Abstract
The potential of mesenchymal stem cells (MSC) in tissue regeneration is increasingly gaining attention. There is now accumulating evidence that MSC make an important contribution to postnatal vasculogenesis. During bone development and fracture healing, vascularization is observed before bone formation. The present study determined the potential of MSC, transduced ex vivo with a recombinant adeno-associated virus 6 (rAAV6) encoding bone morphogenetic protein 2 (BMP2) and vascular endothelial growth factor (VEGF) in a mouse model of segmental bone defect created in the tibiae of athymic nude mice. Mouse MSC that were mock-transduced or transduced with rAAV6-BMP2:VEGF were systemically transplanted following radiographic confirmation of the osteotomy. Effects of the therapy were determined by enzyme-linked immunosorbent assay measurements for BMP2 and VEGF, dual-energy X-ray absorptiometry (DXA) for bone density, three-dimensional microcomputed tomography (µCT) for bone and capillary architecture, and histomorphometry for bone remodeling. Results of these analyses indicated enhanced bone formation in the group that received BMP2+VEGF-expressing MSC compared to other groups. The therapeutic effects were accompanied by increased vascularity and osteoblastogenesis, indicating its potential for effective use while treating difficult nonunion bone defects in humans.
Introduction
Among millions of fractures suffered annually in the United States, 5–10% of these result in impaired healing, including nonunions.1 In most of these cases, bone regeneration needs to be enhanced for healing, and the failure to do so results in severe lifestyle impairment. Bone marrow-derived mesenchymal stem cells (MSC) have been demonstrated to be an attractive therapeutic cell source for tissue regeneration and repair. In recent years, gene transfer has emerged as an effective approach to deliver therapeutic proteins in a more physiological and persistent manner.2,3,4 Bone is a highly vascularized tissue, and angiogenesis plays an important role in bone growth and remodeling.5,6 Vascular endothelial growth factor (VEGF) is a potent angiogenic factor that is shown to be essential in the bone repair for both intramembraneous7 and endochondral bone formation.7,8 Bone morphogenetic proteins (BMPs) are a well-characterized growth factor and have been investigated for their capacity to improve bone healing. Among BMPs, BMP2 is an osteogenic growth factor commonly used in both ectopic and orthotopic sites for bone generation.9,10 It has been successfully tested in fracture sites in rats,11 rabbits,12,13 and dogs.14 However, there is concern that just a one-time exposure to an exogenous growth factor may not induce adequate osteogenic signal in many clinical situations where there is only limited bone-healing potential, which is due to compromised vascularity, limited bone stock, and abundant fibrous tissue.15 Recent work by Patel et al.16 demonstrated that dual delivery of angiogenic and osteogenic growth factors by gelatin microparticles, when incorporated within the scaffold pores, improved blood-vessel formation and bone growth, which in turn suggests an interplay between these growth factors for early bone regeneration. Studies have also demonstrated the importance of supplying extra VEGF for bone formation induced by osteogenic BMPs.17 VEGF, in addition to its role in cartilage resorption during endochondral bone formation,18 influences other steps in bone formation and bone-healing processes. As BMP2 and VEGF are involved in bone formation via different pathways, it is possible to attain a synergistic response of bone regeneration than with a single factor alone during the early stages of fracture healing. We hypothesized that MSC, modified to express both BMP2 and VEGF, can be used for controlled delivery of angiogenic and osteogenic factors and can mimic natural bone healing to promote bone regeneration in segmental defect. Using an ex vivo approach with genetically engineered MSC to express BMP2 and VEGF, the present study demonstrates that transplantation of MSC, expressing both BMP2 and VEGF, in a mouse segmental defect resulted in efficient bone formation that correlated with increased vascularity.
