignment angles was also much narrower in the HN condition than in the NN condition. (d) Cell nuclear morphology in the four conditions after 14 days of culture. The nuclei of hMSCs grown under the two 2% O2 conditions were more elongated than their counterparts in the normal oxygen cultures. Values are means ± SD for six samples of each substrate (*P < 0.05).
After 14 days in culture, cells grown under low-oxygen conditions (HN and HF) exhibited a nuclear morphology that was slightly elongated (Figure 1a), with no significant difference in roundness (RN) (P > 0.05) and elongation factor E (P > 0.05) between the nanopatterned (HN) and flat (HF) surfaces (Figure 1d). In contrast, cells grown at the normoxic condition displayed a more elongated nuclear morphology than their low-oxygen counterparts. The nuclear factor E of hMSCs from NN condition was 2.7 compared to 1.0 for HN (P < 0.01) (Figure 1d) (a spherical nucleus would have an E = 0 and RN = 1; RN > 1 indicates a less than spherical shape). The RN of hMSCs from normoxic conditions was both significantly higher than those from hypoxic conditions (P < 0.01), indicating a less rounded cell nuclear shape. In contrast, no significant differences were observed between the nanopatterned (NN) and flat (NF) surfaces under normoxic condition (Figure 1d).
Gap junction communication and ECM secretion
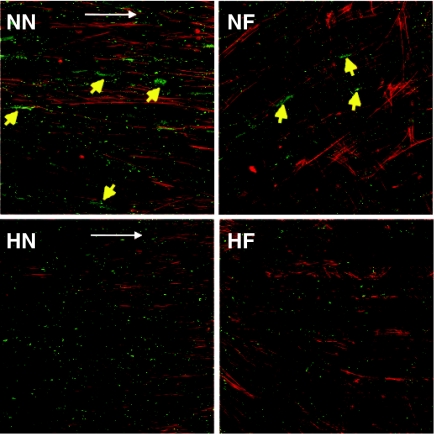
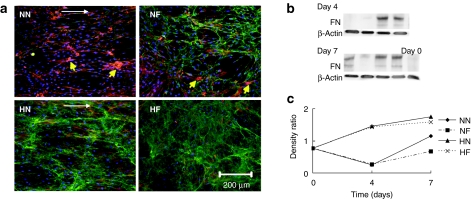
The connexin-43 secreted by cells cultured in conventional normoxic condition formed clusters on both flat (NF) and nanopatterned (NN) surfaces, whereas only distinct, small spots were observed in the 2% O2 conditions (Figure 2). Fibronectin, an important ECM protein responsible for cell adhesion was examined at the early culture period. Immunofluorescent staining at day 7 showed that more abundant fibronectin was secreted in the two hypoxic samples than in the two normoxic samples (Figure 3a). The counterstaining of F-actin illustrated that large gaps were formed between the cells on the two normoxic surfaces (NN and NF). Western blotting confirmed that the fibronectin secreted at days 4 and 7 was significantly higher in the two hypoxic samples (HN and HF) than in the two normoxic samples (NN and NF) (Figure 3b).
Figure 2.
Morphology of connexin-43 (green) at day 14 in hMSCs at conditions of NN, NF, HN and HF. The connexin-43 secreted by hMSCs cultured in the two 20% O2 conditions formed clusters on both nanopatterned (NN) and flat surfaces (NF), whereas only small spots uniformly distributed in the cultures at 2% O2 conditions (HN, HF). The F-actin was conunterstained with phalloidin (red). Yellow arrows indicate the connexin-43 clusters in the samples. White arrows indicate the direction of nanogratings on the surface of poly(dimethylsiloxan).
Figure 3.
Morphology and western blotting of fibronectin. (a) Morphology of fibronectin (green) at day 7 in human mesenchymal stem cells (hMSCs) at conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF). The F-actin was conunterstained with phalloidin (red). Yellow arrows indicate cell clusters. White arrows indicate the direction of nanogratings on the surface of poly(dimethylsiloxan). (b) Western blotting of fibronectin at four different conditions at days 4 and 7. (c) Quantitative analysis of fibronectin expression in hMSCs cultured in the four conditions. The expression of fibronectin was normalized with the expression of endogenous control β-actin. The fibronectin secretion at days 4 and 7 was significantly higher in the two hypoxic samples (HN and HF) than the other two normoxic samples (NN and NF).