Results
MSC phenotyping and expansion of luciferase-expressing MSC for cell tracking
MSC were isolated from the bone marrow and cultured in vitro. The cultured cells appeared to form a morphologically homogeneous population of fibroblast-like cells and were confirmed by flow cytometry analysis as positive for CD105, CD73, CD44, CD29, and ScaI. The cells were also negative for CD45, CD34, and CD31. To track the marked MSC in vivo after systemic transplantation, luciferase-expressing MSC were isolated from a luciferase-transgenic mouse, and a transgene expression from the MSC was confirmed in a Berthold SIRIUS luminometer (Pforzheim, Germany). Before testing MSC in vivo their stem cell plasticity was confirmed by differentiating them into osteoblast, adipocyte, chondrocyte, and myocyte lineages as described earlier.19
Expression of BMP2 and VEGF from transduced MSC, and transplantation into mice with osteotomy
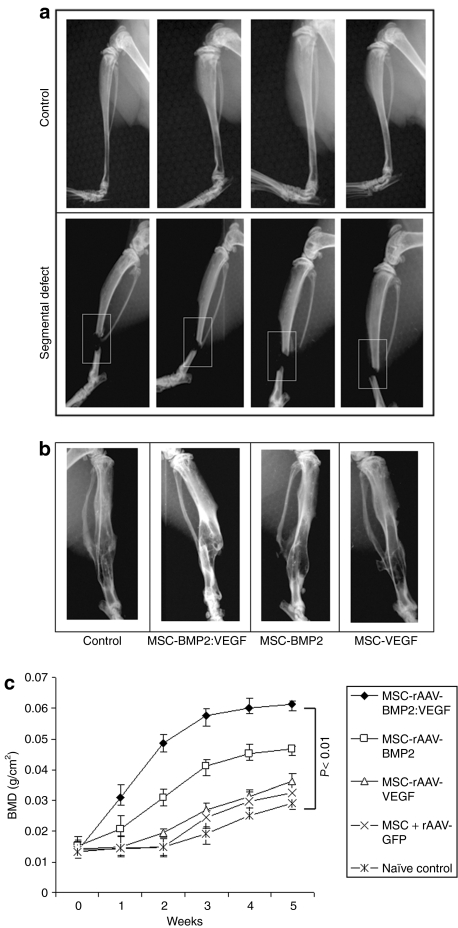
Culture-expanded MSC were mock-transduced or transduced with recombinant adeno-associated virus 6 (rAAV6)-BMP2:VEGF, and culture supernatants were used to determine the expression level of BMP2 and VEGF by enzyme-linked immunosorbent assay. Results indicated the secretion of BMP2 in transduced MSC was at the level of 41 ± 7 ng/106 cells and VEGF at the level of 7.5 ± 1.2 ng/106 cells. MSC were transduced with AAV-green fluorescent protein (GFP) control vector to normalize the basal levels of VEGF and BMP2 secretion. Transplantation of MSC, secreting dual-growth factors, showed augmented bone healing in the BMP2:VEGF group as monitored by X-ray image and dual-energy X-ray absorptiometry (DXA) analyses in mice with osteotomy (Figure 1a–c).
Figure 1.
Bone mineral density (BMD) in the segmental defect area of tibiae during fracture healing. (a) Segmental defect was surgically created in the right tibia of 10–12-week-old nude mice. (b) X-ray imaging of mice after 5 weeks of MSC transplantation showing the fracture healing process. (c) A total of 1 × 106 MSC that were unmodified, transduced with rAAV6-BMP2:VEGF or rAAV6-GFP, were administered in five consecutive days by intravenous injection. Mice were anesthetized with isoflurane for dual-energy X-ray absorptiometry (DXA) analysis. BMD was determined weekly around the fractured area of tibia by noninvasive DXA to follow the bone growth. DXA was performed in a GE Lunar PIXImus machine, and data analyses were performed using PIXImus software version 1.43.020. BMP2, bone morphogenetic protein 2; MSC, mesenchymal stem cells; rAAV, recombinant adeno-associated virus; VEGF, vascular endothelial growth factor.
Homing of systemically transplanted MSC to the fracture site
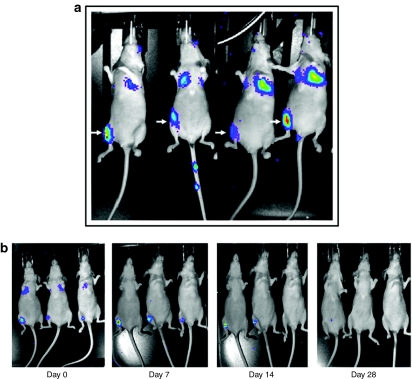
To investigate the homing of systemically transplanted MSC in vivo, the cells were intravenously injected (2 × 105 cells/mouse) after surgically creating a 2–3-mm segmental defect in right tibiae of mice. Bioluminescence imaging, performed after 24 hours of MSC transplantation, shown in Figure 2a, indicated the homing pattern of MSC upon in vivo administration. Initially, most of the cells were trapped in the lungs and liver, in addition to significant number of MSC in the region of osteotomy in the tibia; however, with time, injected MSC were cleared from lung, liver, and other tissues, as shown in Figure 2b and Supplementary Figure 1b,c. Other factors such as bioluminescence imaging and quantification of luciferase signal and immunohistochemistry with luciferase antibody in organs harvested from MSC-transplanted mice also confirmed this effect (Supplementary Figure 1a–c). Total body X-ray analysis, performed 5 weeks after MSC transplantation, indicated no unintended ossification in other soft tissues (Supplementary Figure S2).
Figure 2.
Biodistribution of systemically transplanted luciferase-positive MSC. (a) Luciferase-positive MSCs were intravenously transplanted into nude mice, following creation of tibial segmental defect in the right leg, and bioluminescence image was monitored after 24 hours. Representative images of mice showing homing-in-on of transplanted MSC to the site of segmental defect are shown above. (b) Bioluminescence imaging was performed at indicated time points to track long-term viability and existence of transplanted, luciferase-positive MSC in recipient mice. MSC, mesenchymal stem cells.
VEGF-enhanced neoangiogenesis resulted in proper vasculature around the segmental defect area
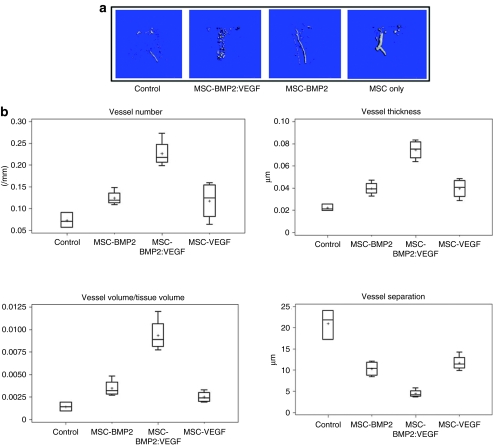
For quantification of angiogenic response in the MSC-transplanted mice, the procedure of microcomputed tomography (µCT)-based angiography using microfil was performed at week 5. Results of this experiment, as shown in Figure 3a, indicated several new sprouting blood vessels, which were spread around the segmental defect area in the BMP2:VEGF group compared to other MSC-injected group, where vessels numbers were low and vessels not well spread out. This suggests that growth factors released from the MSC in response to local stimuli did not fall below the critical physiological levels during the early fracture-healing process because blood vessel network was stable and consistent during the process of remodeling till 5 weeks. Microarchitectural parameters, shown in Figure 3b also corroborated increased vessel number and vessel connectivity density in BMP2:VEGF group compared to control groups (P < 0.03).
Figure 3.
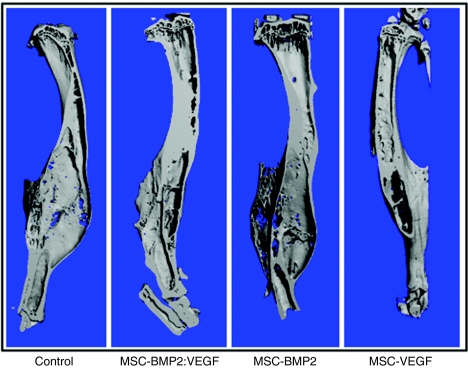
MSC-expressing osteogenic and angiogenic factors show vascularized bone development around segmental defect area of tibia and microarchitectural parameters of Microfil-perfused vasculature of tibia. (a) After intravenous transplantation of MSC, mice were sacrificed after Microfil perfusion from each group, and bones were used for µCT. Representative µCT images of vasculature in Microfil-perfused tibia (around the segmental defect area) show three-dimensional image of region of interest (ROI) extracted from reconstructed vasculature volume after 5 weeks of treatment of mice in control group; those injected with MSC-expressing BMP2 and VEGF, those injected with MSC-expressing BMP2, and those injected with MSC-expressing VEGF are shown. (b) Denoted values of microarchitectural parameters were determined from three-dimensional µCT measurements from groups of mice receiving AAV-BMP2:VEGF treatment or no treatment. Horizontal lines in each box from top to bottom indicate 25th, 50th, and 75th percentiles. Error bars indicate 10th and 90th percentiles. AAV, adeno-associated virus; BMP2, bone morphogenetic protein 2; µCT, microcomputed tomography; MSC, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
VEGF expression synergistically enhanced bone formation with BMP2
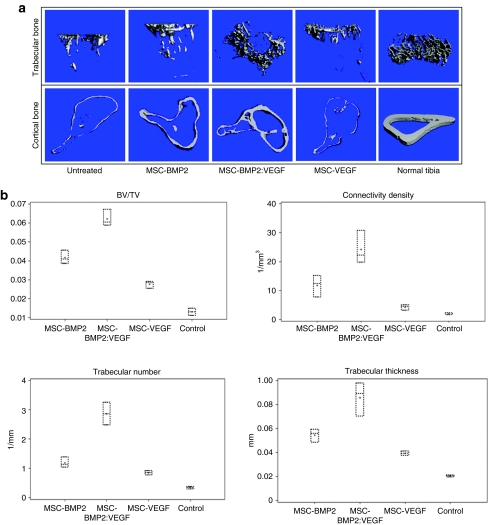
Noninvasive X-ray imaging of fractured tibia after 5 weeks indicated a robust bone growth around the fracture area in BMP2:VEGF group (Figure 1b). Cohorts of mice were evaluated for bone formation in the tibia around the fractured area by noninvasive DXA analysis. Results of DXA analysis of bone growth around the segmental defect area (see Figure 1c) indicated a gradual increase in bone density in all MSC-injected mice, but the group receiving BMP2 and VEGF had the highest bone growth compared to others. In addition, µCT analysis after week 5 reconfirmed the DXA finding, that the dual-therapy promoted highest bone regeneration. Bone formation was observed to be the least in the control and VEGF groups. To quantify the new bone growth as measured by microarchitectural parameters such as trabecular bone volume/tissue volume, trabecular connectivity density, trabecular number and trabecular thickness, and trabecular bone and cortical bone, µCT analysis was performed around the fracture area after 5 weeks of MSC therapy. Results of this analysis also corroborated with highest therapy effects on all these parameters in the group receiving MSC, expressing BMP2 and VEGF (Figure 4a,b).