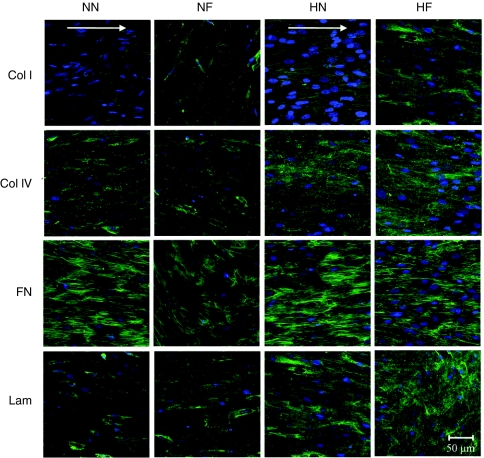
At day 14, there were significant differences in the amount of collagen I detected among the samples. The collagen I sparsely distributed in the NN and NF cultures, but organized into a network in the HN and HF samples. The two normoxic cultures appeared to express collagen I and collagen IV at significantly lower levels (assessed from staining intensity) than those seen in the two hypoxic cultures (Figure 4). For laminin and fibronectin, there were no apparent distinctions in the intensity of stains in the four cultures. Laminin analysis showed no difference in their organization, which displayed a disorganized polymer network, whereas fibronectin formed fibrils as a result of the high cell densities. Fibronectin fibrils in all the cultures also showed increasing alignment as the cells grew to confluency.
Figure 4.
Extracellular matrix (ECM) morphology (green) at day 14 in the conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF). Abundant fibronectin and laminin were secreted in all the samples. There were no apparent distinctions in the intensity of stains in the four cultures. The two 20% O2 cultures appeared to express collagen I and collagen IV at significantly lower levels than those seen in the two 2% O2 cultures. The cell nuclei was conunterstained with 4′,6-diamidino-2-phenylindole (blue). White arrows indicate the direction of nanogratings on the surface of poly(dimethylsiloxan).
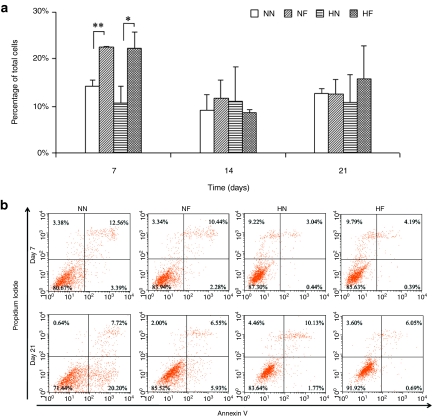
Cell cycle and viability
At day 7, the total percentage of cells in the proliferative state (S and M phases) on the two nanopatterned surfaces was significantly lower (P < 0.05) than those on the two flat surfaces. Beyond day 7, no significant difference was detected when comparison was made between the types of substrates (Figure 5a). Cell viability was evaluated by the flow cytometry–based Annexin-V/propidium iodide (PI) apoptosis assay. In this assay, viable cells are both Annexin-V and PI negative and cells undergoing apoptosis are Annexin-V positive and PI negative, whereas dead cells are both Annexin-V and PI positive. At day 7, there was a significantly higher percentage of dead cells in both NN and NF cultures (Figure 5b). However, the percentage of necrotic and apoptotic cells was significantly lower than their counterparts on HN and HF contains, respectively. After 21 days of culture, the NN samples displayed the lowest percentage of viable cells (71.44%) and the highest percentage of apoptotic cells (20.20%), whereas the HF samples gave the highest percentage of viable cells (91.92%) and the lowest percentage of apoptotic cells (0.69%) (Figure 5b). The percentage of apoptotic cells was 3.4 times higher in the NF (5.93%) than the HN (1.77%) samples.
Figure 5.
Cell proliferation and viability of hMSCs in the four conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF). (a) The percentage of cells in proliferation status (S and M phases) of the cell cycle over the 21-day culture period, obtained from flow cytometry–based propidium iodide (PI) cell cycle assay (supporting data). The total percentage of proliferating cells on the two nanopatterned surfaces was significantly lower than those on the two flat surfaces at day 7. There were no significant difference among all the samples after day 7. (b) Cell viability by flow cytometry–based Annexin-V/PI apoptosis assay at days 7 and 21. Significantly higher percentage of dead cells appeared in the 2% O2 samples, whereas fewer cells were in necrotic apoptotic status at day 7. At day 21 the NN samples displayed the lowest percentage of viable cells, while the HF samples gave the highest percentage of viable cells.
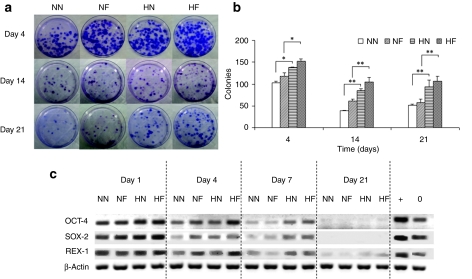
Colony-forming unit-fibroblasts and gene expression
The proportion of primitive cells in the constructs was determined by measuring the colony-forming ability of the cells (Figure 6a). All culture conditions resulted in a progressive reduction of progenitor population with time (Figure 6b). At all time points there were no significant difference in colony numbers between the two normoxic cultures and the two hypoxic samples. However, a significant difference appeared between the hypoxic and normoxic cultures starting from day 4, with 1.5- to 1.8-fold higher progenitor cells observed at hypoxic compared to normoxic conditions (P < 0.05).
Figure 6.
Stemness of human mesenchymal stem cells (hMSCs) in the four conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF). (a) Morphology of colonies formed by the cells from the four culture conditions. The cells were harvested after a predetermined time, and then further cultured for 14 days at a low seeding density. The colonies were stained with crystal violet. (b) The colony-forming ability of hMSCs. A significant difference appeared between the 2% O2 and 20% O2 cultures starting from day 4. (c) The RNA expression levels of stem cell genes Oct-4, Rex-1, and Sox-2, in hMSCs over the 21-day culture period. The expression of genes was normalized with the expression of endogenous control β-actin. Human embryonic stem cell sample was used as a positive control. Culture under 2% O2 condition enhanced all of the mRNA expression levels in hMSCs grown on both aligned pattern and flat surfaces.
To assess the stemness of hMSCs, we analyzed three stem cell markers that correlate with pluripotency:24 Oct-4, Rex-1, and Sox-2, using the WiCELL hES cell line (H-9) as a control. Reverse transcriptase (RT)-PCR analysis showed that hypoxic conditions maintained a higher expression of all these stem cell markers in hMSCs grown on both nanopatterned and flat surfaces in the first 7 days compared to their normoxic counterparts (Figure 6c). The expression levels in all samples decreased with time and were barely (Rex-1) or not (Oct-4 and Sox-2) detectable after 14 days.
Differentiation
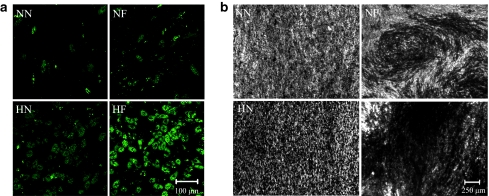
To determine whether the cells still maintained their multilineage capabilities after extended periods of culture, differentiation assays were carried out on cells cultured under the four conditions. The cells from all experimental conditions displayed ability to differentiate along both osteoblastic and adipogenic lineages. Cells cultured under hypoxic conditions (HN and HF) showed more advanced adipogenesis with significantly more lipid-vacuole formation than the normoxic counterparts (NN and NF) (Figure 7a). Von Kossa assay also showed more advanced osteogenesis with darker mineralized deposits (Figure 7b). On the nanopatterned surfaces, the cells from the hypoxic condition (HN) were still better aligned and with more calcium uniformly deposited than the normoxic counterpart (NN). On the flat surfaces, a larger and more uniform mineral deposited area was observed on the hypoxic sample (HF) than the normoxic one (NF).
Figure 7.
Multipotency of human mesenchymal stem cells (hMSCs) after 21-day culture in the four conditions of nanopatterned surface at 20% O2 (NN), flat surface at 20% O2 (NF), nanopatterned surface at 2% O2 (HN), and flat surface at 2% O2 (HF). (a) Adipocyte differentiation showed large lipid droplet clusters in the HN and HF constructs compared to the NN and NF samples. (b) Von Kossa staining indicated mineralization (dark regions) of the extracellular matrix (ECM) secreted by the cells. Significant calcium deposition was displayed within the ECM in the HN and HF cultures, while very little mineralization was displayed in the NN and NF samples. Samples were induced in adipoinductive and osteoinductive media for 21 days.