Figure 4.
µCT analysis of cortical and trabecular bones of fractured tibia and microarchitectural parameters of trabecular bone around the fractured tibia after MSC therapy. (a) Tibia from MSC-transplanted cohorts of mice were used for µCT analysis of cortical bone and trabecular bones. Representative images from indicated groups show three-dimensional images of tibia extracted from reconstructed bone volume, 8 weeks after treatments. (b) Denoted values of microarchitectural parameters were determined from three-dimensional µCT measurements from groups of mice receiving indicated treatments or no treatment (control). Horizontal lines in each box from top to bottom indicate 25th, 50th, and 75th percentiles. Error bars indicate 10th and 90th percentiles. BMP2, bone morphogenetic protein 2; µCT, microcomputed tomography; MSC, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
VEGF increased bone mineral density and bone mineral content by increasing cell recruitment
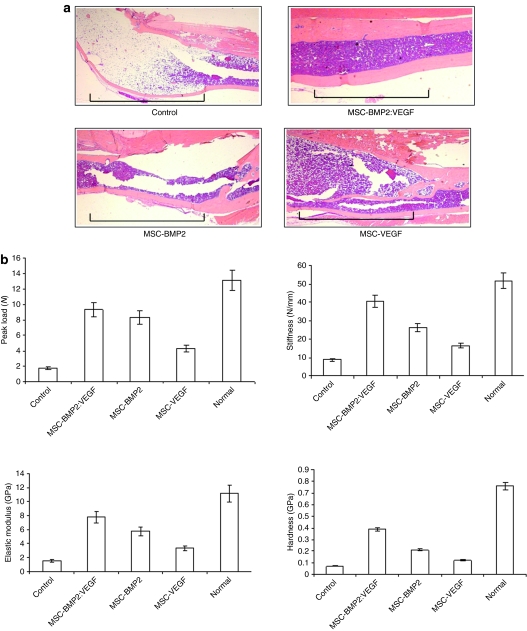
End-point µCT analysis of healed bones (see Figure 5) indicated the benefit of transplanting MSC-expressing BMP2 and VEGF on bone growth. Tibial bone defect was corrected with new bone growth leading to less deformed skeletal integrity compared to control tibiae, where callus was inflated due to inefficient bone-healing process. Histology of the healing area (see Figure 6a) indicated most efficient bone growth in the BMP2:VEGF group compared to other MSC administered groups. This group also had densely packed bone marrow with abundant marrow cells than other groups (Figure 6a).
Figure 5.
µCT analysis of fixed tibia after MSC therapy. Tibiae from MSC-transplanted cohorts of mice were used for µCT analysis. Representative images from indicated groups show three-dimensional images of tibiae extracted from reconstructed bone volume, 16 weeks after the treatment. BMP2, bone morphogenetic protein 2; µCT, microcomputed tomography; MSC, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
Figure 6.
Osteogenic and angiogenic factors induce synergistic enhancement of bone healing in a segmental defect of tibia. (a) Histology of bone sections after H&E staining indicates significant increase of newly formed bone in the area of osteotomy, following treatment with MSC-expressing BMP2 and VEGF, compared to other groups. The region of segmental defect is indicated by horizontal lines in each panel. (b) Three-point bending tests (peak load, stiffness) and nanoindentation (elastic modulus and hardness) were performed on tibia isolated from the MSC-transplanted and control mice after 16 weeks of treatment. Data shown represent mean ± SD. *P < 0.05. BMP2, bone morphogenetic protein 2; H&E, hematoxylin and eosin; MSC, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
Combination therapy increases the biomechanical quality of the bone
To assess the biomechanical quality of new bone growth following the MSC therapy of segmental defect in mice, tibiae were isolated from mice after 16 weeks of therapy and subjected to three-point bending test. Results as shown in Figure 6b demonstrated a significant increase in peak load, stiffness, and toughness of tibial bones in the dual-therapy group, compared to other MSC-injected groups (P < 0.05), and this increase in bone quality was the result of a delivery of both growth factors, BMP2 and VEGF, as a single-growth factor was not sufficient to attain comparable bone growth and biomechanical properties.