Discussion
The structural organization of tissues plays a critical role in dictating their function. Cell alignment is an important feature in many tissues. With the multipotency of hMSC, an aligned hMSC sheet would be a useful enabling technology for functional tissue engineering. To form an aligned hMSC sheet, the first crucial step is to grow hMSCs into confluency and high density, such that the cells can form tight junctions with each other, and secrete an abundance of ECM proteins to hold the cells together for harvesting.6,19,20 Topography is a potent cue to guide cell alignment.17,25,26,27 However, hMSCs tend to grow into a patchy structure (Figure 1), and differentiate along neuronal, myogenic, and osteogenic pathways when cultured on synthetic nanostructures.15,17,21 For many tissue engineering applications, it is highly desirable to maintain the undifferentiated state of the hMSCs so the cells can respond to the microenvironmental cues for optimal site-specific tissue development. Low-oxygen tension can effectively support hMSC survival, maintain their primitive status and improve the secretion of ECM proteins.18,22,28 Several in vitro studies have demonstrated that hMSCs show an upregulated expression of growth factors and cytokines, such as vascular endothelial growth factor and hepatocyte growth factor, under hypoxia.23,29,30 The underlining mechanism involves AKT signaling pathway and hypoxia-inducible factors.23,31,32
Cell alignment and uniformity on synthetic nanogratings
Substrate grating can effectively orient cells.26,27 Other than the width, the depth of the grating is also an important parameter. At the microscale, deep gratings appear to produce a nonuniform cell sheet.25 Portion of the cell layer grown on the ridges tends to be thinner, rendering the cell sheet more prone to tearing during handling and processing. Furthermore, deep grooves would likely lead to an increase in the time required for an intact sheet to form.25 We used a grating depth of 250 nm in this study, which appeared to be sufficient to orient the cells without causing heterogeneity of the cell sheet.
Low-oxygen (2%) culture can considerably improve the uniformity of cell layers. In our previous study,18 we have shown that on flat surfaces hMSCs grown under normoxic conditions formed clusters of cells that retracted from neighboring groups, forming small “islets”, whereas hMSCs from hypoxic conditions exhibited uniform cell orientation within various regions at an early stage (day 7), and multilayers at day 11. In this study, similar phenomena were observed on the nanopatterned surfaces. Despite the high confluency at day 14, hMSCs were densely packed and uniformly aligned within the whole area, at the centimeter scale, when cultured under 2% O2 (Figure 1). The distribution of the cell nuclear alignment angles confirmed that the percentage of aligned cells was significantly higher in the 2% O2 than the 20% O2 condition (75% versus 58%); and the range of alignment angles was also much narrower (30° versus 90° with respect to the grating axis).
Connexin-43 is a gap junction protein secreted by hMSCs that plays an important role in cell–cell communication.18 It resides intracellularly and not at gap junctional plaques in the noncommunicating cells.33 On the other hand, connexons are formed when the cells have uniform communications.34,35 In the 20% O2 group, the connexin-43 protein was mostly confined within the cells on both nanopatterned and flat surfaces (Figure 2). In contrast, connexons, visible as bright fluorescence puncta, were uniformly distributed in the two low O2 groups, indicating an established cell–cell communication in the cell layers.
The uniformity and high alignment of hMSCs grown on nanopatterned surfaces under low O2 conditions are attributed to the significant secretion and more uniform distribution of the ECM protein fibronectin in the early stage (Figure 3). Fibronectin contains cell adhesion domains that play an important role in promoting cell migration.36 Less secretion of fibronectin from hMSCs cultured under normal O2 would result in weak cell–substrate interaction in the beginning and strong cell–cell adhesion at the later stage, the consequence of which is a higher degree of cell clustering (Figure 3). This effect is even more acute on the nanopatterned surfaces. In contrast, under 2% O2 condition the cells exhibited higher cell–substrate adhesion and improved motility,23 leading to uniform cell layers on both flat and nanopatterned PDMS surfaces.