Discussion
MSC are nonhematopoietic adult stem cells with broad differentiation potential and, hence, are regarded as possible effectors for tissue repair in vivo. For most therapeutic purposes, systemic infusion of MSC remains the practical mode of administration. However, this also requires that MSC must be capable of migrating and homing in on to the targeted tissue upon systemic delivery. We are still in the process of learning about the cellular cues that enable MSC to be directed to the site of tissue damage and the mechanisms by which MSC then exert therapeutic effect. What limit the homing in, and engraftment, on MSC in vivo is unclear, and the exact molecular mechanisms underlying MSC homing also remain undefined thus far. However, use of ectopic homing signal α4β1-integrin on MSC increased the bone-homing potential of these cells, as observed in our earlier studies.19 The objective of the present study was that osteogenic response by osteoinductive factors would be augmented with concomitant induction of vascular network by the angiogenic factor VEGF, and this would facilitate proper nutrient supply required for the migration and recruitment of cells necessary to support bone-forming osteoprogenitors. Fracture healing is a complex physiological process that involves an orchestrated series of cellular events.20 Molecular mechanisms governing therapeutic effects of MSC are not well elucidated. However, it is attributed to a large number of potent mediators secreted from MSC in response to surrounding local stimuli that would potentially be more relevant to MSC therapeutic properties than transdifferentiation of MSC into homed tissue. In our transplantation model, MSC were not found to be integrated into newly formed bone but were present in a supportive role during early bone formation. MSC express high levels of growth factors such as hepatocyte growth factor, transforming growth factor, fibroblast growth factor-2 (FGF2), VEGF, matrix metalloproteinase-2, and matrix metalloproteinase-9, which may be involved to a certain extent in MSC-induced therapeutic response.
The use of luciferase-marked cells to track the homing and engraftment indicated that, upon intravenous administration of MSC, the cells homed in on to other organs, in addition to the fracture site, but luciferase signal from these nonspecifically homed organs became undetectable after 1 week of transplantation, possibly because these organs are not the natural niche for MSC. The gradual decrease of transplanted MSC over time also corroborates our previous studies.21 These analyses indicate that therapeutic effects observed in this study may also be due to the recruitment of endogenous bone progenitors stimulated by BMP2 and VEGF and other growth factors/cytokines expressed by transplanted MSC during the initial phase of bone healing. Bone regeneration requires co-operation and contribution from resident bone cells and components of bone marrow and thus leading to the development of proper vascular network around the injury, which will facilitate recruitment of osteoprogenitor cells for bone development. After transplantation, the transplanted MSC, expressing BMP2 and VEGF, possibly remained as a potential source for necessary growth factors and cytokines required for bone regeneration. The beneficial effects of MSC might be mediated, at least in part, by their ability to supply required amount of both angiogenic and osteogenic. Whether MSC that homed in on to regions other than the fracture site contributed to therapeutic effects by paracrine mechanism needs to be determined.
The use of synthetic radiopaque agent Microfil at an early time point allowed visualization and quantification of the vasculature, which showed dual therapy with BMP2 and VEGF had significant effect on neoangiogenesis with even distribution of blood vessels around the healing area with uniform vascular diameter and size. Vascularization is observed at the transition of preosteoblast to mature osteoblasts during both bone development and fracture healing. This suggests the effect of growth factors during dynamic interaction between several cell types in the bone marrow. Interpretation of synergistic increase in bone regeneration results also necessitates careful considerations, as BMP2 and VEGF can have multiple effects on different cell types, and what role each growth factors have during the process of bone healing needs to be further investigated. VEGF may cause proliferation and migration of endothelial cells, promote chemotaxis,22,23,24 and influence osteoblast differentiation.25,26,27 Similarly, BMP2 also has several important functions driving osteoprogenitor cell proliferation and differentiation and can, at the same time, play an indirect role in stimulating osteoblast production of VEGF28 and also engaging endothelial cells by chemotaxis.29 Increased bone volume and number of bone marrow cells in the dual therapy group suggests that increased vascularity during the bone-healing process may have resulted from continuous flow of all the necessary nutrients and cells needed for bone regeneration. The difficulty in interpretation of their role could be linked to the diversity of cells recruited in situ around the fracture area and to the elusive nature of the paracrine signals that are exchanged between the MSC and cells involved in fracture repair, for example, endothelial, osteoprogenitor, and stromal cells. Bone marrow–recruited pericytes have a major role during elongation, vessel maturation, and vascular remodeling.30,31 Furthermore, pericyte density was shown to have an impact on vessel morphology, which occurs in response to VEGF.32,33 Thus, MSC could facilitate the anchoring, maintenance, organization, and differentiation of endothelial cells into stable vascular structures because MSC also express immature pericyte markers.34 MSC being an integral component of bone marrow stromal system also shows a marked degree of phenotypic plasticity; a specific example is the ability to manipulate MSC in culture to differentiate into either adipocyte or chondrocyte and then subsequently “regress” the cells and direct them along the osteogenic pathways.35 Although generally a 2–3-mm-long segmental defect usually fails to heal, in our study, we observed modest bone growth in control mice also. This is possibly because mice used in our study were relatively of younger age. Evidence in recent literature also suggests that influence of age on MSC number and proliferation capabilities are at peak during young age but gradually decrease with age. It remains possible, however, to further improve the therapy effects by using other proangiogenic signals such as FGF and hypoxia-inducible factor-1α. In this study, FGF was not chosen despite its potential in inducing more mature vessels because, at low concentrations, FGF arrests the differentiation of MSC but maintains the stem cells in a pluoripotent state.36 In addition, the effects of this therapy could be maximized by local injection of modified MSC at the fracture site in situations where the defect is restricted to a specific region.
Overall, the present study demonstrates the potential of MSC-expressing BMP2 and VEGF in order to enhance bone healing in segmental defect. Future evaluation of this strategy in multiple fracture and nonunion fracture models should increase the potential of this approach for clinical use.
Material and Methods
Cells and reagents. Human embryonic kidney-293 cell line was purchased from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% newborn calf serum. Restriction endonucleases and other modifying enzymes were purchased from either NEB (Beverly, MA) or Promega (Madison, WI). Resources and material procured to conduct the experiment are as follows: BMP2 and VEGF Enzyme-Linked Immunosorbent Assay kits, purchased from R&D Systems (Minneapolis, MN), the α4-integrin antibody, purchased from e-Biosciences (San Diego, CA); GFP antibody, purchased from Abcam (Cambridge, MA); and luciferase antibody, purchased from Chemicon (Billerica, MA).
Construction of plasmids and production of recombinant AAV2. All AAV2 plasmids were constructed using pSub201 as the backbone.37 The complementary DNA-encoding rat BMP2 was kindly provided by Dr Chen (University of Texas at San Antonio, San Antonio, TX), and the complementary DNA-encoding mouse VEGF was excised from pBLAST49-mVEGF (InvivoGen, San Diego, CA). For bicistronic expression of BMP2 and VEGF, the coding sequence of BMP2 was cloned under cytomegalovirus promoter, and, VEGF sequence, following an internal ribosome entry site sequence downstream. The plasmid-encoding GFP has been described recently.37 Packaging of rAAV6 was done in an adenovirus-free system as described.37 Purification of the virions was done in a discontinuous iodixanol gradient centrifugation followed by heparin-affinity chromatography. Particle titers of the purified virions were determined by quantitative slot-blot analysis as described.37
Primary mouse MSC culture and gene transfer. Nude mice were purchased from the National Cancer Institute—Frederick Cancer Research Facility (Frederick, MD), and GFP transgenic mice [C57BL/6-Tg(ACTbEGFP)1Osb/J] were purchased from the Jackson Laboratories (Barr Harbor, ME). Luciferase-positive mouse MSC was purchased from Xenogen (Alameda, CA). All animal protocols were approved by the Institutional Animal Care and Use Committee. To obtain bone marrow-derived MSC, 4–6-week-old male mice were sacrificed; bone marrow was flushed from the femur and tibia; and the marrow mononuclear cells were purified by Ficoll gradient. Bone marrow stromal cells were grown in Stemline MSC expansion medium (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum and 10−9 mol/l FGF2 to maintain the cells in pluripotent and undifferentiated state.36 Residual macrophages from the MSC culture were removed by IMAC using antimouse CD11b beads (BD IMag; BD Biosciences, San Diego, CA). After 14 days, the adherent stromal cells were split before attaining confluence to avoid possible onset of differentiation. The cells were routinely prepared and used for in vitro and in vivo studies as low-passage cultures (passages 4–8). Undifferentiated MSC were transduced with 1,000 multiplicity of infection (1 multiplicity of infection = 50 genomic particles) of rAAV6-BMP2:VEGF or rAAV6-GFP. Virus infection was performed in Opti-MEM for 2 hours at 37 °C following which complete medium with FGF2 was added. The cells were grown for ten more days before transplantation into mice.
Creation of segmental defect in tibia. Approximately 10–12-week-old athymic nude mice were used for creating segmental defects. Each animal was anesthetized with an isoflurane and oxygen mixture transferred onto a heating pad (maintained at 37 °C) in the operating field. Right tibiae of mice were fractured using a three-point bending apparatus and a 2–3-mm-long segmental defect was created. The fracture was stabilized with external pins and surgical sutures and tapes. Tylenol was added to drinking water as postoperative analgesia. Animals had free access to food and water and were monitored daily in the postoperative phase, to look for any complications or abnormal behavior.
Transplantation of MSC. A total of six mice were included in each group. Mock-transduced or rAAV6 (GFP or BMP2:VEGF)-transduced MSC were resuspended in a volume of 100 µl of normal saline and intravenously administered into recipient mice through tail vein. Before in vivo administration, the cells were transiently transfected with the plasmid encoding the mouse α4-integrin. Cohorts of mice received a total of 1 × 106 MSC in five consecutive days (2 × 105 cells/injection) of untransduced, AAV6-GFP-transduced or AAV6-BMP2:VEGF-transduced MSC. Each week after transplantation, animals were subjected to DXA and after 16 weeks bones from each group were subjected to µCT analysis. Identification of homed MSC from the donor GFP mice was performed by GFP antibody staining, and luciferase MSC, by bioluminescence imaging.