Gratings at the nanoscale significantly elongate hMSCs and their nuclei, leading to reduced proliferation and upregulation of neuronal-like phenotypes.17 The topography-mediated effects have been attributed to the mechanical forces that propagate to the nucleus through actin-intermediate filament system during the reorganization of cell shape, which further rearranges the nuclear matrix through deformation of cell nuclei over time.17,37,38 In this study, the hMSCs grown under the same oxygen tension, regardless on nanopatterned or flat surfaces, exhibited similar nuclear shape with no significant difference in the morphology parameters of elongation and roundness (Figure 1d). However, the nuclei of hMSCs from 2% O2 condition displayed a rounder shape, whereas those from 20% O2 were more elongated after 21-day culture. The difference in cell orientation and nuclear shape suggests that different physical forces are experienced by the cells between the two oxygen culture conditions.
The development of tissues and organs is guided by the organization and composition of ECM proteins.39 The physical state of the ECM, not only its molecular composition, functions as a regulator of cell–matrix adhesion.40 On vertical TiO2 nanotubes where the ECM deposition is nonuniform, the hMSCs extend their filopodia to search for the ECM proteins, thus forming extraordinarily elongated shapes.21 We have also observed that during the first 2 days after cell seeding, the cells on nanopatterned surfaces under 2% O2 were less spread than their normal-oxygen counterparts (data not shown). In agreement with the results of Oh et al., it is apparent that hMSCs grown under 2% O2 secrete more fibronectin that reduce their need to reach for ECM protein aggregates. Thus, they are able to maintain a nucleus that is more spherical. There also appears to be significant differences in the level of collagen I and IV secreted by the cells under the two O2 tensions. Transcriptional induction of collagen is proportional to the roundness of the nucleus.41,42 Thus, the nuclear elongation might affect the synthesis of collagen under normal-oxygen tension, which eventually influences the hMSC phenotypes in the cell layer.
Cell viability, stemness and pluripotency
Human MSCs possess great metabolic flexibility that enables them to survive in ischemic environments under hypoxia and glucose depletion,28 which may explain the lower percentage of apoptotic cells in the hypoxic samples at day 21. The higher expression of stem cell gene markers Oct-4, Rex-1, and Sox-2 in the hypoxic samples also suggests that the 2% O2 condition is a more favorable microenvironment for hMSC self-renewal than under normoxic condition. The more advanced adipogenesis and osteogenesis observed in the differentiation assays for the hypoxic samples corroborates with the gene expression profiling and confirms the benefit of creating an hMSC layer under low-oxygen tension for retention of a higher degree of stemness.
In summary, we report in this study the application of nanotopography and low O2 (2%) tension culture conditions to create a confluent, well-aligned cell layer comprising hMSCs in a relatively undifferentiated state. The cell layers are more uniform and contain greater amount of ECM proteins than their counterparts from conventional cultures. With the well-preserved multilineage differentiation ability of the hMSCs, and coupled with other technologies that detach the cell layer from the substrate43 and create three-dimensional structures,12 this approach will expand the capability of cell sheet engineering for the regeneration of complex tissues.
Materials and Methods
Production of nanopatterned PDMS with soft lithography. The nanopattern was generated by using electron beam lithography on the poly(methyl methacrylate)-coated Si wafer as previously described,16,17 and then replicated on PDMS (Ellsworth Adhesives, Germantown, WI) using soft lithography. The gratings on the patterned PDMS surfaces were 250 nm in depth, 350 nm in width, and with a 700 nm pitch. The three-dimensional structure (Supplementary Figure S1) was imaged by atomic force microscope (Digital Instruments Dimension 3100; Veeco, Plainview, NY). Before cell culture, the PDMS samples were coated with bovine collagen I (BD Biosciences, San Jose, CA) at 20 µg/cm2, cut into disks with diameters of 1.6 cm, and sterilized using 70% ethanol.