Bioluminescent imaging. In vivo bioluminescence imaging was conducted in a cryogenically cooled IVIS-100 system (Xenogen) to detect luciferase expression in MSC using Living Image, an acquisition and analysis software (Xenogen). Briefly, mice were anesthetized with isoflurane and were intraperitoneally injected with 2.5-mg luciferin potassium salt (Xenogen) in phosphate-buffered saline. Imaging was performed after intravenous injection of luciferase-positive MSC. Image acquisition times were in the range of 10–240 seconds. The data acquisition software was calibrated to ensure that pixels remained saturated during image collection. Light emission from the tissue regions (relative photons/second) were measured using Living Image software (Xenogen). The intensity of light emission was represented with a pseudocolor scaling of bioluminescent images. The bioluminescent images superimposed on black-and-white images of mice were collected at the same time.
Quantification of BMP2 and VEGF levels in serum samples of transplanted animals. BMP2 and VEGF expressions in MSC, and in mouse serum, were collected from the animals at different time points and were determined using BMP2 and VEGF Quantikine kit Enzyme-Linked Immunosorbent Assay kits (R&D Systems), as per the manufacturer's instructions. A standard curve was generated using purified BMP2 and VEGF proteins to determine the concentrations of BMP2 and VEGF in serum.
DXA and µCT analyses of bone. For DXA analysis, animals were briefly anesthetized with isoflurane (2%)–oxygen mixture and placed in a prostrate position on the imaging plate. Bone mineral density, bone mineral content, and other body composition was assessed in vivo by DXA (GE-Lunar PIXImus, version 1.45; GE-Lunar, Madison, WI) periodically. To assess bone density, mass, geometry, and microarchitecture, intact tibia from each mouse was scanned using high-resolution µCT imaging system (µCT40; SCANCO Medical, Wayne, PA). Histomorphometric parameters, including bone volume, trabecular connectivity, trabecular thickness, trabecular separation, and degree of anisotropy, were evaluated.
Microfil perfusion and imaging of blood vessels. Cohorts of mice were sacrificed after 5 weeks to evaluate the blood-vessel formation. Mice were anesthetized with isoflurane, the thoracic cavity was opened surgically, and inferior vena cava was incised. The vasculature was flushed with normal saline containing heparin (100 U/ml) at a flow rate of 2 ml/minute via a needle inserted into the left ventricle. The specimens were then pressure-fixed with 10% buffered formalin. Formalin was flushed from the vessels in heparinized saline, and vasculature was injected with a radiopaque silicone rubber compound containing lead chromate (Microfil MV-122; Flow Tech, Carver, MA) solution prepared in a volume ratio of 4:5 of Microfil diluent with 5% curing agent. Samples were stored at 4 °C for contrast agent polymerization. Tibial bones were dissected and soaked for 48 hours in 10% buffered formalin to ensure complete tissue fixation. Tissues were subsequently treated in a formic acid-based solution, Cal-Ex II (Fisher Scientific, Pittsburgh, PA), to decalcify the bone and facilitate image thresholding of the vasculature from the surrounding tissues. Images were obtained using a high-resolution µCT imaging system (µCT 40; SCANCO Medical). Histomorphometric parameters, including vessel volume, connectivity, number, thickness, separation, and degree of anisotropy, were evaluated.
Biomechanical testing. All samples were fixed in formalin and stored in ethanol until mechanical testing. The bones were tested to failure by three-point bending on an 858 MiniBionix Materials Testing System (MTS Systems, Eden Prairie, MN). The samples were flexed at a rate of 0.03 mm/second with a support span of 10 mm for tibia with tension on the anteromedial surface. The load applicators were rollers of 2-mm diameter to avoid indenting the bone. Force–displacement data were plotted from which structural properties, including stiffness, peak load, and yield load, were obtained.38 Stiffness was calculated as the slope of the linear portion of the load–displacement curve. Peak load and peak displacement were taken as the maximum load and displacement values attained during the test. Yield load was obtained by using a line drawn at 0.002 mm, parallel to the linear portion of the load–displacement curve. Toughness was measured as the area under the curve until fracture, whereas energy to yield was obtained as the area under the curve until yield.
Histology. Formalin-fixed tissues were decalcified in EDTA solution for 2 weeks and embedded in paraffin. Longitudinal sections of 5-µm thicknesses were cut from paraffin-embedded blocks of frontal sections of tibia, using a Leica 2265 microtome. Sections were then stained with hematoxylin and eosin for morphological examination.
Statistical analysis. All data are reported as mean ± SD. Bone mineral density and bone mineral contents were analyzed using analysis of variance. Comparison of differences between two variables was performed using the two-tailed, two-sample (with equal variances), independent t-tests. Results were considered significant at P <0.05.
SUPPLEMENTARY MATERIALFigure S1a. Luciferase-positive MSC were transplanted intravenously and mice were sacrificed after 24 hours and lung, liver, spleen, heart, kidney, and adipose tissues were harvested.Figure S1b. Microscopic identification of transplanted MSC in soft tissues.Figure S1c. Quantitative analysis of luciferase expression in host tissues per microgram of protein.Figure S2. Whole-body CT scan to visualize unintended ossification.