Cell culture. Bone marrow–derived hMSCs were provided by Tulane University Health Sciences Center. Briefly, bone marrow aspirates of about 2 ml were drawn from healthy donors ranging in age from 19 to 49 years under an institutional review board–approved protocol. Plastic adherent nucleated cells were separated from the aspirate and cultured using complete media [α-minimum essential medium with 20% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies, Rockville, MD)] at 37 °C and 5% CO2.18,44,45 The 5–6th passage of hMSCs from up to three donors were used. The cells were cultured at 95% air (20% O2)–5% CO2. For hypoxia studies, hMSCs were cultured in chambers that were flushed with humidified gas mixtures of composition 2% O2–5% CO2–93% N2. The cultures from the four conditions were abbreviated as: NN: 20% O2, normoxic, nanopatterned surface; NF: 20% O2, normoxic, flat surface; HN: 2% O2, hypoxic, nanopatterned surface; HF: 2% O2, hypoxic, flat surface.
Immunocytochemistry staining. The expression of various ECM proteins as well as connexin-43 was examined by immunocytochemistry staining, following a previously published protocol.18,45 Briefly, hMSCs grown on both PDMS surfaces were washed with Dulbecco's phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 15 minutes, blocked, and incubated with the primary antibody against fibronectin/collagen I/collagen IV/laminin/paxillin (Abcam, Cambridge, MA) or connexin-43 (Sigma, St Louis, MO) for 1 hour at 37 °C. Cells were washed and incubated with a mixture of fluorescein isothiocyanate–conjugated secondary antibodies conjugated to Alexa Fluor 488 (Abcam) and phalloidin conjugated to Alexa Fluor 568 (Jackson Laboratories, West Grove, PA). The samples were then washed and incubated in 4′,6-diamidino-2-phenylindole (Sigma) solution to counter stain the cell nuclei. Finally the samples were mounted and viewed using a Zeiss 510 confocal microscope (Carl Zeiss Microimaging, Thornwood, NY).
Cell nuclei analysis. Several separate regions of each sample were photographed using a Zeiss 510 confocal microscope with a ×40 magnification. The images were analyzed with ImageJ NIH image processing software (Bethesda, MD). Briefly, a threshold overlay was added to the cell nuclei, and then they were analyzed. Parameters such as the area and the perimeter, including major and minor axes, were processed. The cell alignment was calculated by taking the angle between the major axis of each cell nucleus and the main direction of the nanogratings. Cells were considered aligned if this angle was <15°. The elongation (E) parameter describes the extent to which the cell nucleus is lengthened or stretched out. It was calculated as the ratio of the long axis over the short axis minus one. The nuclear roundness (RN) parameter describes the irregularity of an object compared to a circle.46 Area (A) and perimeter (P) of the filled region that describes the projected cell nuclear were used to calculate RN, RN = P/[(4πA)0.5]. RN = 1 for a circle, and RN > 1 indicates the nuclear shape is less round. The percentage of cell alignment, the E-factor, and RN were measured. For each type of condition, an average of 300 cells was counted.
CFU-F. To determine colony-forming unit-fibroblast (CFU-F) numbers, hMSCs were harvested aseptically from PDMS discs using a solution containing 0.5% trypsin/0.25% collagenase/1 mmol/l EDTA in PBS. Cells were filtered using a cell strainer to ensure cell separation, and 800 cells were plated into a 10 cm Petri dish (14 cells/cm2). Samples for each condition were done in duplicate. The cells were grown for 12–14 days at 37 °C and 5% CO2 in a humidified incubator. Upon harvesting, cells were washed with PBS and stained with a 0.5% crystal violet solution for 10–15 minutes at room temperature. Cells were washed twice with PBS and imaged with a digital camera. The visible, intensely stained colonies were counted. Triplicate matrices from each chamber were used for each data point, and two independent runs were repeated under identical conditions.
Western blot analysis. The samples were removed from media and washed twice in PBS solution. Total protein was extracted from the matrices in a lysing buffer (Sigma) containing 1% Triton X-100 and protease inhibitors (Sigma). Cell lysate samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred electrophoretically onto nitrocellulose membranes (0.2 mm). The membranes were then blocked using a Tris buffer containing 0.1% Tween-20 and 5% dry milk. Membranes were then incubated with primary antibody overnight at 4 °C, washed with blocking buffer and incubated with a horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. Protein bands were determined by reacting the secondary antibody with an ECL plus substrate (GE Healthcare/Amersham, Pittsburgh, PA) to provide a chemiluminescent signal, which was detected using a FluorChem Imaging System (Alpha Innotech, San Leandro, CA). Blots were read on a densitometer and normalized to β-actin contents to quantify relative amounts of proteins. The results were reported as the ratio (density of the target band: density of β-actin band) for quantitative comparisons.
RT-PCR. The mRNA was extracted from 150 µl of original samples using the RNeasy MiniKit (QIAGEN, Valencia, CA). The purified mRNA was amplified in a one step RT-PCR reaction (QIAGEN) following the manufacturer's recommendations. Briefly, 5 µl ultrapure water containing 200 ng RNA was added to the RT-PCR mixture. This mixture contains 1 µl QIAGEN OneStep RT PCR enzyme mix, 5 µl of 5× QIAGEN OneStep RT-PCR buffer, 1 µl dNTP solution, 0.2 µl forward and reverse primer solutions, and 12.6 µl ultrapure water, that gives a final volume of 25 µl. The thermo cycler conditions used were: 50 °C for 30 minutes for reverse transcription, 95 °C for 15 minutes for the activation of the HotStart DNA polymerase. For the PCR we used a specific number of cycles (depending on the gene marker) consisting of the following cycles: 94 °C for 1 minute, an annealing temperature depending on the gene of interest for 30 seconds, 72 °C for 1 minute, followed by an extension period of 10 minutes at 72 °C. For RNA extraction and RT-PCR procedures, we included sufficient number of negative controls (using double distilled water). These negative controls did not show positive results, which indicated the absence of cross-contamination. PCR-products were run on a 2% agarose gel, stained with ethidium bromide, and visualized under UV-light using a FluorChem Imaging System (Alpha Innotech). The DNA bands were quantified using AlphaEaseFC (Alpha Innotech). Intensities were normalized to β-actin. The primer sequences for target genes are listed in Supplementary Table S1.
Flow cytometry. Flow cytometry was used for both cell cycle and apoptosis assays. Apoptosis assays were performed using an Annexin-V-fluorescein isothiocyanate apoptosis antibody (BD Biosciences) following the manufacturer's protocol. Briefly, hMSCs were trypsinized from the PDMS discs, counted, and resuspended in a binding buffer. The cell suspension was incubated with Annexin-V and PI for 15 minutes in the dark, and analyzed using a FACScan flow cytometer. For cell cycle assay, hMSCs were trypsinized, collected, and fixed with ice-cold absolute ethanol at 20 °C for 20 hours. The cells were then centrifuged and resuspended in a PBS buffer containing 20 µg/ml RNAase and 50 µg/ml PI. The samples were analyzed by flow cytometry within 72 hours. A peak-fitting program was used to model each of the cell cycle phases by gating analysis of the cytometric data. Statistical analysis of each iteration was performed to improve the data accuracy.
Differentiation assays. Differentiation assays were carried out after a 21-day culture period to determine whether the cell populations from both normal- and low-oxygen conditions still retained their multilineage potential. Both groups were differentiated under the same oxygen conditions (20% O2). For osteogenesis, the samples were cultured in osteoinductive media (Invitrogen, Carlsbad, CA). After 3 weeks, cells were fixed in 4% formaldehyde, placed in 5% silver nitrate solution, and exposed to UV-light for von Kossa assays. The samples were then incubated in sodium thiosulfate solution, mounted on a microscope slide, and viewed with a Nikon microscope (Nikon Instruments, Lewisville, TX).
To determine the adipogenic potential, cells were cultured with adipogenic induction medium for 21 days (Invitrogen). Adipocyte lipid-vacuole formation was detected using Nile Red staining. The cells were fixed in 4% glutaraldehyde. Stock Nile Red solution (1 mg/ml in acetone) was diluted in PBS at a ratio of 1:100 and the cells were incubated for 30 minutes. Cells were washed with PBS, mounted on a microscope slide, and viewed using a Nikkon microscope.
Statistics/data analysis. Experimental results were expressed as means ± SD of the means of samples. All the collected data were analyzed by analysis of variance for multiple comparisons, and statistical significance was accepted at P < 0.05.
SUPPLEMENTARY MATERIALFigure S1. Morphology of the nanogratings on the PDMS surfaces.Table S1. Primer sequence for target genes used in this study.
Supplementary Materials
Morphology of the nanogratings on the PDMS surfaces.
Primer sequence for target genes used in this study.