Supplementary Material
Luciferase-positive MSC were transplanted intravenously and mice were sacrificed after 24 hours and lung, liver, spleen, heart, kidney, and adipose tissues were harvested.
Microscopic identification of transplanted MSC in soft tissues.
Quantitative analysis of luciferase expression in host tissues per microgram of protein.
Whole-body CT scan to visualize unintended ossification.
Acknowledgments
Financial support of the National Institutes of Health Grant R01AR50251 and the U.S. Army Department of Defense Grants BC044440 and PC050949 are gratefully acknowledged.
REFERENCES
- Praemer A, Furner S., and , Rice DP. Musculoskeletal Conditions in the United States. American Academy of Orthopaedic Surgeons: Rosemont; 1999. [Google Scholar]
- Nolta JA.ed.) (2006Gene Therapy for Mesenchymal Stem Cells. Springer Science [Google Scholar]
- Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84:1093–1103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., and , Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Ferrara N., and , Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z., and , Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Lieberman JR., and , Friedlaender GE. Bone Regeneration and Repair Biology and Clinical Applications. Gene Transfer Approaches to Enhancing Bone Healing. Humana Press; 2005. [Google Scholar]
- Schmoekel HG, Weber FE, Schense JC, Grätz KW, Schawalder P., and , Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yoshida G, Toyoda H., and , Takaoka K. Generation of tendon-to-bone interface “enthesis” with use of recombinant BMP-2 in a rabbit model. J Orthop Res. 2007;25:1415–1424. doi: 10.1002/jor.20447. [DOI] [PubMed] [Google Scholar]
- Fu TS, Chen WJ, Chen LH, Lin SS, Liu SJ., and , Ueng SW. Enhancement of posterolateral lumbar spine fusion using low-dose rhBMP-2 and cultured marrow stromal cells. J Orthop Res. 2009;27:380–384. doi: 10.1002/jor.20644. [DOI] [PubMed] [Google Scholar]
- Chang SC, Chuang H, Chen YR, Yang LC, Chen JK, Mardini S, et al. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J Surg Res. 2004;119:85–91. doi: 10.1016/j.jss.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Baas J, Elmengaard B, Jensen TB, Jakobsen T, Andersen NT., and , Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials. 2008;29:2915–2922. doi: 10.1016/j.biomaterials.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Jiang J, Fan CY., and , Zeng BF. Osteogenic differentiation effects on rat bone marrow-derived mesenchymal stromal cells by lentivirus-mediated co-transfection of human BMP2 gene and VEGF165 gene. Biotechnol Lett. 2008;30:197–203. doi: 10.1007/s10529-007-9532-1. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME., and , Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A, Carano D., and , Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- Kumar S., and , Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- Dimitriou R, Tsiridis E., and , Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Kumar S, Nagy TR., and , Ponnazhagan S. Gene Ther. e-pub ahead of print 10 September 2009.; 2009. Therapeutic potential of genetically modified adult stem cells for osteopenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AM, Bouchier-Hayes DJ., and , Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., and , Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C, et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- Hsiong SX., and , Mooney DJ. Regeneration of vascularized bone. Periodontol 2000. 2006;41:109–122. doi: 10.1111/j.1600-0757.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE., and , Löwik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- Midy V., and , Plouët J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem Biophys Res Commun. 1994;199:380–386. doi: 10.1006/bbrc.1994.1240. [DOI] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- Raida M, Heymann AC, Günther C., and , Niederwieser D. Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med. 2006;18:735–739. doi: 10.3892/ijmm.18.4.735. [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, et al. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]
- Krüger M., and , Bechmann I. Pericytes. Central Nervous System Diseases and Inflammation. Biomedical and Life Sciences: Springer US.; 2008. [Google Scholar]
- Gerhardt H., and , Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Bergers G., and , Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P., and , Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielby R, Jones E., and , McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38 Suppl 1:S26–S32. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ren C, Kumar S, Shaw DR., and , Ponnazhagan S. Genomic stability of self-complementary adeno-associated virus 2 during early stages of transduction in mouse muscle in vivo. Hum Gene Ther. 2005;16:1047–1057. doi: 10.1089/hum.2005.16.1047. [DOI] [PubMed] [Google Scholar]
- Kalajzcic I, Kalajzcic J, Hurley M, et al. Stage specific inhibition of osteoblast lineage differentiation by FGF2 and Noggin. J Cell Biochem. 2003;88:1168–1176. doi: 10.1002/jcb.10459. [DOI] [PubMed] [Google Scholar]
- Turner CH., and , Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Luciferase-positive MSC were transplanted intravenously and mice were sacrificed after 24 hours and lung, liver, spleen, heart, kidney, and adipose tissues were harvested.
Microscopic identification of transplanted MSC in soft tissues.
Quantitative analysis of luciferase expression in host tissues per microgram of protein.
Whole-body CT scan to visualize unintended ossification.