Acknowledgments
Young Clinical Scientist Award from Flight Attendant Medical Research Institute (FAMRI) to F.Z. (062518_YCSA) is acknowledged, as well as support by NIH (HL83008) and AOSPINE Foundation. We also thank Tulane University Health Sciences Center for providing the human mesenchymal stem cells.
REFERENCES
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatripnck LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Chan BP., and , Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17 Suppl 4:467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L., and , McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts–from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389–395. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, McAllister TN., and , de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Puleo C, Zhang Z., and , Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- Akahane M, Nakamura A, Ohgushi H, Shigematsu H, Dohi Y., and , Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2:196–201. doi: 10.1002/term.81. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chen CH, Lin WW, Hwang SM, Hsieh PC, Lai PH, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 2008;77:515–524. doi: 10.1093/cvr/cvm046. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chang Y, Wang CC, Huang CH, Huang CC, Yeh YC, et al. Construction and characterization of fragmented mesenchymal-stem-cell sheets for intramuscular injection. Biomaterials. 2007;28:4643–4651. doi: 10.1016/j.biomaterials.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Dang JM., and , Leong KW. Myogenic Induction of Aligned Mesenchymal Stem Cell Sheets by Culture on Thermally Responsive Electrospun Nanofibers. Adv Mater Weinheim. 2007;19:2775–2779. doi: 10.1002/adma.200602159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Reano RM, Pang SW, Yee AF, Chen CS., and , Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26:5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Pang SW., and , Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B., and , Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, et al. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Auger FA, Rémy-Zolghadri M, Grenier G., and , Germain L. The self-assembly approach for organ reconstruction by tissue engineering. J Regen Med. 2000;1:75–86. [Google Scholar]
- Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Izadpanah R, Bunnell B., and , Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- Rosová I, Dao M, Capoccia B, Link D., and , Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolf CM, Cho E., and , Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, et al. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials. 2008;29:2565–2572. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RB, Gooden MD, Lara SL., and , Wight TN. Microgrooved fibrillar collagen membranes as scaffolds for cell support and alignment. Biomaterials. 2005;26:3131–3140. doi: 10.1016/j.biomaterials.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Dadhania M, Rourke P, Desai TA., and , Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomater. 2005;1:93–100. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325–1336. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Xu M, Uemura R, Dai Y, Wang Y, Pasha Z., and , Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Grayson WL, Fröhlich M., and , Vunjak-Novakovic G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25:32–42. doi: 10.1002/btpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MC., and , Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD., and , Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Jordan K, Chodock R, Hand AR., and , Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114 Pt 4:763–773. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- Simek J, Churko J, Shao Q., and , Laird DW. Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J Cell Sci. 2009;122 Pt 4:554–562. doi: 10.1242/jcs.036970. [DOI] [PubMed] [Google Scholar]
- Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA., and , Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson CD., and , Curtis AS. Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp Cell Res. 2003;284:274–282. doi: 10.1016/s0014-4827(02)00053-8. [DOI] [PubMed] [Google Scholar]
- Putnam AJ, Schultz K., and , Mooney DJ. Control of microtubule assembly by extracellular matrix and externally applied strain. Am J Physiol, Cell Physiol. 2001;280:C556–C564. doi: 10.1152/ajpcell.2001.280.3.C556. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A., and , Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- Katz BZ, Zamir E, Bershadsky A, Kam Z, Yamada KM., and , Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CH, Collier JH, Sfeir CS., and , Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci USA. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani L, Grattarola M., and , Nicolini C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. Int J Biochem Cell Biol. 2004;36:1447–1461. doi: 10.1016/j.biocel.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Nagase K, Kobayashi J., and , Okano T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J R Soc Interface. 2009;6 Suppl 3:S293–S309. doi: 10.1098/rsif.2008.0499.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., and , Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng. 2005;91:482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- Zhao F, Pathi P, Grayson W, Xing Q, Locke BR., and , Ma T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005;21:1269–1280. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- Andersson AS, Bäckhed F, von Euler A, Richter-Dahlfors A, Sutherland D., and , Kasemo B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials. 2003;24:3427–3436. doi: 10.1016/s0142-9612(03)00208-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphology of the nanogratings on the PDMS surfaces.
Primer sequence for target genes used in this study.